T-cell acute lymphoblastic leukemia (T-ALL) originates from multiple gene alterations occurring in normal precursor T cells, and represents 20% of adult ALL cases. Several recurrent cytogenetic abnormalities, cryptic cytogenetic aberrations, micro-deletions and other genomic changes were described using fluorescence in situ hybridization (FISH) arrays and gene expression and mutation analysis. Among the alterations affecting cell growth and differentiation mechanisms,1, 2 a key pathogenetic role was demonstrated for NOTCH1-activating mutation, detectable in about 60% of the cases, with or without associated FBXW7 gene inactivation. Other frequent findings are CDKN2A/2B deletions and T-cell receptor (TCRB, TCRA–TCRD) gene translocations involving transcription factor (TAL1, TAL2, LYL1, LMO1, LMO2, LMO3, TLX1, TLX3, NKX2.1, NKX2.2, HOXA, MYC), tumor suppressor (WT1, LEF1, ETV6, RUNX1, GATA3) and signal transduction (PTEN, ABL1, NRAS, JAK1, JAK3) genes. Variously combined, these abnormalities concur to identify distinct molecular and prognostic subsets that could be targeted by new biology response modifiers to enhance the probability of cure.
Translocation t(8;14)(q24;q11), first reported in pediatric patients and detectable in about 1% of T-ALL cases, is the hallmark of an extremely aggressive syndrome characterized by hyperleukocytosis, lymphoma-like presentation, rapid neurological progression and poor response to chemotherapy.3 In t(8;14)(q24;q11), the MYC proto-oncogene, which is also a target of NOTCH1 activation and maps at 8q24, is activated and fused to TCRA/D genes, exerting transcriptional repression of cell cycle inhibitors p27 and p21. Because of its rarity, t(8;14)+ T-ALL is almost unknown (or under-recognized) in adults. In the MRC-ECOG study recruiting 782 successfully karyotyped patients, no t(8;14)+ T-ALL was recognized, although there were 102 patients with unspecified abnormal karyotypes,4 and no t(8;14)+ T-ALL was identified in two large series from the same group (n=356) and GIMEMA (n=90).5, 6
We identified an adult patient with t(8;14)+ T-ALL in whom a thorough clinico-laboratoristic investigation was performed, including the combined interphase (CI) FISH study of several genes potentially involved in T-ALL pathogenesis and the molecular evaluation of minimal residual disease (MRD). This 44-year-old male suffering from backache and malaise for 3 days before referral presented with a white blood cell (WBC) count of 251 × 109/l (100% lymphoblasts), hemoglobin 16.4 g/dl, platelets 159 × 109/l, modest diffuse lymphadenopathies, and enlarged spleen and liver palpable 5 and 3 cm below the costal margin, respectively. The chest film showed a mediastinal mass (34% of the transverse chest diameter), while routine biochemistry indicated liver involvement (aspartate transaminase 216 U/l, alanine transaminase 188 U/l, alkaline phosphatase 493 U/l, gamma glutamyl-trasferase 303 U/l, bilirubin 0.7 mg/dl), preserved renal function (urea 36 mg/dl, creatinine 1.28 mg/dl, creatinine clearance 99 ml/min, sodium 144 mmol/l, potassium 3.8 mmol/l, chloride 104 mmol/l, calcium 10.4 mmol/l), and high risk for tumor lysis syndrome (lactate dehydrogenase 5079 U/l, uric acid 9.1 mg/dl).
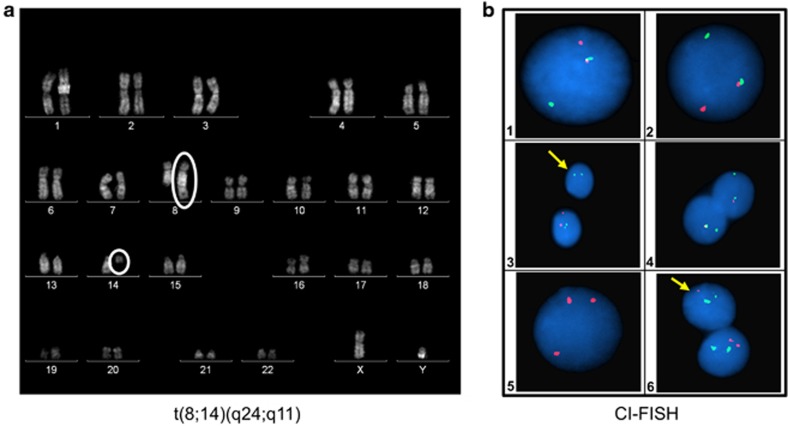
Flow cytometry analysis confirmed T-ALL with late cortical immunophenotype: cyCD3 94%, CD7 97%, CD2 92%, CD5 95%, CD4 70%, CD8 94%, CD1a 82%, sCD3 92% nuclear TdT was poorly expressed (11%), CD56 was 3% and myeloid/stem cell antigens were CD33 7%, CD117 70% and CD34 70% (CD13 not assessed). The cytogenetic analysis, performed according to Quinacrine-banding of 24 and 48 h unstimulated cell cultures, showed a 46 XY, t(8;14)(q24;q11)[13]/46 XY[3] karyotype (Figure 1a). The concurrent involvement of MYC proto-oncogene and TCRA/D genes was confirmed by FISH on metaphases exposed to LSI MYC/IGH/CEP8 tricolor dual fusion and LSI MYC and TCRA/D break apart Vysis probes: t(8;14)(D8Z1+,5′MYC+,3′TCRA/D+5′TCRA/D+,3′MYC+)[10]. Thirty-one genes, mapping as indicated in brackets and known to be implicated in T-ALL pathogenesis, were studied by CI FISH as previously reported:7 TCRB (7q34), TCRAD (14q11), TAL1 (1p32), FBXW7 (4q31), LEF1 (4q25), TLX3 (5q35), GRIK2 (6q16), CASP8AP2 (6q15), C-MYB (6q23), IKAROS (7p11), CDKN2A (9p21), JAK2 (9p24), ABL1 (9q34), NUP214 (9q34), NOTCH1 (9q34), PTEN (10q23), WT1 (11p13), LMO2 (11p13), NUP98 (11p15), CALM (11q14), MLL (11q23), ETV6 (12p13), RB1 (13q14), NF1 (17q12), PTPN2 (18p12), AF10 (10p13), C-MYC (8q24), BCL11B (14q32), AML1 (21q22), ERG (21q22) and PHF6 (Xp11). The analysis confirmed t(8;14)(q24;q11) involving TCRA/D and MYC genes in 98% and 82% of the cells studied, respectively. NOTCH1 and FBXW7 genes were not mutated (this being also excluded by denaturing high-performance liquid chromatography and sequencing), while additional aberrations consisted of SIL-TAL1 gene deletion (82%), biallelic CDKN2A/B gene deletion (88%), 10p13/AF10 gene gain (86%) and del(10)(q23)/PTEN deletion in a leukemic subclone (12%) (Figure 1b). Two molecular case-specific probes were generated to perform serial MRD evaluations (probe 1: TAL deletion type 1, sensitivity 10−5; probe 2: Jbeta 2.3, sensitivity 10−5).
Figure 1.
(a) t(8;14)(q24;q11) in a patient with T-ALL. (b) CI FISH results (the full list of gene-specific CI-FISH probes is available upon request to the authors): 1. TCRA/D (RP11-242H9+RP11-447G18, 14q11) break-apart FISH assay showing a split signal. 2. C-MYC (RP11-367L7+RP11-26E5, 8q24) break-apart-test abnormal pattern consisting of one fusion, one orange and one green signal. 3. Interphase nucleus showing (arrow) a biallelic deletion of RP11-149I2 (CDKN2A/B/9p21), Spectrum Orange probe. In green, ABL1 (RP11-57C19+RP11-83J21, 9q34). 4. SIL-TAL1 FISH probe detecting a SIL/1p33 deletion (G248P80397F3, Spectrum Orange). 5. AF10/10p13 Spectrum Orange specific probes (RP11-249M6+RP11-418C1) showing the presence of three signals. 6. Nuclei hybridized with RP11-380G5/PTEN, Spectrum Orange, and RP5-926B9+RP5-1002G3/NF1, Spectrum Green, proving (arrow) a PTEN/10q23 monoallelic deletion.
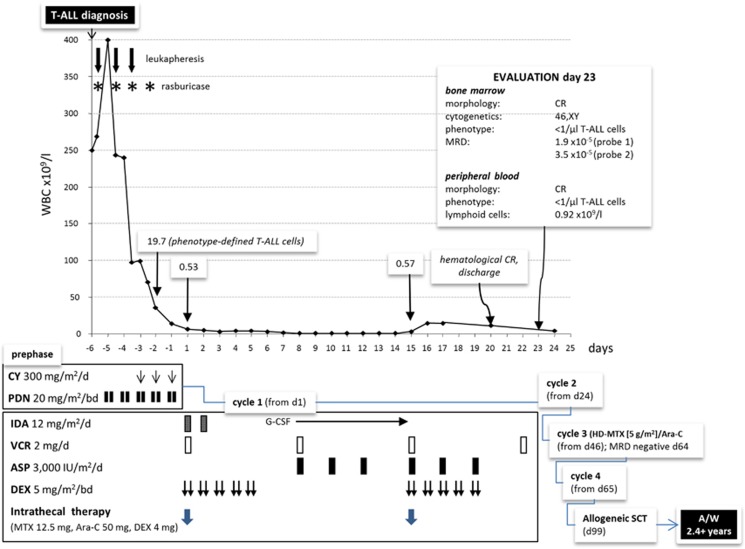
Although leukapheresis and rasburicase were immediately applied to prevent an acute tumor lysis syndrome, the WBC count increased to 400 × 109/l after 14 h, for an extrapolated doubling time of circulating blast cells of 23 h. Two more leukaphereses were performed and prephase therapy started. Treatment response is detailed in Figure 2. The induction block of the Northern Italy Leukemia Group (NILG) ALL protocol 10/07 (ClinicalTrials.gov NCT-00795756)8 led to a prompt hematological response (neutrophils and platelets >1 and >100 × 109/l, respectively) 20 days after diagnosis, the patient being discharged home 22 days after admission. On day 23 a complete hematologic, cytogenetic and molecular remission (CR) was confirmed, with MRD signals <10−4. Additional MRD tests were performed after cycle 3 and after allogeneic SCT at day 30, 100 and 180. A complete MRD clearing was documented after cycle 3 and maintained in all subsequent evaluations. Because with modern regimens T-ALL relapse is rarely observed after 18–24 months5, 9 and the patient is disease-free at 29 months from CR and off-therapy 26 months after SCT, the probability of cure appears very high.
Figure 2.
Schematic representation of clinical course and therapeutic response. Following an early rise in total WBC count soon after diagnosis, a rapid, complete hematological, cytogenetic (46,XY[20]), immunophenotypic (<1 CD1a/CD4/CD8/CD7/CD45+ cell × 103/μl) and molecular MRD (1.9 × 10−5 with probe; 3.5 × 10−5 with probe 2) remission was achieved after pre-phase and chemotherapy block 1. Three postremission consolidation blocks were administered in tight sequence, recycling on days 24 (block 2: cyclophosphamide, idarubicin, dexamethasone, cytarabine, 6-mercaptopurine, triple intrathecal therapy), 46 (block 3: high-dose methotrexate 5 g/m2 and cytarabine 2 g/m2) and 65 (block 4: like cycle 2). Because of being classified as very high risk, the patient was eligible for allogeneic stem cell transplantation (SCT) from a compatible sibling donor. On day 99 from diagnosis and following conditioning with total body irradiation, cyclophosphamide and anti-thymocyte globulin, the patient received a peripheral blood unmanipulated stem cell graft from his fully HLA/DR-matched sister (nucleated cells 10.7 × 108/kg, CD3+ T cells 209.7 × 106/kg, CD34+ cells 4.3 × 106/kg). Apart from mild chronic graft-vs-host disease, no major or life-threatening complication occurred in the post-transplantation period, and he remains well and alive in CR1 more than 2 years from diagnosis.
T-ALL carrying t(8;14) is very rare in adults and confers a dismal outlook. In the August 2013 update of the Mitelman registry,10 5 adult cases are reported in patients older than 15 years (range 17–35 years), compared with 31 childhood cases. The WBC count of the adult patients ranged between 46.6–320 and only one survived (67 months). Additional chromosomal alterations were detected in four: del(6)(q13q21),del(9)(p22); add(9)(p21),del(10)(q?),−14,+21; +i(7)(q10),−4,−Y,del(6)(q15q?23); and t(1;4)(p32;p12). The case with t(8;14) as sole abnormality, like ours, had the highest WBC count (320 × 109/l). An additional molecular study was performed in one case, excluding alterations of SIL-TAL1, HOX11L2, HOX11, CALM-AF3, MLL and CALM-AF5 genes.
Our report suggests that cure is possible in adult patients with this hyperkinetic ALL subset, possibly the fastest growing ever reported. The disease was of apparent thymic origin, as indicated by the enlarged mediastinum, the late cortical CD1a+ sCD3+ phenotype, and the preserved hemoglobin and platelet count, indicating a late marrow involvement. Its enormous proliferative capability was the most striking feature, to put in relation with the underlying gene abnormalities. The main lesion was t(8;14)(q24;q11), which differs from t(8;14)(q24;q32) of Burkitt leukemia/lymphoma (BL) for the different breakpoint in chromosome 14, fusing MYC to TCRA/D genes instead of immunoglobulin genes. BL is the most rapidly proliferating of all B-cell neoplasms, and is also TdT-negative, like a small fraction of T-ALLs, which is another interesting analogy with the current T-ALL case.11 Thus, all high WBC count and/or TdT-negative T-ALLs should be accurately screened for t(8;14)(q24;q11) and MYC rearrangements, in order to identify this elusive and highly malignant ALL syndrome. CI FISH appears particularly suitable as a rapid and sensitive diagnostic method, simultaneously providing useful information on associated genetic abnormalities, especially when no other cytogenetic alteration is detectable. In this case, the absence of NOTCH1 and FXWB7 gene mutations excludes a pathogenetic role of the former and portrays a worse clinical outcome. This case would belong to the recently recognized NOTCH1-independent/MYC-mediated T-ALL subsets, in which a concurrent PTEN downregulation, here present in a leukemic subclone, or other mutations, can act as a major pathway of MYC activation.12, 13 The concurrent CDKN2A/2B biallelic gene deletion is associated with the loss of other tumor suppressor genes (P16INK4A, P14ARF), to complete the picture of a hyperproliferative acute T-cell malignancy, with a central pathogenetic role for MYC activation.
In spite of the explosive growth rate, treatment was successful by employing a new intensive regimen preliminarily associated with an excellent outcome of adult T-ALL (74% overall and disease-free survival at 2 years in 24 patients) and high MRD early negativity rate (78% of high-risk patients with MRD <10−4 at week 10, that is, after course 3).8 The treatment schedule featured an extensive use of cyclophosphamide, dexamethasone, and antimetabolites such as cytarabine—both standard and high-dose, 6-mercaptopurine and methotrexate 5 g/m2 in keeping with pharmacokinetic studies in T-ALL,14 together with the shortest possible intercycle intervals to counteract the high rebound potential of the leukemic clone. Similar treatment concepts allowed to improve significantly the outcome of BL, the B-cell counterpart of t(8;14)/MYC+ ALL.15
In conclusion, t(8;14)(q24;q11)+ T-ALL is a rare, highly malignant syndrome sometimes observed in adult patients. The case herein described would exemplify the outer limits of the clinical aggressiveness of the disease. Even so, prompt recognition and careful start of management with modern T-ALL-specific therapy can warrant good therapeutic results, including an early complete molecular response. CI FISH analysis was essential to highlight distinct routes of molecular pathogenesis centered around MYC gene activation, and as such may contribute to identify new meaningful NOTCH1-independent targets within these disregulated metabolic pathways.
The authors declare no conflict of interest.
References
- Kraszewska MD, Dawidowska M, Szczepański T, Witt M. T-cell acute lymphoblastic leukaemia: recent molecular biology findings. Br J Haematol. 2012;156:303–315. doi: 10.1111/j.1365-2141.2011.08957.x. [DOI] [PubMed] [Google Scholar]
- Van Vlierberghe P, Ferrando A. The molecular basis of T cell acute lymphoblastic leukemia. J Clin Invest. 2012;122:3398–3406. doi: 10.1172/JCI61269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BJ, Raimondi SC, Heerema N, Nowell PC, Minowada J, Steinherz PE, et al. Pediatric leukemia/lymphoma with t(8;14)(q24;q11) Leukemia. 1992;6:613–618. [PubMed] [Google Scholar]
- Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- Marks DI, Paietta EM, Moorman AV, Richards SM, Buck G, DeWald G, et al. T-cell acute lymphoblastic leukemia in adults: clinical features, immunophenotype, cytogenetics, and outcome from the large randomized prospective trial (UKALL XII/ECOG 2993) Blood. 2009;114:5136–5145. doi: 10.1182/blood-2009-08-231217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale A, Guarini A, Ariola C, Mancini M, Mecucci C, Cuneo A, et al. Adult T-cell acute lymphoblastic leukemia: biologic profile at presentation and correlation with response to induction treatment in patients enrolled in the GIMEMA LAL 0496 protocol. Blood. 2006;107:473–479. doi: 10.1182/blood-2005-04-1754. [DOI] [PubMed] [Google Scholar]
- Gorello P, La Starza R, Varasano E, Chiaretti S, Elia L, Pierini V, et al. Combined interphase fluorescence in situ hybridization elucidates the genetic heterogeneity of T-cell acute lymphoblastic leukemia in adults. Haematologica. 2010;95:79–86. doi: 10.3324/haematol.2009.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassan R, Masciulli A, Spinelli 0, Intermesoli T, Oldani E, Rossi G, et al. New first line chemotherapy program with lineage targeted methotrexate infusions is feasible and improves the early minimal residual disease response and survival in acute T-lymphoblastic leukemia Haematologica 201196(Suppl 2238(abstract 0557).20952517 [Google Scholar]
- Ben Abdelali R, Asnafi V, Leguay T, Boissel N, Buzyn A, Chevallier P, et al. Pediatric-inspired intensified therapy of adult T-ALL reveals the favorable outcome of NOTCH1/FBXW7 mutations, but not of low ERG/BAALC expression: a GRAALL study. Blood. 2011;118:5099–5107. doi: 10.1182/blood-2011-02-334219. [DOI] [PubMed] [Google Scholar]
- Mitelman F, Johansson B, Mertens F.(eds). Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer, 2013, , http://cgap.nci.nih.gov/Chromosomes/Mitelman .
- Zhou Y, Fan X, Routbort M, Cameron Yin C, Singh R, Bueso-Ramos C, et al. Absence of terminal deoxynucleotidyl transferase expression identifies a subset of high-risk adult T-lymphoblastic leukemia/lymphoma. Mod Pathol. 2013;26:1338–1345. doi: 10.1038/modpathol.2013.78. [DOI] [PubMed] [Google Scholar]
- Bonnet M, Loosveld M, Montpellier B, Navarro JM, Quilichini B, Picard C, et al. Posttranscriptional deregulation of MYC via PTEN constitutes a major alternative pathway of MYC activation in T-cell acute lymphoblastic leukemia. Blood. 2011;117:6650–6659. doi: 10.1182/blood-2011-02-336842. [DOI] [PubMed] [Google Scholar]
- Rakowski LA, Garagiola DD, Li CM, Decker M, Caruso S, Jones M, et al. Convergence of the ZMIZ1 and NOTCH1 pathways at C-MYC in acute T lymphoblastic leukemias. Cancer Res. 2013;73:930–941. doi: 10.1158/0008-5472.CAN-12-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson E, Relling MV, Synold TW, Liu Q, Schuetz JD, Sandlund JT, et al. Accumulation of methotrexate polyglutamates in lymphoblasts is a determinant of antileukemic effects in vivo. A rationale for high-dose methotrexate. J Clin Invest. 1996;97:73–80. doi: 10.1172/JCI118409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intermesoli T, Rambaldi A, Rossi G, Delaini F, Romani C, Pogliani EM, et al. High cure rates in Burkitt lymphoma and leukemia: NILG study of the German short intensive rituximab-chemotherapy program. Haematologica. 2013;98:1718–1725. doi: 10.3324/haematol.2013.086827. [DOI] [PMC free article] [PubMed] [Google Scholar]