Abstract
During the primary mixed leukocyte reaction, T lymphocytes of the lyt-2- helper subclass proliferate in response to transplantation antigens on allogeneic dendritic cells. We have isolated populations of antigen-specific proliferating lymphoblasts and recultured them in fresh medium. Within 2 days, the blasts become smaller in size, lose responsiveness to T-cell growth factor or interleukin 2, but retain vigorous reactivity to the original alloantigen. Two new biologic properties of these "memory" lymphocytes have been noted. First, they primarily respond to alloantigen on dendritic cells, whereas freshly sensitized lymphoblasts react to allogeneic dendritic cells, macrophages, and B lymphocytes. Second, the memory lymphocytes quickly aggregate with dendritic cells that are either syngeneic or allogeneic, but not with B cells. The aggregates that form with syngeneic dendritic cells disassemble within hours and do not release interleukin 2 or proliferate. The aggregates that form with allogeneic dendritic cells remain intact, release large amounts of interleukin 2 on the first day of culture, and synthesize DNA on the second day. Therefore, dendritic cells actively cluster memory lymphocytes by an antigen-independent mechanism, and this may underlie the heightened functional activity of each cell type.
Full text
PDF
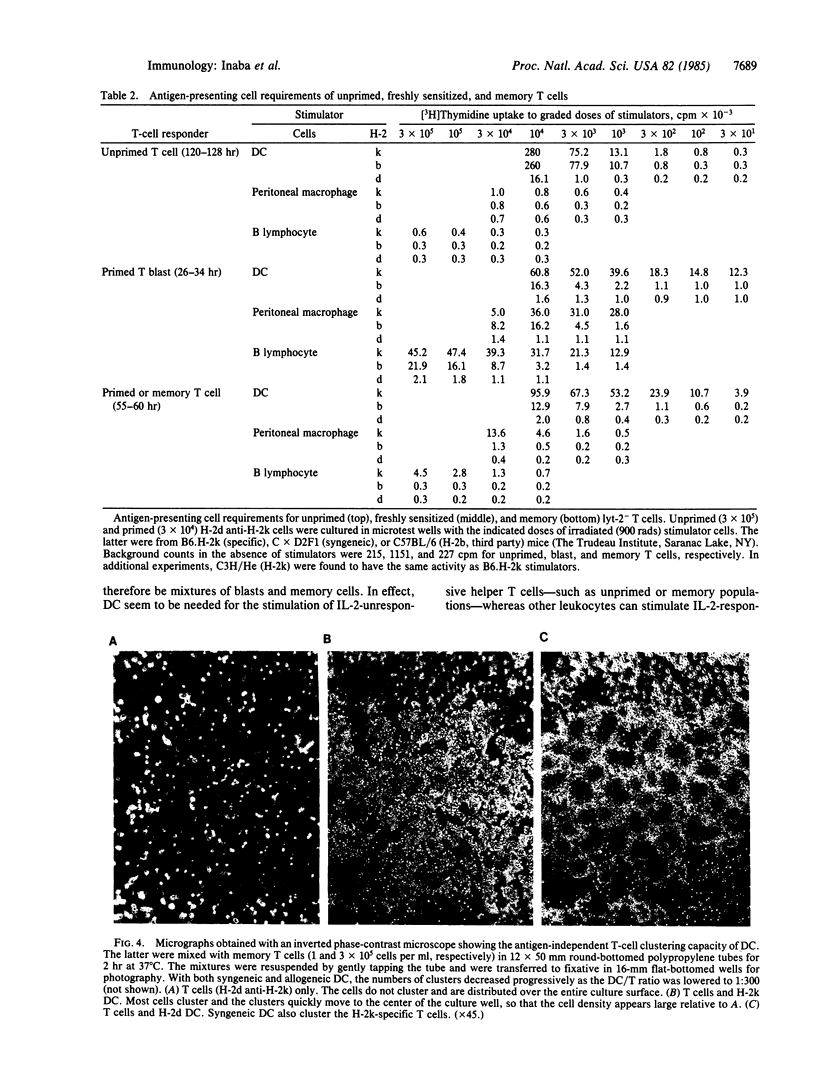

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustman D. L., Steinman R. M., Gebel H. M., Hauptfeld V., Davie J. M., Lacy P. E. Prevention of rejection of murine islet allografts by pretreatment with anti-dendritic cell antibody. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3864–3868. doi: 10.1073/pnas.81.12.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford W. L., Simmonds S. J., Atkins R. C. Early cellular events in a systemic graft-vs.-host reaction. II. Autoradiographic estimates of the frequency of donor lymphocytes which respond to each Ag-B-determined antigenic complex. J Exp Med. 1975 Mar 1;141(3):681–696. doi: 10.1084/jem.141.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Protein-specific helper T-lymphocyte formation initiated by dendritic cells. Science. 1985 Aug 2;229(4712):475–479. doi: 10.1126/science.3160115. [DOI] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Van Voorhis W. C., Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., Bowers W. E. Accessory and stimulating properties of dendritic cells and macrophages isolated from various rat tissues. J Exp Med. 1982 Jul 1;156(1):1–19. doi: 10.1084/jem.156.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert W. E., LaBadie J. H., O'Brien J. P., Beyer C. F., Bowers W. E. Rat dendritic cells function as accessory cells and control the production of a soluble factor required for mitogenic responses of T lymphocytes. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5414–5418. doi: 10.1073/pnas.77.9.5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight S. C., Mertin J., Stackpoole A., Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6032–6035. doi: 10.1073/pnas.80.19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechler R. I., Batchelor J. R. Restoration of immunogenicity to passenger cell-depleted kidney allografts by the addition of donor strain dendritic cells. J Exp Med. 1982 Jan 1;155(1):31–41. doi: 10.1084/jem.155.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason D. W., Pugh C. W., Webb M. The rat mixed lymphocyte reaction: roles of a dendritic cell in intestinal lymph and T-cell subsets defined by monoclonal antibodies. Immunology. 1981 Sep;44(1):75–87. [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Gutchinov B., Cohn Z. A. Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med. 1980 Oct 1;152(4):1070–1084. doi: 10.1084/jem.152.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Unkeless J. C., Witmer M. D., Gutchinov B., Cohn Z. A. Studies of the cell surface of mouse dendritic cells and other leukocytes. J Exp Med. 1981 Jul 1;154(1):168–187. doi: 10.1084/jem.154.1.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C. W., MacPherson G. G., Steer H. W. Characterization of nonlymphoid cells derived from rat peripheral lymph. J Exp Med. 1983 Jun 1;157(6):1758–1779. doi: 10.1084/jem.157.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Inaba K. Stimulation of the primary mixed leukocyte reaction. Crit Rev Immunol. 1985;5(4):331–348. [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Voorhis W. C., Valinsky J., Hoffman E., Luban J., Hair L. S., Steinman R. M. Relative efficacy of human monocytes and dendritic cells as accessory cells for T cell replication. J Exp Med. 1983 Jul 1;158(1):174–191. doi: 10.1084/jem.158.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. B., Nowell P. C. Quantitative studies on the mixed lymphocyte interaction in rats. V. Tempo and specificity of the proliferative response and the number of reactive cells from immunized donors. J Exp Med. 1971 Mar 1;133(3):442–453. doi: 10.1084/jem.133.3.442. [DOI] [PMC free article] [PubMed] [Google Scholar]