Abstract
Traditional nanosized photocatalysts usually have high photocatalytic activity but can not be efficiently recycled. Film-shaped photocatalysts on the substrates can be easily recycled, but they have low surface area and/or high production cost. To solve these problems, we report on the design and preparation of efficient and easily recyclable macroscale photocatalysts with nanostructure by using Ta3N5 as a model semiconductor. Ta3N5-Pt nonwoven cloth has been prepared by an electrospinning-calcination-nitridation-wet impregnation method, and it is composed of Ta3N5 fibers with diameter of 150–200 nm and hierarchical pores. Furthermore, these fibers are constructed from Ta3N5 nanoparticles with diameter of ~25 nm which are decorated with Pt nanoparticles with diameter of ~2.5 nm. Importantly, Ta3N5-Pt cloth can be used as an efficient and easily recyclable macroscale photocatalyst with wide visible-light response, for the degradation of methylene blue and parachlorophenol, probably resulting in a very promising application as “photocatalyst dam” for the polluted river.
Environmental problems associated with harmful pollutants in water pose severe threats to human health. Among the water treating methods, photocatalysis offers a “green” and energy saving technology for completely eliminating organic pollutants in water1,2,3. A prerequisite for the development of photocatalysis application is to gain access to excellent photocatalysts. Generally, two kinds of photocatalysts have been well developed. One kind is the nanosized semiconductor photocatalysts, including nanoparticles4, nanotubes5, nanowires6, nanosheets7, nanospheres8 and nanocomposites9,10,11. Recently, we have also prepared some nano-sized photocatalysts, such as Bi2WO6 superstructures12,13, and AgBr-Ag-Bi2WO6 nanojunction system14. They always show relatively high photocatalytic activity due to their nanoscaled particle size and large specific surface area. Unfortunately, it is very difficult to recycle these nanosized photocatalysts in practical application (such as degrading organic pollutants in lake and/or river), resulting in second-contamination and limiting their large-scale application. The other kind is semiconductor films on the substrates, such as nanoparticles-based composite films on ITO glass15,16, nanowires/nanotubes-based film grew on metal foil17,18. These film-shaped photocatalysts on the substrate can be easily recycled, but they suffer from the problems, such as relatively low surface area and/or high production cost. Thus, it is quite necessary to develop novel kind of photocatalysts. Ideal photocatalysts should have a broad range of visible-light response, superior photocatalytic activity, high photostability, low cost and easily recycling characteristics, and etc.
It is well known that micro/nano-fibers and nonwoven cloth can be easily prepared via electrospinning technique that represents a simple, cost-effective and versatile method for the large-scale production of fibers. Traditional micro/nano-fibers are polymer or polymer/inorganic composite fibers19,20,21. Few kinds of semiconductor nanofibers including TiO222, Bi4Ti3O1223, TiO2/SnO224 and GaN25 have been prepared for photocatalysis or photodetector. In photocatalytic application, semiconductor nanofibers have also suffered from the problems such as relatively low surface area and recycle difficulty. It should be noted that macroscale nonwoven cloth are usually composed of polymer or polymer/inorganic composite fibers, and they have already found use in applications (such as drug carriers, tissue engineering and ultrafiltration) and can be easily recycled. If semiconductor nonwoven cloth is composed of nanofibers that are constructed from semiconductor nanoparticles with plenty of hierarchical nanopores, it will have both high surface area and easily recycling characteristics for photocatalytic application. These features trigger our interest in the novel concept of developing efficient and easily recyclable macroscale semiconductor photocatalysts with nanostructure.
Among semiconductor photocatalysts, Ta3N5 with a narrow band gap of approximately 2.1 eV can absorb and utilize a large fraction of visible light up to 600 nm, and Ta3N5 nanomaterials26,27,28,29 and/or films30,31,32,33,34,35 have been prepared as visible-light-driven (VLD) photocatalysts. Herein, by using Ta3N5 as a model semiconductor, we report the design and preparation of Ta3N5-Pt nonwoven cloth that is composed of nanofibers constructed from Ta3N5 nanoparticles, hierarchical nanopores and Pt nanoparticles. The macroscale Ta3N5-Pt nonwoven cloth exhibits large surface area (23.1 m2 g−1). Furthermore, it can be used as an efficient, stable and easily recyclable macroscale semiconductor photocatalyst with nanostructure, for the degradation of both methylene blue (MB) dye and parachlorophenol (4-CP) under visible light irradiation. This finding promotes the design and development of novel kind of macroscale photocatalysts with nanostructure for practical application, for example, degrading pollutants in lake and/or river.
Results
Synthesis and characterization of the nonwoven cloth
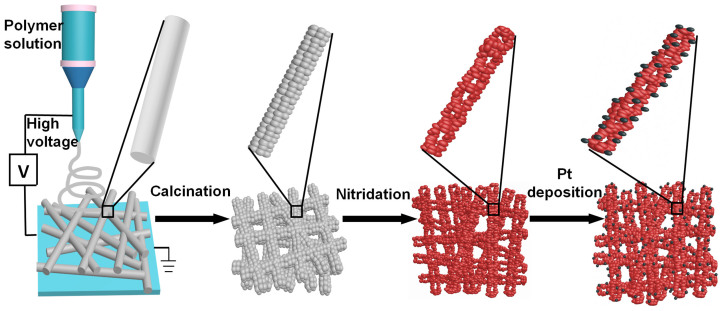
Ta3N5-Pt nonwoven cloth was prepared by an electrospinning-calcination-nitridation-wet impregnation method, as demonstrated in Figure 1. First step was to prepare PVP/Ta2O5/Ta(OEt)4 composite nonwoven cloth, by electrospinning the solution (ethanol-acetic acid mixture (3.3:1, volume ratio) containing 10 wt% tantalum ethanolate (Ta(OEt)4) and 5 wt% polyvinylpyrrolidone (PVP, MW ≈ 1300000 g mol−1)) at a high voltage of 15 kV, and followed by the hydrolysis process. The as-prepared PVP/Ta2O5/Ta(OEt)4 composite nonwoven cloth is white, and its typical photograph (area: ~10 × 9.5 cm2) is shown in Figure 2a. In fact, in our case, the area of the nonwoven cloth can be easily tuned in a broad range (10−4 ~ 1 m2) by changing the collecting region of aluminum foil during the electrospinning process. This macroscopic nonwoven cloth is composed of plenty of individual straight fibers with smooth surface and diameters ranging from 250 to 300 nm, as revealed in scanning electron microscopy (SEM) images (Figures 2b, 2c).
Figure 1. Schematic illustration of the preparation of Ta3N5-Pt nonwoven cloth.
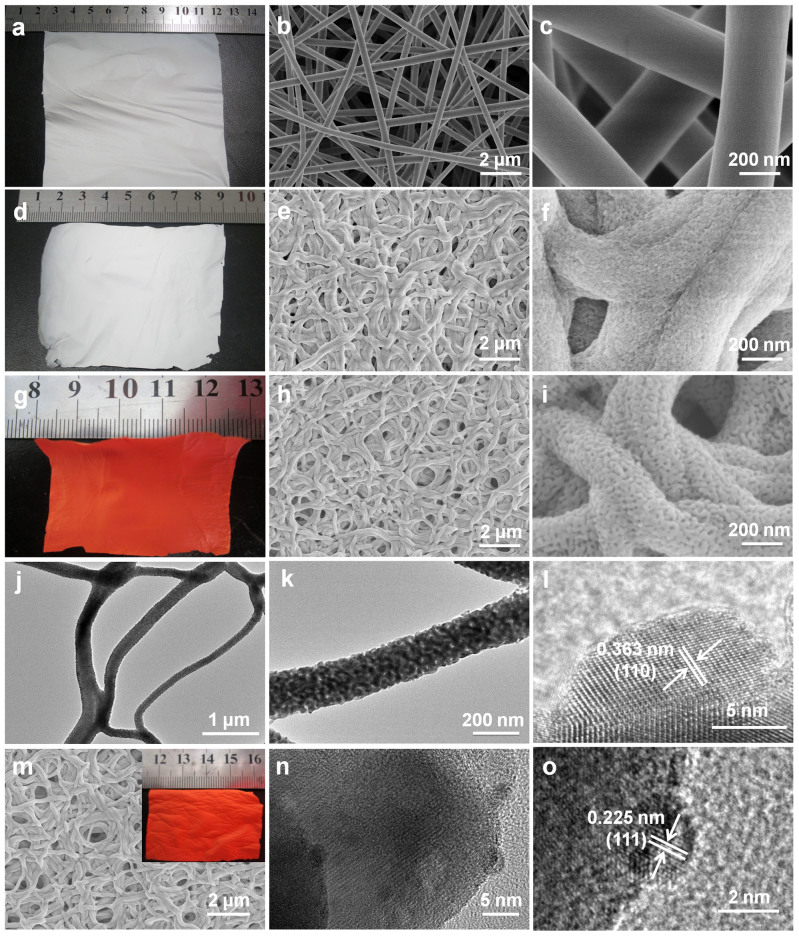
Figure 2. The photos and microscopy images of samples.
(a–c) The photo and SEM images of as-prepared PVP/Ta2O5/Ta(OEt)4 composite nonwoven cloth. (d–f) The photos and SEM images of Ta2O5 nonwoven cloth after the anneal in air at 600°C for 6 h. (g–i) The photo and SEM images of Ta3N5 nonwoven cloth after the nitridation at 800°C under NH3 flow (500 mL min−1) for 8 h. (j–l) TEM images of nanofibers from Ta3N5 nonwoven cloth and HR-TEM image of one nanoparticle in the nanofiber from Ta3N5 nonwoven cloth. (m–o) SEM image of Ta3N5-Pt nonwoven cloth, TEM image of Ta3N5 fiber decorated with Pt nanoparticles and HR-TEM image of one Pt nanoparticle on the surface of the nanofiber from Ta3N5-Pt nonwoven cloth.
The second step was to calcine the composite nonwoven cloth at 600°C in air for 6 h, for removing polymer component and obtaining inorganic nonwoven cloth based on Ta2O5 fibers. After the calcination process, this Ta2O5 nonwoven cloth still has the macroscopic morphology (Figure 2d) similar to that (Figure 2a) of the as-prepared PVP/Ta2O5/Ta(OEt)4 composite cloth, indicating that the calcination process has no obvious adverse effect on the macroscopic morphology. However, after the calcination, there are obvious changes in the microstructure of the cloth. This Ta2O5 nonwoven cloth consists of the pores and bent fibers that interweave and/or stick together (Figures 2e, 2f), which results from the disappearance of PVP component and the high-temperature anneal of Ta2O5 component. Furthermore, the diameters of Ta2O5 fibers shrink to 200–250 nm, and the fibers with rough surface are composed of nanoparticles with diameter of about 10 nm (Figures 2e, 2f).
The third step was to further nitridize Ta2O5 cloth at 800°C under NH3 flow (500 mL min−1) for 8 h to obtain Ta3N5 cloth. It should be noted that Ta2O5 nonwoven cloth was tailored to ~4.5 × 3 cm2 to fit the small inter-diameter (~5 cm) of the quartz furnace tube during the nitridation process. Obviously, such Ta3N5 cloth is still free-standing and can be easily transferred and/or recycled for further practical application. Its color turned from white to red-orange, as demonstrated vividly in Figure 2g, indicating the conversion from Ta2O5 to Ta3N5. SEM images (Figures 2h, 2i) reveal that Ta3N5 cloth is also composed of hierarchical pores (diameter: 0.2–1 μm) and fibers. The diameters of Ta3N5 fibers were reduced to 150–200 nm, and these fibers also interweave and/or stick together. Importantly, Ta3N5 fibers are comprised of plenty of nanoparticles with diameters of ~25 nm and nanopores with diameters of ~15 nm (Figure 2i), probably resulting in high surface area. Further information about Ta3N5 fibers was obtained from the transmission electron microscopy (TEM) images (Figures 2j–l). The TEM images (Figures 2j–l) confirm that Ta3N5 cloth is composed of fibers that are constructed from nanoparticles and nanopores, which agrees well with that revealed by the SEM images. The high-resolution TEM image (Figure 2l) taken from one nanoparticle in the fiber (Figure 2k) shows clear lattice fringes with an interplane spacing of 0.363 nm, which is corresponding to the (110) crystal plane of monoclinic Ta3N5. It should be noted that when the nitridation temperature was above 900°C, significant collapse of fibers occurred, resulting in the distortion of Ta3N5 cloth (Supplementary Figure S1).
At last, Ta3N5 cloth was decorated with Pt nanoparticles (~0.5 wt%) by the photocatalytic reduction of H2PtCl6 in methanol aqueous solution under a 300 W xenon lamp light irradiation. The decoration process of Pt has no obvious effects on the shape of cloth, as confirmed by photo (the inset of Figure 2m) and SEM image (Figure 2m). However, from the TEM image (Figure 2n), one can find that there are plenty of nanoparticles with the size of about 2.5 nm on the surface of fibers. The high-resolution TEM image (Figure 2o) shows clear lattice fringes with an interplane spacing of 0.225 nm, which is corresponding to the (111) crystal plane of cubic Pt. Thus, one can confirm the formation of Ta3N5-Pt nonwoven cloth with well-defined heterostructure.
The phase and pore structure, and optical characterizations
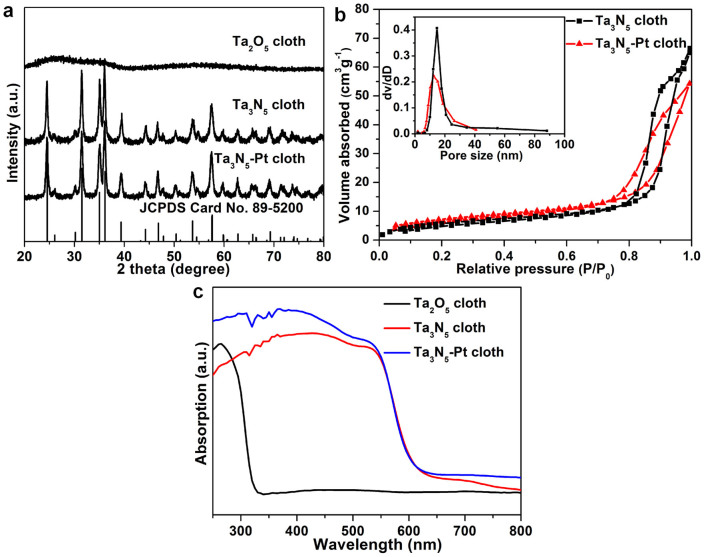
The phase structure of Ta3N5-Pt nonwoven cloth was further investigated. Figure 3a shows the X-ray diffraction (XRD) patterns of Ta2O5, Ta3N5 and Ta3N5-Pt nonwoven cloths. Obviously, Ta2O5 cloth is amorphous, while Ta3N5 cloth is well crystallized. All of the diffraction peaks for Ta3N5 cloth are characteristic of the monoclinic Ta3N5 (JCPDS Card No. 89-5200) (System: monoclinic, System group: C2/m(12), a = 10.229 Å, b = 3.875 Å, c = 10.229 Å). In addition, no characteristic peaks peculiar to the source materials or other impurities are observed. After the decoration of Pt nanoparticles, the XRD pattern of the Ta3N5-Pt cloth is quite similar to that of the Ta3N5 cloth. No obvious diffraction peaks from Pt can be detected, which should be attributed to the low amount of Pt on the Ta3N5-Pt cloth.
Figure 3. The phase and pore structure, and optical characterizations.
(a) The X-ray diffraction (XRD) patterns of Ta2O5 nonwoven cloth, Ta3N5 nonwoven cloth, Ta3N5-Pt nonwoven cloth and standard XRD pattern of Ta3N5 (JCPDS No. 89-5200). (b) Nitrogen adsorption–desorption isotherms of the Ta3N5 cloth and Ta3N5-Pt cloth. (c) The ultraviolet–visible diffuse reflectance spectra of Ta2O5 cloth, Ta3N5 cloth and Ta3N5-Pt cloth.
Subsequently, the nitrogen adsorption/desorption isotherms of Ta3N5 cloth and Ta3N5-Pt cloth were investigated (Figure 3b). The Brunauer–Emmett–Teller (BET) surface area of Ta3N5 cloth is calculated to be 22.0 m2 g−1. After the deposition of Pt nanoparticles, Ta3N5-Pt cloth exhibits a slight increase of BET surface area (23.1 m2 g−1). Thus, although both Ta3N5 cloth and Ta3N5-Pt cloth are macroscale, they have high surface area compared with those of bulk powders (Figure S4) and traditional fibers, resulting from their nanostructure. Moreover, the pore size distributions, which are calculated from the desorption branches, reveal the existence of nanopores in both Ta3N5 cloth and Ta3N5-Pt cloth (the inset of Figure 3b). The nanopores in Ta3N5 cloth have the diameter of about 15 nm, while those in Ta3N5-Pt cloth have the diameter of about 13 nm, which agrees with that revealed by the SEM and TEM images (Figures 2h–k, 2m). The presence of nanopores in fibers and macro-pores among fibers may greatly improve the physicochemical properties and/or serve as transport paths for small molecules.
The optical properties of Ta2O5, Ta3N5 and Ta3N5-Pt cloths were studied by using an UV-Vis-NIR spectrometer (Figure 3c). The spectrum of Ta2O5 cloth is similar to what has been reported previously for Ta2O5 samples26, and it exhibits a short-wavelength absorption edge at approximately 330 nm. Importantly, Ta3N5 cloth shows a large red shift from 330 to 600 nm, due to the band gap narrowing caused by the substitution of N for O atoms36, which agrees well with the reported value for the bandgap (Eg ≈ 2.1 eV) of Ta3N5 samples26,30. Furthermore, after the decoration of Pt, no obvious change of absorption spectrum has been observed. These facts indicate that both Ta3N5 cloth and Ta3N5-Pt cloth have a broad region of visible-light photo-response, and therefore can be expected to act as excellent VLD photocatalysts.
Photocatalytic activity
In order to investigate the potential of Ta3N5-Pt cloth as VLD photocatalyst, the photocatalytic activity of macroscopic Ta3N5-Pt cloth was evaluated by immersing the cloth in the solution containing MB dye or colorless 4-CP as the model pollutant (Figure 4). For comparison, bulk Ta3N5 powder, bulk Ta3N5 powder decorated with Pt nanoparticles (denoted as bulk Ta3N5-Pt powder), and mesoporous SiO2 powder decorated with Pt nanoparticles (denoted as SiO2-Pt powder) were also prepared and used as the photocatalysts. These bulk powders were also characterized by XRD, SEM, BET, UV-Vis-NIR spectrometer or TEM (Supplementary Figures S2–S6).
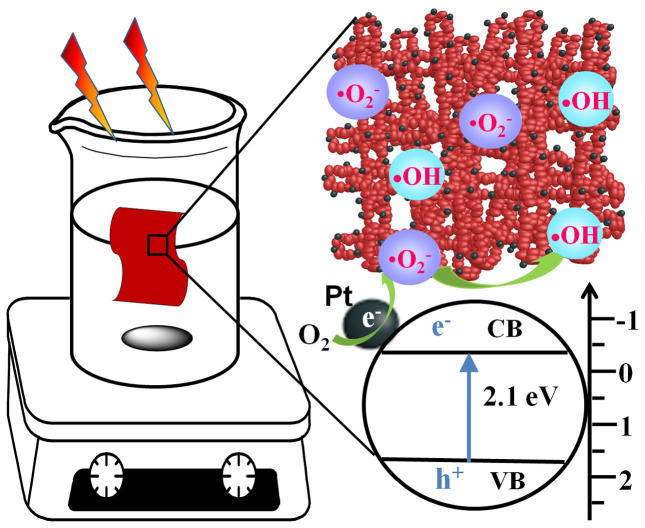
Figure 4. Schematic illustration of experimental setups and photocatalytic process.
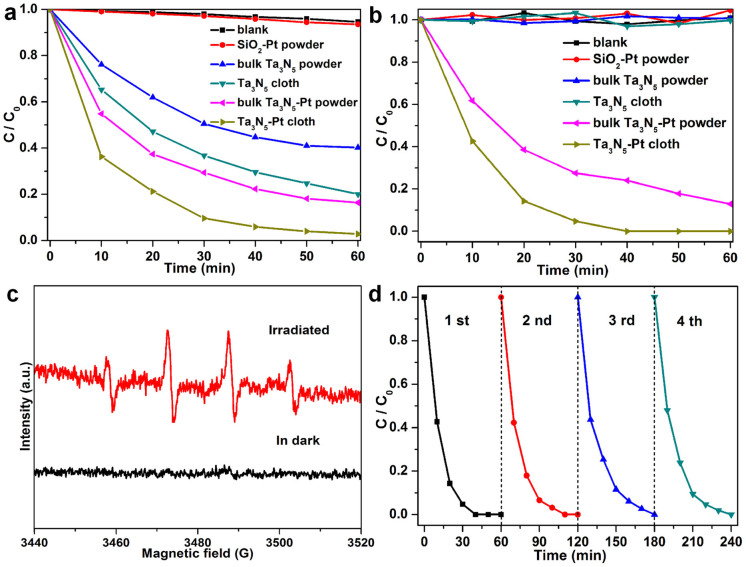
When MB dye was used as the model of organic pollutant, the photocatalytic activity of macroscopic Ta3N5-Pt cloth was evaluated by immersing the cloth (20 mg, size: ~2.5 × 3.5 cm2) in 60 mL aqueous solution containing 10 mg L−1 methylene blue (MB) dye under visible light irradiation (λ > 400 nm). When dissolved in distilled water, MB dye displays a major absorption band centered at 663 nm, which is used to monitor the photocatalytic degradation. With the macroscopic Ta3N5–Pt cloth as the photocatalyst, the temporal evolution of the absorption spectra of MB is shown in Supplementary Fig. S7. A rapid decrease of MB absorption at wavelength of 663 nm is observed, accompanied with an absorption band shift to shorter wavelengths. The color of MB solution gradually changes from initially blue to transparent as the reaction proceeds (the inset of Fig. S7), indicating that Ta3N5–Pt cloth exhibits excellent photocatalytic activity for the degradation of MB. For comparison, the photodegradation of MB without photocatalyst (blank test) and with SiO2-Pt, bulk Ta3N5 powder, bulk Ta3N5-Pt powder, or Ta3N5 cloth, was also measured under the other identical conditions, respectively (Figure 5a). The blank test indicates that the degradation of MB is extremely slow without photocatalyst under visible light illumination. By using bulk Ta3N5 powder as the VLD photocatalyst, the photodegradation efficiency of MB can just approach 59.8% after 60 min of reaction. When using Ta3N5 cloth as the photocatalyst, 79.4% of MB is photocatalytically degraded after 60 min. This indicates that Ta3N5 cloth exhibits higher photocatalytic activity than bulk Ta3N5 powder, which can be attributed to its higher BET surface area and hierarchical nanopores. Interestingly, after the decoration with Pt nanoparticles, the Ta3N5–Pt cloth can degrade 97.2% of MB after 60 min, indicating the highest photocatalytic activity. To investigate the role of Pt nanoparticles in the photocatalytic process, the photocatalytic degradation of MB was conducted in the presence of SiO2-Pt powder and bulk Ta3N5-Pt powder, respectively. Obviously, the SiO2-Pt is inactive under visible light irradiation, and the photodegradation of MB is even similar to that of the blank test, which reveals that both SiO2 and Pt have no photocatalytic activity. However, after the deposition of Pt, bulk Ta3N5-Pt powder exhibits the improved photodegradation efficiency (83.7%) of MB after 60 min, compared with that (59.8%) of bulk Ta3N5 powder. These facts indicate that Pt nanoparticles can greatly improve the photocatalytic activities of Ta3N5, which results from the fact that Pt act as electron trap to facilitate the separation of photogenerated electron-hole pairs and promote interfacial electron transfer process37,38.
Figure 5. Photocatalytic performances of Ta3N5-Pt cloth.
The degradation efficiency of (a) MB aqueous solution (10 mg L−1, 60 mL) and (b) 4-CP aqueous solution (1.28 mg L−1, 60 mL), versus the exposure time under visible light irradiation (λ > 400 nm), in the absence of photocatalyst and in the presence of SiO2–Pt powder, bulk Ta3N5 powder, bulk Ta3N5-Pt powder, Ta3N5 cloth or Ta3N5-Pt cloth. (c) DMPO spin-trapping ESR spectra recorded at ambient temperature in the aqueous solution with Ta3N5 cloth for DMPO-·OH under visible light irradiation (λ = 532 nm). (d) Cycling photocatalytic test of Ta3N5–Pt nonwoven cloth (20 mg).
When colorless parachlorophenol (4-CP) was used as the model of organic pollutant, the photocatalytic activity of macroscopic Ta3N5-Pt cloth was evaluated by immersing the cloth (20 mg, size: ~2.5 × 3.5 cm2) in 60 mL aqueous solution containing 1.28 mg L−1 4-CP under visible light irradiation (λ > 400 nm). 4-CP as a typical pollutant has no photolysis and no visible light absorption characteristics in the photodegradation process. When the macroscopic Ta3N5–Pt cloth was used as the photocatalyst, the temporal degradation of 4-CP was determined by high-performance liquid chromatography (HPLC) profiles (Supplementary Fig. S8). The peak with retention time of 8.5 min is attributed to the initial 4-CP and is used to monitor the photocatalytic degradation. As the reaction proceeds, the peak decreases rapidly in the reaction, indicating that Ta3N5–Pt cloth exhibits high photocatalytic activity for the degradation of 4-CP. For comparison, the photodegradation of 4-CP without photocatalyst and with SiO2-Pt, bulk Ta3N5 powder, Ta3N5 cloth or bulk Ta3N5-Pt powder, was also measured with otherwise identical conditions, respectively (Figure 5b). The photodegradation of 4-CP without photocatalyst and with SiO2-Pt, bulk Ta3N5 powder or Ta3N5 cloth is extremely slow and nearly no 4-CP is degraded after 60 min. Surprisingly, after decoration with Pt nanoparticles, the photocatalytic performances of both bulk Ta3N5-Pt powder and Ta3N5-Pt cloth are dramatically improved for the degradation of 4-CP. After 60 min of visible light irradiation, the Ta3N5–Pt powder exhibits higher photodegradation efficiency of 4-CP (87.2%) than that by Ta3N5 cloth, which can be attributed to the enhanced separation of photogenerated electron-hole pairs in Ta3N5-Pt heterostructure. With macroscopic Ta3N5-Pt cloth as photocatalyst, only a 40 min period was required to decompose all the 4-CP in the solution (Supplementary Fig. S8 and Fig. 5b), further demonstrating the highest photocatalytic activity. Compared with bulk Ta3N5-Pt powder, the outstanding photocatalytic activity for the degradation of 4-CP by Ta3N5-Pt cloth can be ascribed to its relatively higher BET surface area (23.1 m2 g−1) and special hierarchical structure.
It is well known that mineralization is the ultimate goal in pollutant treatment. Total organic carbon (TOC) value as an important index for the mineralization of organic species, was studied in the photodegradation of 4-CP (60 mL, 20 mg L−1) by 250 mg of Ta3N5-Pt cloth (Supplementary Fig. S9). It is clear that the TOC concentration of the solution continuously decreases, indicating that 4-CP is steadily mineralized by Ta3N5-Pt cloth photocatalyst under visible light irradiation. After 120 min of irradiation, the TOC concentration decreases from 10.76 mg L−1 to 3.66 mg L−1, reaching a high mineralization ratio of 66%. This fact demonstrates that Ta3N5-Pt cloth can efficiently degrade and mineralize organic pollutants under the irradiation of visible light.
It has been reported that the conduction and valence band edges of Ta3N5 at pH 0, are at approximately −0.4 V and +1.7 V versus NHE, respectively39. Since the redox potential value of photogenerated hole (φ(h+)) is approximately equal to that (+1.7 V) of the valance band, the φ(h+) is lower than φ(OH·/H2O)(+2.38 V versus NHE)14. As a result, the ·OH radicals can not be produced via the direct oxidation of H2O molecules by photo-induced holes. The photogenerated electron (φ(e−)) is more negative than φ(O2/·O2−)(−0.33 V versus NHE), which allows the production of ·O2− via the reduction of O2 by conduction band electrons. To confirm this conjecture, the electron spin resonance (ESR) technique (with 5,5-dimethyl-pyrroline N-oxide, DMPO) was used to obtain the information on the active radicals involved in the solution with Ta3N5 cloth irradiated by visible light or un-irradiated. Because ·O2− in water is very unstable and undergoes facile disproportionation rather than slow reaction with DMPO40, the involvement of ·O2− was examined in DMSO in which the DMPO-·O2− has a longer life time41. The characteristic peaks of the DMPO-·O2− adducts were observed in DMSO solution with Ta3N5 cloth irradiated by visible light (Supplementary Fig. S10), while no ·O2− signal was detected in dark under otherwise identical conditions, which are in good agreement with the previous report42. Recently, there are several reports revealed that the ·OH can be generated from ·O2− with the assistance of the photoinduced electrons43,44,45. In our case, to confirm the presence of ·OH in the photocatalytic process, the aqueous solution with Ta3N5 cloth irradiated by visible light or in dark was measured by ESR. As shown in Figure 5c, the four characteristic peaks of DMPO-·OH (1:2:2:1 quartet pattern) were also observed in aqueous solution with Ta3N5 cloth irradiated with visible light, while no ·OH signal was detected in dark under otherwise identical conditions. This fact demonstrates that the ·OH can be produced from ·O2−, which is similar to the previous reports43,44,45. These ESR results confirm that ·OH and ·O2− were produced in the solution with Ta3N5 cloth under the irradiation of visible light, and they are supposed to finally induce the decomposition of organic pollutants.
Most importantly, the macroscopic Ta3N5-Pt cloth (present area: ~4.5 × 2.6 cm2) can be easily transferred and/or recycled in photocatalytic application. To evaluate the stability and reusability of macroscopic Ta3N5-Pt cloth, a recycling test was performed, as shown in Figure 5d. The photodegradation of 4-CP was monitored for four cycles (each cycle lasted 60 min). After each cycle, the macroscopic Ta3N5-Pt cloth was taken out and washed with water. Then the cloth was immersed in the same volume (60 mL) of fresh 4-CP solution again. The photocatalytic activity of Ta3N5-Pt cloth does not significantly decrease in the cycling test and the photodegradation efficiency of 4-CP can still reach 100% for the fourth cycle. Thus, during four cycles, there is no significant loss of photocatalytic activity. The SEM image and the XRD patterns (Supplementary Fig. S11) further confirm that there are no obvious changes in the morphology and the crystalline phase of Ta3N5-Pt cloth before and after recycling reactions, indicating excellent stability and reusability of Ta3N5-Pt cloth.
Discussion
On the basis of the above results and energy band diagram, the photocatalytic process of Ta3N5-Pt cloth can be proposed, as shown in Figure 4. The photocatalytic activity of macroscopic Ta3N5-Pt cloth was evaluated by immersing the cloth in aqueous solution containing model pollutant due to its macroscale size (present area: ~4.5 × 2.6 cm2). Ta3N5 with the narrow band-gap (2.1 eV) has a broad range of visible-light photo-response and can exhibit efficient visible-light photoabsorption. The photocatalytic reaction is initiated by the absorption of visible-light photons with energy equal or higher than the band-gap in Ta3N5 semiconductor, which results in the creation of photogenerated holes in its valence band (VB) and electrons in its conduction band (CB). Because of the small particle size of Ta3N5 nanoparticles (~25 nm), the charge carriers can quickly travel to the surface of the catalyst from the interior. Then CB-electrons easily flow into metal Pt through the Schottky barrier because the CB (or the Fermi level) of Ta3N5 is higher than that of the loaded metal Pt, which is consistent with the previous study on electron transfer from semiconductor (such as TiO2) to Pt37,38. This process of fast electron transfer contributes to enhancing interfacial charge transfer and realizing the efficient separation of VB-holes and CB-electrons in the heterostructures37,38. Thus, plenty of CB-electrons in Pt component are available to reduce O2 to produce ·O2−, which can be further transformed into ·OH with the assistance of the photoinduced electrons43,44,45. Under successive attacks by ·O2− and ·OH, MB and 4-CP were effectively photodegraded, as demonstrated in Figures 5a,b and Supplementary Figures S7–S9.
It is noteworthy that hierarchical pores in Ta3N5-Pt cloth are supposed to play an important role in this photocatalytic process. As mentioned above, there are plenty of nanopores with diameter of ~13 nm inside Ta3N5-Pt fibers and micro-pores with sizes of 0.2–1 μm beside Ta3N5 fibers, probably resulting in two positive effects. The one effect is that micro-pores with sizes of 0.2–1 μm increase the photoscattering and absorption of visible light (Supplementary Fig. S12), since the photoabsorption can be enhanced if the nanoarrays are aligned with photonic-crystal microstructures, and/or the faceted end planes of well-shaped crystals serve as good laser-cavity mirrors46,47 The other results from the fact that the hierarchical combination of smaller nanopores and larger macro-pores can be considered as transport paths48. It has been reported that chemical reactions can occur more easily when the transport paths, through which reactant molecules move in or out of the nanostructured materials, are included as an integral part of the architectural design49. The textural transport paths have been revealed to have the beneficial effect on photocatalysis48,50. We believe that the presence of transport paths in Ta3N5-Pt cloth also benefits the pollutant molecules to get to the reactive sites on the framework walls of photocatalysts, which results in excellent photocatalytic activity. Furthermore, these transport paths as well as macroscale size probably make Ta3N5-Pt cloth used as “microfiltration membrane” with photocatalytic activity, probably resulting in a very promising application as “photocatalyst dam” for the polluted river in the future (Supplementary Fig. S13).
In summary, macroscopic Ta3N5-Pt nonwoven cloth with hierarchical nanopores has been synthesized by an electrospinning-calcination-nitridation-wet impregnation method. Such free-standing cloth is composed of nanofibers constructed from Ta3N5 nanoparticles, hierarchical nanopores and Pt nanoparticles. Under visible light illumination, it exhibits excellent photocatalytic activities on MB and 4-CP degradation. Furthermore, it can be easily transferred and/or recycled, with good stability. It should be noted that the present Ta3N5-Pt cloth is still relatively fragile, further work should be carried out for obtaining Ta3N5-Pt cloth with better strength and flexibility, and work in this direction is already ongoing. More importantly, this work provides some insight into the design and development of novel, efficient and easily recyclable macroscale photocatalysts with nanostructure, for future practical photocatalytic application, for example, as “photocatalyst dam” for the photodegradation of organic pollutants in the polluted river.
Methods
Materials synthesis
Synthesis of Ta3N5 nonwoven cloth
At first, 10 wt% Ta(OEt)4 was dissolved in an ethanol-acetic acid mixture (3.3:1, volume ratio). Then, 5 wt% polyvinylpyrrolidone (PVP, MW ≈ 1300000 g mol−1) was added to the above solution. After vigorously stirring for 24 h, the precursor solution was loaded into a plastic syringe and the feeding rate was kept constant at 0.3 ml h−1 using a syringe pump. A high voltage of 15 kV was applied between the orifice and grounded aluminum foil at a distance of 20 cm. The collected PVP/Ta2O5/Ta(OEt)4 composite cloth was calcined at 600°C in air for 6 h to obtain Ta2O5 nonwoven cloth. The Ta2O5 cloth was further nitridized at 800°C under an ammonia flow (500 mL min−1) for 8 h to obtain Ta3N5 nonwoven cloth.
Synthesis of Ta3N5-Pt nonwoven cloth
Pt (0.5 wt%) was loaded on Ta3N5 nonwoven cloth by the photocatalytic reduction of H2PtCl6 in methanol aqueous solution under a 300 W xenon lamp light irradiation for 4 h.
Mesoporous SiO2 was prepared according to the reference51. SiO2-Pt powder: Pt (0.5 wt%) was loaded on SiO2 by the photocatalytic reduction of H2PtCl6 in methanol aqueous solution under a 300 W xenon lamp light irradiation for 4 h.
Bulk Ta3N5 powder was prepared by thermal nitridation of bulk Ta2O5 powder synthesized in our laboratory. Bulk Ta2O5 powder: 2.5 g Ta(OEt)4 was dissolved in 30 ml absolute alcohol to obtain Ta(OEt)4 solution, then the ethanolic Ta(OEt)4 solution was quickly added into 30 ml aqueous solution with pH8 under magnetic stirring, the precipitate was collected by centrifugation, washed with ethanol and distilled water, and dried in an oven at 100°C, then the dried precipitate was calcined at 800°C for 3 h; Bulk Ta3N5 powder: Bulk Ta2O5 powder was nitridized at 850°C under an ammonia flow (500 mL min−1) for 15 h to obtain bulk Ta3N5 powder.
Bulk Ta3N5-Pt powder
The procedure of Pt loading is the same as that of Ta3N5-Pt nonwoven cloth except that the Ta3N5 nonwoven cloth was replaced by bulk Ta3N5 powder.
Characterizations
X-ray diffraction (XRD) measurements were recorded on a D/max-2550 PC X-ray diffractometer using Cu Kα radiation (λ = 0.15418 nm). The scanning electron microscope (SEM) characterizations were performed on a Hitachi S-4800 field emission scanning electron microscope. The transmission electron microscope (TEM) analyses were performed by a JEOL JEM-2010F high-resolution transmission electron microscope. The optical diffuse reflectance spectrum were conducted on a UV-VIS-NIR scanning spectrophotometer (UV-3101PC, Shimadzu) using an integrating sphere accessory. Nitrogen absorption–desorption measurement were conducted on a Micromeritics ASAP 2020 nitrogen adsorption apparatus (USA). The BET surface area was determined by a multipoint BET method using the adsorption data in the relative pressure (P/P0) range of 0.05–0.3. A desorption isotherm was used to determine the pore size distribution via the Barret–Joyner–Halender (BJH) method, assuming a cylindrical pore model. Electron paramagnetic resonance (EPR) signals of paramagnetic species spin-trapped with 5,5-dimethyl-pyrroline N-oxide (DMPO) were recorded with a Bruker ESR 300E spectrometer. The irradiation source was a Quanta-Ray Nd:YAG pulsed laser system (λ = 532 nm, 10 Hz). The total organic carbon (TOC) values were detected by a Shimadzu TOC-VCPH total organic carbon analyzer.
Photocatalytic tests
Photocatalytic activities of the photocatalysts were evaluated by degradation of Methylene Blue (MB) dye and parachlorophenol (4-CP) contaminant in an aqueous solution under visible light irradiation using a 300 W xenon lamp (Beijing Perfect Light Co. Ltd., Beijing) with a cut-off filter (λ > 400 nm) as light source. In each experiment, Ta3N5 cloth (20 mg, size: ~2.5 × 3.5 cm2), Ta3N5-Pt cloth (20 mg, size: ~2.5 × 3.5 cm2), mesoporous SiO2-Pt (20 mg), bulk Ta3N5 powder (20 mg) or bulk Ta3N5-Pt powder (20 mg) as photocatalyst was added into 60 mL of MB aqueous solution (10 mg L−1) and 60 mL of parachlorophenol aqueous solution (1.28 mg L−1). The temperature of the reaction solution was controlled at 22 ± 2°C by cooling water. Before illumination, the suspension was mildly magnetically stirred in the dark for 3 h to ensure that an adsorption/desorption equilibrium was established between the photocatalysts and the target contaminant (MB and 4-CP). When the remaining MB and 4-CP concentration needed to be measured, at given irradiation time intervals (10 min), 3 mL aliquots were collected and centrifuged to remove the remaining solids for analysis. Then, for the photocatalytic test of MB, the absorption UV-vis spectra of the solution were recorded on a U-2910 UV-vis spectrophotometer (Hitachi, Japan). For the photocatalytic test of 4-CP, the 4-CP concentrations in the solutions were analyzed by high-performance liquid chromatography (HPLC) using an Agilent 1100 series (USA) equipped with a diode array detector (DAD) with wavelength set at 280 nm directly after filtration through a 0.22 μm hydrofacies syringe filter. The mobile phase was methanol (80%) and water (20%) and the flow rate was 0.5 mL min−1; In the TOC test, 250 mg of Ta3N5-Pt cloth was added into 60 mL of parachlorophenol aqueous solution (20 mg L−1). In the stability and reusability test of the catalyst, four consecutive cycles were tested. The catalysts were washed thoroughly with water and dried after each cycle, and then the cloth was immersed in the same volume (60 mL) of fresh parachlorophenol aqueous solution (1.28 mg L−1) again.
Author Contributions
L.S.Z. and Z.G.C. designed the experiments. S.J.L. performed the experiments, calculations and data analysis. H.L.W., K.B.X. and J.Q.H. assisted with some of the experiments. Z.G.C., J.S.L. and L.S.Z. guided the work and analysis. S.J.L. and L.S.Z. wrote the paper.
Supplementary Material
Electronic Supplementary Information
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 21377023, 21107013, 21171035, 41073060, and 51272299), Specialized Research Fund for the Doctoral Program of Higher Education (Grant Nos. 20100075110010 and 20110075120012), project of the Shanghai Committee of Science and Technology (13JC1400300), Innovation Program of Shanghai Municipal Education Commission (Grant No. 13ZZ053), the Fundamental Research Funds for the Central Universities and DHU Distinguished Young Professor Program.
References
- Hoffmann M. R., Martin S. T., Choi W. & Bahnemann D. W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 95, 69–96 (1995). [Google Scholar]
- Asahi R., Morikawa T., Ohwaki T., Aoki K. & Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 293, 269–271 (2001). [DOI] [PubMed] [Google Scholar]
- Kubacka A., Fernández-García M. & Colón G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 112, 1555–1614 (2012). [DOI] [PubMed] [Google Scholar]
- Chen X., Liu L., Yu P. Y. & Mao S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011). [DOI] [PubMed] [Google Scholar]
- Roy P., Berger S. & Schmuki P. TiO2 nanotubes: synthesis and applications. Angew. Chem. Int. Ed. 50, 2904–2939 (2011). [DOI] [PubMed] [Google Scholar]
- Wu H. B., Hng H. H. & Lou X. W. Direct synthesis of anatase TiO2 nanowires with enhanced photocatalytic activity. Adv. Mater. 24, 2567–2571 (2012). [DOI] [PubMed] [Google Scholar]
- Liu G. et al. Visible light responsive nitrogen doped anatase TiO2 sheets with dominant {001} facets derived from TiN. J. Am. Chem. Soc. 131, 12868–12869 (2009). [DOI] [PubMed] [Google Scholar]
- Sun J. H. et al. Bioinspired hollow semiconductor nanospheres as photosynthetic nanoparticles. Nat. Commun. 3, 1139 (2012). [Google Scholar]
- Xiong Z. & Zhao X. S. Nitrogen-doped titanate-anatase core–shell nanobelts with exposed {101} anatase facets and enhanced visible light photocatalytic activity. J. Am. Chem. Soc. 134, 5754–5757 (2012). [DOI] [PubMed] [Google Scholar]
- Li R. G. et al. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 4, 1432 (2013). [DOI] [PubMed] [Google Scholar]
- Xi G. et al. In situ growth of metal particles on 3D urchin-like WO3 nanostructures. J. Am. Chem. Soc. 134, 6508–6511 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang L. S., Wang W. Z., Zhou L. & Xu H. L. Bi2WO6 nano- and microstructures: shape control and associated visible-light-driven photocatalytic activities. Small 3, 1618–1625 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang L. S. et al. Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 17, 2526–2532 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang L. S. et al. Effective photocatalytic disinfection of E. coli K-12 using AgBr-Ag-Bi2WO6 nanojunction system irradiated by visible light: the role of diffusing hydroxyl radicals. Environ. Sci. Technol. 44, 1392–1398 (2010). [DOI] [PubMed] [Google Scholar]
- Jiang D. L., Zhang S. Q. & Zhao H. J. Photocatalytic degradation characteristics of different organic compounds at TiO2 nanoporous film electrodes with mixed anatase/rutile phases. Environ. Sci. Technol. 41, 303–308 (2007). [DOI] [PubMed] [Google Scholar]
- Zhang L. W., Wang Y. J., Cheng H. Y., Yao W. Q. & Zhu Y. F. Synthesis of porous Bi2WO6 thin films as efficient visible-light-active photocatalysts. Adv. Mater. 21, 1286–1290 (2009). [Google Scholar]
- Kitano M. et al. Synthesis of nanowire TiO2 thin films by hydrothermal treatment and their photoelectrochemical properties. Catal. Lett. 119, 217–221 (2007). [Google Scholar]
- Zhang Z. H., Zhang L. B., Hedhili M. N., Zhang H. N. & Wang P. Plasmonic gold nanocrystals coupled with photonic crystal seamlessly on TiO2 nanotube photoelectrodes for efficient visible light photoelectrochemical water splitting. Nano Lett. 13, 14–20 (2013). [DOI] [PubMed] [Google Scholar]
- Agarwal S., Wendorff J. H. & Greiner A. Progress in the field of electrospinning for tissue engineering applications. Adv. Mater. 21, 3343–3351 (2009). [DOI] [PubMed] [Google Scholar]
- Bognitzki M. et al. Nanostructured fibers via electrospinning. Adv. Mater. 13, 70–72 (2001). [Google Scholar]
- Jo E. et al. Core-sheath nanofibers containing colloidal arrays in the core for programmable multi-agent delivery. Adv. Mater. 21, 968–972 (2009). [Google Scholar]
- Li D. & Xia Y. N. Fabrication of titania nanofibers by electrospinning. Nano Lett. 3, 555–560 (2003). [Google Scholar]
- Hou D. F., Luo W., Huang Y. H., Yu J. C. & Hu X. L. Synthesis of porous Bi4Ti3O12 nanofibers by electrospinning and their enhanced visible-light-driven photocatalytic properties. Nanoscale 5, 2028–2035 (2013). [DOI] [PubMed] [Google Scholar]
- Liu Z. Y., Sun D. D., Guo P. & Leckie J. O. An efficient bicomponent TiO2/SnO2 nanofiber photocatalyst fabricated by electrospinning with a side-by-side dual spinneret method. Nano Lett. 7, 1081–1085 (2007). [DOI] [PubMed] [Google Scholar]
- Wu H. et al. GaN nanofibers based on electrospinning: facile synthesis, controlled assembly, precise doping, and application as high performance UV photodetector. Adv. Mater. 21, 227–231 (2009). [Google Scholar]
- Hara M. et al. TaON and Ta3N5 as new visible light driven photocatalysts. Catal. Today 78, 555–560 (2003). [Google Scholar]
- Ma S. S. K., Hisatomi T., Maeda K., Moriya Y. & Domen K. Enhanced water oxidation on Ta3N5 photocatalysts by modification with alkaline metal salts. J. Am. Chem. Soc. 134, 19993–19996 (2012). [DOI] [PubMed] [Google Scholar]
- Wang D. et al. Core/shell photocatalyst with spatially separated co-catalysts for efficient reduction and oxidation of water. Angew. Chem. Int. Ed. 52, 11252–11256 (2013). [DOI] [PubMed] [Google Scholar]
- Wu C. H. et al. Ta3N5 nanowire bundles as visible-light-responsive photoanodes. Chemistry - An Asian Journal 8, 2354–2357 (2013). [DOI] [PubMed] [Google Scholar]
- Feng X. J. et al. Ta3N5 nanotube arrays for visible light water photoelectrolysis. Nano Lett. 10, 948–952 (2010). [DOI] [PubMed] [Google Scholar]
- Higashi M., Domen K. & Abe R. Fabrication of efficient TaON and Ta3N5 photoanodes for water splitting under visible light irradiation. Energy Environ. Sci. 4, 4138–4147 (2011). [Google Scholar]
- Kado Y. et al. Enhanced water splitting activity of M-doped Ta3N5 (M = Na, K, Rb, Cs). Chem. Commun. 48, 8685 (2012). [DOI] [PubMed] [Google Scholar]
- Zhen C., Wang L. Z., Liu G., Lu G. Q. & Cheng H.-M. Template-free synthesis of Ta3N5 nanorod arrays for efficient photoelectrochemical water splitting. Chem. Commun. 49, 3019–3021 (2013). [DOI] [PubMed] [Google Scholar]
- Hou J. G., Yang C., Wang Z., Jiao S. Q. & Zhu H. M. Cobalt-bilayer catalysts decorated Ta3N5 nanorod array as integrated electrodes for photoelectrochemical water oxidation. Energy Environ. Sci. 6, 3322–3330 (2013). [Google Scholar]
- Li Y. et al. Cobalt phosphate-modified barium-doped tantalum nitride nanorod photoanode with 1.5% solar energy conversion efficiency. Nat. Commun. 4, 2566 (2013). [DOI] [PubMed] [Google Scholar]
- Fang C. M. et al. The electronic structure of tantalum (oxy)nitrides TaON and Ta3N5. J. Mater. Chem. 11, 1248–1252 (2001). [Google Scholar]
- Linsebigler A. L., Lu G. Q. & Yates J. T. Photocatalysis on TiO2 surfaces: principles, mechanisms, and selected results. Chem. Rev. 95, 735–758 (1995). [Google Scholar]
- Wang W.-N. et al. Size and structure matter: enhanced CO2 photoreduction efficiency by size-resolved ultrafine Pt nanoparticles on TiO2 single crystals. J. Am. Chem. Soc. 134, 11276–11281 (2012). [DOI] [PubMed] [Google Scholar]
- Chun W.-J. et al. Conduction and valence band positions of Ta2O5, TaON, and Ta3N5 by UPS and electrochemical methods. J. Phys. Chem. B 107, 1798–1803 (2003). [Google Scholar]
- Ma W. H. et al. An efficient approach for the photodegradation of organic pollutants by immobilized iron ions at neutral pHs. Chem. Commun. 1582–1584 (2003). [Google Scholar]
- Ben-Hur E., Carmichael A., Riesz P. & Rosenthal I. Photochemical generation of superoxide radical and the cytotoxicity of phthalocyanines. Int. J. Radiat. Biol. 48, 837–846 (1985). [DOI] [PubMed] [Google Scholar]
- Lhachatryan L., Vejerano E., Lomnicki S. & Dellinger B. Environmentally persistent free radicals (EPFRs). 1. generation of reactive oxygen species in aqueous solutions. Environ. Sci. Technol. 45, 8559–8566 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrini O., Oliveros E. & Braun A. M. Photochemical processes for water treatment. Chem. Rev. 93, 671–698 (1993). [Google Scholar]
- Fujishima A., Zhang X. & Tryk D. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 63, 515–582 (2008). [Google Scholar]
- Sun S. et al. Visible light-induced efficient contaminant removal by Bi5O7I. Environ. Sci. Technol. 43, 2005–2010 (2009). [DOI] [PubMed] [Google Scholar]
- Huang M. H. et al. Room-temperature ultraviolet nanowire nanolasers. Science 292, 1897–1899 (2001). [DOI] [PubMed] [Google Scholar]
- Tian Q. W. et al. Hydrophilic flower-like CuS superstructures as an efficient 980 nm laser-driven photothermal agent for ablation of cancer cells. Adv. Mater. 23, 3542–3547 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang L. Z. & Yu J. C. A sonochemical approach to hierarchical porous titania spheres with enhanced photocatalytic activity. Chem. Commun. 2078–2079 (2003). [DOI] [PubMed] [Google Scholar]
- Rolison D. R. Catalytic nanoarchitectures--the importance of nothing and the unimportance of periodicity. Science 299, 1698–1701 (2003). [DOI] [PubMed] [Google Scholar]
- Wang X. C., Yu J. C., Ho C. M., Hou Y. D. & Fu X. Z. Photocatalytic activity of a hierarchically macro/mesoporous titania. Langmuir 21, 2552–2559 (2005). [DOI] [PubMed] [Google Scholar]
- Han L. et al. One-pot morphology-controlled synthesis of various shaped mesoporous silica nanoparticles. J. Mater. Sci. 48, 5718–5726 (2013). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic Supplementary Information