Abstract
Purpose
This study compared serum vascular endothelial growth factor (VEGF) concentration between patients given the bilateral and unilateral intravitreal injections of bevacizumab.
Methods
In a prospective manner, serum VEGF levels in treatment-naive patients with age-related macular degeneration who underwent bilateral or unilateral intravitreal injections of bevacizumab were investigated. After informed consent, peripheral blood was collected from in patients who underwent bilateral or unilateral intravitreal injection of bevacizumab before and 1 month after the injection. Serum VEGF levels were measured by enzyme-linked immunosorbent assay after centrifugation. In addition, best-corrected visual acuity (BCVA) and central retinal thickness (CRT) before and 1 month after the injection were compared between each group.
Results
Twenty patients received bilateral injections (40 eyes) and 20 patients received unilateral injections. The VEGF concentrations (pg/mL) before the bilateral injection were 235.75 ± 183.16 and 252.53 ± 233.52 for the unilateral injection. They were significantly reduced to 153.88 ± 113.26 and 189.42 ± 251.72 after 1 month, respectively (p = 0.037 and 0.019), which are showing no significant difference between the two groups (p = 0.771). And there were no significant intergroup difference in pre- and postoperative BCVA and CRT.
Conclusions
The bilateral simultaneous intravitreal injection of bevacizumab did not differ greatly from unilateral intravitreal injection in the influence on serum VEGF levels and the therapeutic outcome.
Keywords: Bevacizumab, Intravitreal injections, Vascular endothelial growth factor
Vascular endothelial growth factor (VEGF) is an important mediator of ocular angiogenesis and thus is important in the etiology of various ophthalmic diseases including retinal and choroidal neovascularization. Intravitreal injection of anti-VEGFs is the standard treatment for wet age-related macular degeneration (AMD). One major problem with this treatment is the typical need for frequent injections. The interval between injections varies depending upon the severity of disease and the physician; however, all patients are burdened with frequent hospital visits [1-6].
AMD tends to occur in both eyes. The incidence rate of bilateral AMD has been estimated to be at least 30% and 45% in Asian and European countries, respectively [7]. Also, 26% of patients diagnosed with unilateral AMD were reported to develope choroidal neovascularization in the opposite eye within five years [8]. Thus, patients undergoing anti-VEGF treatment in both eyes should visit the hospital once every 1 to 2 weeks, which imposes economic and time burdens on patients. In addition, there is a need to inject anti-VEGF into both eyes at the same time on each treatment day.
Recently, some hospitals have been performing bilateral simultaneous intravitreal injections to reduce costs and save time. A series of studies have reported that there is no significant difference in the occurrence rate of complications between bilateral simultaneous intravitreal injections and unilateral intravitreal injections [9-11], and some ophthalmologists perform these injections in an office setting [11,12].
Bevacizumab (Avastin; Genentech, San Francisco, CA, USA) is a widely used anti-VEGF drug that consists of the full-length antibody. Researchers have found that unilaterally-injected bevacizumab can reach the contralateral eye via systemic circulation [13-17]. However, the effect of bilaterally-injected bevacizumab on the systemic circulation is not well known.
To compare the systemic effects of bilateral and unilateral intravitreal injections of bevacizumab, we investigated the serum concentration of VEGF from the peripheral blood of patients undergoing bilateral or unilateral intravitreal injections of bevacizumab.
Materials and Methods
This study was approved by the institutional review board of the Soonchunhyang University Hospital, and we adhered to the tenets of the Declaration of Helsinki. Informed consent was obtained from all patients before intravitreal injections and blood collections. In a prospective manner, we investigated preoperative and postoperative serum VEGF levels in treatment-naive AMD patients who underwent bilateral or unilateral intravitreal bevacizumab injections at the Soonchunhyang University Hospital between May 2008 and December 2010. After informed consent was obtained, peripheral blood was collected before and one month after the injection. The collected blood was centrifuged at an authorized institute, and serum VEGF levels were measured by enzyme-linked immunosorbent assay according to the manufacturer's protocol. Patients with systemic malignancy, infection, inflammatory disorder, or other ophthalmologic diseases and who had previous eye surgery were excluded. Patients who could not be followed for three months were also excluded.
Every patient underwent a complete ophthalmologic examination including best-corrected visual acuity (BCVA) and a central retinal thickness (CRT) measurement using spectral optical coherence tomography (Heidelberg Engineering, Dossenheim, Germany) before and one month after the procedure. Every time a patient visited the hospital, ophthalmic complications such as anterior chamber inflammation, endophthalmitis, retinal break, and retinal detachment were checked. Also, the patient was questioned about the occurrence of systemic complications such as heart disease, cerebrovascular disease, and infectious disease.
Intravitreal injection methods
Intravitreal injections were performed by two operators (KSC and SJL) under the same conditions for all patients. Both eyes were anesthetized with the instillation of 0.5% proparacaine hydrochloride (Alcaine; Alcon, Fort Worth, TX, USA). The eyelids and eyelashes on both sides were prepared with 10% povidone-iodine, and 5% povidone-iodine was instilled onto the conjunctival surface. The operators performed all procedures using aseptic techniques. After the eyelid speculum and sterile drape were placed appositely, prepared bevacizumab (1.25 mg/0.05 mL) was injected using a 30-gauge needle at the superotemporal quadrant 3 to 3.5 mm posterior to the limbus. The bevacizumab ampule was opened just before the procedure, and the solution was dispensed into 1-mL syringes and was kept in aseptic conditions. Any remaining drug was disposed. The procedure on the opposite eye was performed after resterilization and with new gloves, drapes, surgical instruments, and syringes. Every patient was instructed to apply levofloxacin (Cravit; Santen, Osaka, Japan) into their eyes four times a day for one week post-injection.
Statistical analysis
Statistical data were analyzed using SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA). Mean changes in serum VEGF level, BCVA, and CRT were analyzed by the Wilcoxon signed-ranks test and intergroup differences by the Mann-Whitney test.
Results
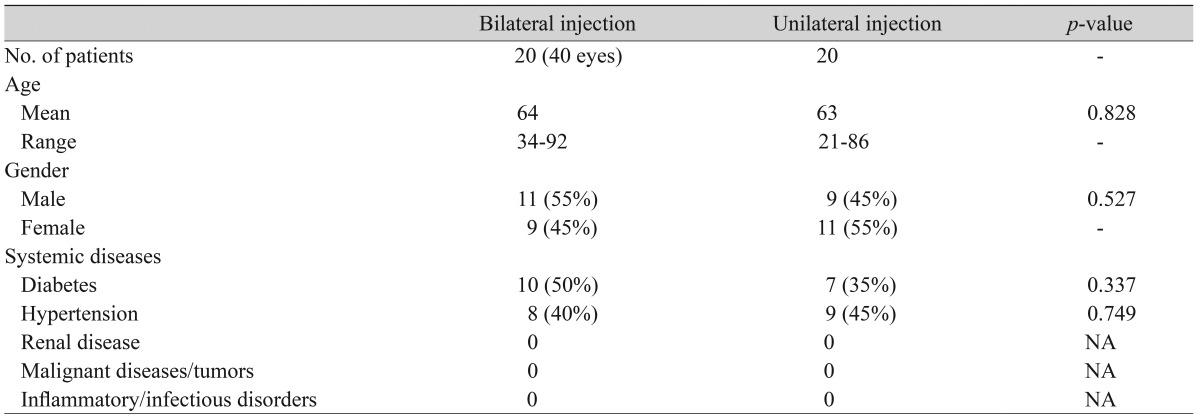
Twenty patients had bilateral intravitreal injections of bevacizumab (40 eyes) for bilateral wet AMD. The median age was 64 years (range, 34 to 92 years). There were 11 males and nine females. Over the same period, 20 patients underwent unilateral bevacizumab injections (nine males and 11 females). The median age was 63 years (range, 21 to 86 years). Between the two groups, there was no significant difference in the prevalence of diabetes (50% and 35%, p = 0.337) or hypertension (40% and 45%, p = 0.749). There were no patients with renal disease. At the preoperative examination, BCVA, CRT, and serum VEGF levels did not show statistical intergroup differences (Table 1).
Table 1.
Baseline characteristics
NA = not applicable.
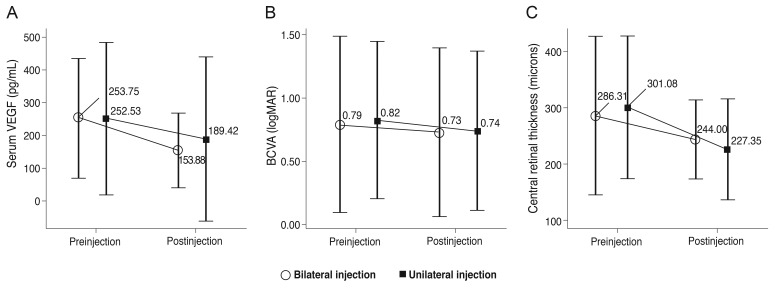
The serum VEGF level before and one month after the bilateral injection was 235.75 ± 183.16 pg/mL and 153.88 ± 113.26 pg/mL, respectively, representing a significant decrease after the procedure (p = 0.037). Similarly, in the unilateral group, the serum VEGF level significantly decreased after the procedure (252.53 ± 233.52 pg/mL to 189.42 ± 251.72 pg/mL, p = 0.019). However, there was no significant intergroup difference (p = 0.771).
The mean preoperative BCVA before the bilateral and unilateral injections was 0.79 ± 0.70 and 0.82 ± 0.62, respectively. The BCVA one month after the bilateral and unilateral injections was 0.73 ± 0.67 and 0.74 ± 0.63, respectively. There were statistically significant improvements in BCVA in both groups (bilateral injection group, p = 0.008; unilateral injection group, p = 0.007). The mean preoperative CRT (µm) was 286.31 ± 140.80 in the bilateral group and 301.08 ± 127.58 in the unilateral group. CRT improved to 244.00 ± 69.73 and 227.35 ± 89.94 in the bilateral and unilateral groups, respectively, which were both statistically significant (p ≤ 0.001, 0.001, respectively). However, there were no significant intergroup differences in the pre- and postoperative BCVA and CRT values (Table 2 and Fig. 1).
Table 2.
Mean serum concentration of VEGF and therapeutic outcome after bilateral intravitreal injection of bevacizumab
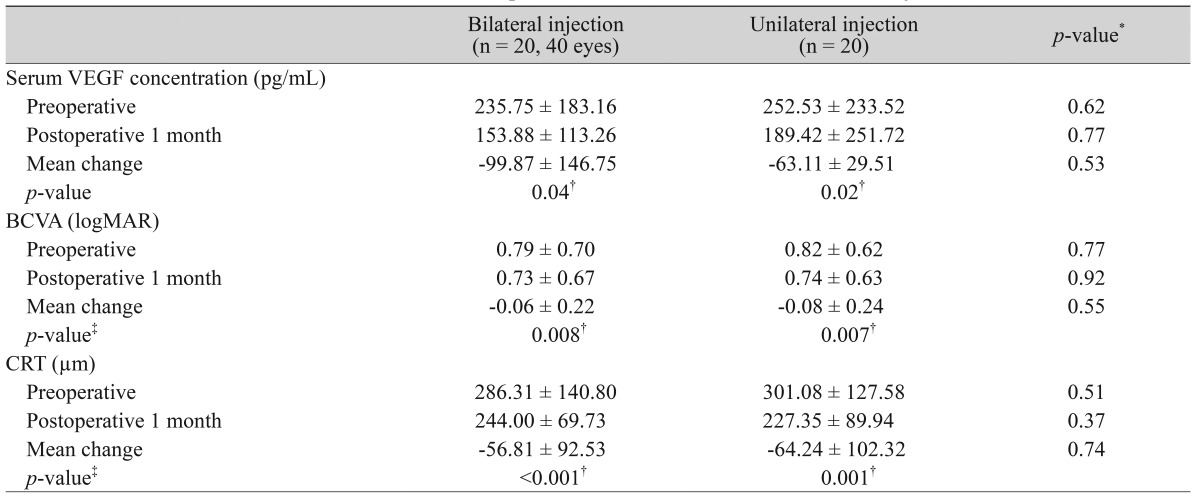
VEGF = vascular endothelial growth factor; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution; CRT = central retinal thickness.
*Mann-Whitney test; †p < 0.05; ‡Wilcoxon signed ranks test.
Fig. 1.
Mean serum concentration of vascular endothelial growth factor (VEGF), best-corrected visual acuity (BCVA), and central retinal thickness before and 1 month after intravitreal injection of bevacizumab for wet age-related macular degeneration. logMAR = logarithm of the minimum angle of resolution.
A case of sudden intraocular pressure (IOP) elevation occurred in a 60-year-old woman who underwent bilateral intravitreal injection for wet AMD. The attack occurred in the right eye one day after the injection. The patient complained of a sudden headache with decreased visual acuity, and the IOP increased to 50 mmHg in the right eye. Slit lamp examination showed a shallow anterior chamber with diffuse corneal edema. Gonioscopy revealed a closed anterior chamber angle in 360 degrees. The patient had a history of an acute glaucoma attack in the opposite eye 20 years prior and had been treated with laser iridotomy. The medical record showed bilateral shallow anterior chambers prior to the intravitreal injection. After laser iridotomy was performed on the right eye, the IOP was maintained at a steady-state for more than one year, during which the patient received three additional bilateral simultaneous intravitreal injections. Serious complication events such as anterior chamber inflammation, endophthalmitis, retinal break, retinal detachment, cerebrovascular disease, and myocardial infarction did not occur in either group.
Discussion
With the increasing use of bevacizumab for VEGF-mediated eye diseases, it is important to know the adverse systemic effects of intravitreal injection. In the CATT (Comparison of Age-related Macular Degeneration Treatments Trials) trial, acute myocardial infarction occurred in 0.7% of patients injected with 1.25 mg of bevacizumab monthly and in 0.3% of patients injected with bevacizumab as needed. Also, stroke events occurred in 0.7% and 0.7% of patients injected monthly and as needed, respectively, and venous thrombotic events occurred in 1.4% and 0.3% of the patients, respectively. These results were not statistically significant, but it remains unknown whether an increase in the systemic dose due to bilateral injections causes an increased risk [18].
The pharmacokinetics of bevacizumab have been determined in other studies. In human nonvitrectomized eyes, the aqueous half-life of 1.5 mg intravitreally injected bevacizumab is 8 to 10 days [19,20]. Recent studies have found that bevacizumab can flow into the serum via systemic circulation. In a study conducted on newborn infants, bevacizumab concentration in the serum increased with time after injection into the vitreous cavity. Also, bevacizumab concentration reached its peak during the second week after injection, which was inversely proportional to the VEGF level [21]. In another recent study in adult patients with diabetes, 24 hours after intravitreal injection of bevacizumab, serum levels of VEGF were lower than basal levels, and the maximal reduction of serum VEGF was noted on the seventh postoperative day. Then, 28 days after the injection, VEGF levels increased to levels similar to those before the treatment [22]. The VEGF-lowering effect of bevacizumab has been known to diminish in the first few weeks after the injection. However, some studies have reported significantly decreased levels of blood VEGF even one month or more after intravitreal injection of bevacizumab [23,24]. In our study, it was impossible to obtain blood samples in the early postoperative period for ethical and technical reasons, and thus the data may be insufficient to estimate the pharmacokinetics of intravitreally-injected bevacizumab. However, our results were in accordance with previous studies showing that the effect of intravitreally-injected bevacizumab can last for one month.
Some researchers have reported a bilateral response after unilateral injection of bevacizumab [13-17]. Considering the fact that unilaterally-injected bevacizumab can reach the contralateral eye, it can be deduced that, after bilateral injection, a synergistic therapeutic effect can occur. However, there were no significant intergroup differences in the preoperative and postoperative BCVA and CRT levels in our study.
In this study, there was no significant difference in the preoperative and postoperative serum VEGF levels between bilateral and unilateral intravitreal injections. These results suggest that bilateral injections have similar systemic effects compared to unilateral injections. However, it would take many more patients in order to assess the incidence of systemic complications. The reason why significant changes in serum level were not observed, even though more medicine was injected into the vitreous cavity, may be due to the relatively large distribution volume of the systemic circulation. The effect of bilateral injections in children or increasing the drug dose is still unknown. Additional causes for no changes in serum level could also be the blood sampling period of one month after the injection, which is a limitation of this study. Since the serum half-life of bevacizumab is known to be less than one week [22,24], the difference in serum VEGF level between the two groups one month after the injection would have been less than that in the early postoperative period. Future studies that include multiple blood tests from the early postoperative period will help more clearly determine the serum drug levels.
During this study, one serious complication, a sudden elevation of IOP requiring emergency treatment, occurred. Ordinarily, the IOP increases temporarily immediately after the intravitreal injection of an anti-VEGF but decreases naturally with time [25,26]. However, recent studies have shown that IOP elevations can occur over a prolonged period. An increase in vitreous volume, an inflammatory reaction of the aqueous humor or meshwork, direct damage to outflow tracks, and meshwork obstruction caused by high-molecular weight medicines such as bevacizumab are cited as the causes of the IOP increase [27-29]. It is rare for acute angle-closure glaucoma to occur after bilateral simultaneous intravitreal injection, with only one case being reported to date [30]. The case in this study occurred in a patient who had a previous angle-closure glaucoma attack in the opposite eye. The vitreous humor increased in volume after the intravitreal injection and narrowed the anterior chamber angle. This may have created conditions susceptible to angle-closure attack. Accordingly, in patients who have a history of glaucoma or a shallow anterior chamber angle, intravitreal injection requires meticulous attention.
In recent years, researchers have reported on the safety of bilateral simultaneous intravitreal injections of anti-VEGFs. Several studies have revealed that the complication rate after bilateral intravitreal injections was not higher than that of unilateral injection [9-12,31]. However, there is still controversy on bilateral intravitreal injections of anti-VEGFs due to possible complications. Arteriothrombotic or venous thrombotic events, hypertension, and death are considered to be serious complications. For patients having cardiovascular risk factors, intravitreal injections require meticulous attention. If the patient has a history of a major systemic events such as stroke, cardiac arrest, or uncontrolled hypertension within the previous three months, intravitreal anti-VEGF injection should be avoided [32-35].
Our study has several limitations including a nonrandomized uncontrolled study, a small number of subjects, and short-term follow-up. In addition, postoperative blood tests were performed only once at one month post-injection. Since it was shown in a newborn study that an increase in the serum concentration of bevacizumab after being injected into the vitreous cavity reaches its peak during the second week [21], our results may not demonstrate the lowest level of serum VEGF.
To our knowledge, there have been no studies comparing the serum VEGF levels in bilateral and unilateral intravitreal injections. In addition, BCVA and CRT did not differ between patients undergoing bilateral and unilateral intravitreal injections. Our results are encouraging; however, in order to understand the exact pharmacokinetic features and the impact of bilateral intravitreal injection on the systemic circulation, larger future studies are needed.
In conclusion, bilateral and unilateral intravitreal injections of bevacizumab do not show significant differences with regard to postoperative serum VEGF level and therapeutic outcome. This study has again demonstrated the effectiveness of bilateral simultaneous intravitreal injection. In-depth study of bilateral injection of bevacizumab with a large number of patients is still required.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Lalwani GA, Rosenfeld PJ, Fung AE, et al. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: year 2 of the PrONTO Study. Am J Ophthalmol. 2009;148:43–58. doi: 10.1016/j.ajo.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Engelbert M, Zweifel SA, Freund KB. "Treat and extend" dosing of intravitreal antivascular endothelial growth factor therapy for type 3 neovascularization/retinal angiomatous proliferation. Retina. 2009;29:1424–1431. doi: 10.1097/IAE.0b013e3181bfbd46. [DOI] [PubMed] [Google Scholar]
- 3.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–248. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: phase III clinical trial results. Ophthalmol Clin North Am. 2006;19:361–372. doi: 10.1016/j.ohc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–1431. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 6.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–1444. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 7.Kawasaki R, Wang JJ, Amirul FM, et al. Is bilateral age-related macular degeneration less common in Asians than Caucasians? Ophthalmic Epidemiol. 2011;18:253–258. doi: 10.3109/09286586.2011.602505. [DOI] [PubMed] [Google Scholar]
- 8.Macular Photocoagulation Study Group. Five-year follow-up of fellow eyes of patients with age-related macular degeneration and unilateral extrafoveal choroidal neovascularization. Arch Ophthalmol. 1993;111:1189–1199. doi: 10.1001/archopht.1993.01090090041018. [DOI] [PubMed] [Google Scholar]
- 9.Mahajan VB, Elkins KA, Russell SR, et al. Bilateral intravitreal injection of antivascular endothelial growth factor therapy. Retina. 2011;31:31–35. doi: 10.1097/IAE.0b013e3181ed8c80. [DOI] [PubMed] [Google Scholar]
- 10.Davis RP, Schefler AC, Murray TG. Concomitant bilateral intravitreal anti-VEGF injections for the treatment of exudative age-related macular degeneration. Clin Ophthalmol. 2010;4:703–707. doi: 10.2147/opth.s10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lima LH, Zweifel SA, Engelbert M, et al. Evaluation of safety for bilateral same-day intravitreal injections of antivascular endothelial growth factor therapy. Retina. 2009;29:1213–1217. doi: 10.1097/IAE.0b013e3181b32d27. [DOI] [PubMed] [Google Scholar]
- 12.Bakri SJ, Risco M, Edwards AO, Pulido JS. Bilateral simultaneous intravitreal injections in the office setting. Am J Ophthalmol. 2009;148:66–69. doi: 10.1016/j.ajo.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 13.Hosseini H, Lotfi M, Esfahani MH, et al. Effect of intravitreal bevacizumab on retrobulbar blood flow in injected and uninjected fellow eyes of patients with neovascular age-related macular degeneration. Retina. 2012;32:967–971. doi: 10.1097/IAE.0b013e31822c28d6. [DOI] [PubMed] [Google Scholar]
- 14.Matsuyama K, Ogata N, Matsuoka M, et al. Effects of intravitreally injected bevacizumab on vascular endothelial growth factor in fellow eyes. J Ocul Pharmacol Ther. 2011;27:379–383. doi: 10.1089/jop.2010.0194. [DOI] [PubMed] [Google Scholar]
- 15.Al-Dhibi H, Khan AO. Bilateral response following unilateral intravitreal bevacizumab injection in a child with uveitic cystoid macular edema. J AAPOS. 2009;13:400–402. doi: 10.1016/j.jaapos.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Wu Z, Sadda SR. Effects on the contralateral eye after intravitreal bevacizumab and ranibizumab injections: a case report. Ann Acad Med Singapore. 2008;37:591–593. [PubMed] [Google Scholar]
- 17.Avery RL, Pearlman J, Pieramici DJ, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 18.CATT Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–1908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31:1877–1884. doi: 10.1097/IAE.0b013e318217373c. [DOI] [PubMed] [Google Scholar]
- 20.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthalmol. 2008;146:508–512. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 21.Sato T, Wada K, Arahori H, et al. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153:327–333. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Davidovic SP, Nikolic SV, Curic NJ, et al. Changes of serum VEGF concentration after intravitreal injection of Avastin in treatment of diabetic retinopathy. Eur J Ophthalmol. 2012;22:792–798. doi: 10.5301/ejo.5000118. [DOI] [PubMed] [Google Scholar]
- 23.Ma Y, Zhang Y, Zhao T, Jiang YR. Vascular endothelial growth factor in plasma and vitreous fluid of patients with proliferative diabetic retinopathy patients after intravitreal injection of bevacizumab. Am J Ophthalmol. 2012;153:307–313. doi: 10.1016/j.ajo.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama K, Ogata N, Matsuoka M, et al. Plasma levels of vascular endothelial growth factor and pigment epithelium-derived factor before and after intravitreal injection of bevacizumab. Br J Ophthalmol. 2010;94:1215–1218. doi: 10.1136/bjo.2008.156810. [DOI] [PubMed] [Google Scholar]
- 25.Gismondi M, Salati C, Salvetat ML, et al. Short-term effect of intravitreal injection of Ranibizumab (Lucentis) on intraocular pressure. J Glaucoma. 2009;18:658–661. doi: 10.1097/IJG.0b013e31819c4893. [DOI] [PubMed] [Google Scholar]
- 26.Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin) Retina. 2007;27:1044–1047. doi: 10.1097/IAE.0b013e3180592ba6. [DOI] [PubMed] [Google Scholar]
- 27.Choi DY, Ortube MC, McCannel CA, et al. Sustained elevated intraocular pressures after intravitreal injection of bevacizumab, ranibizumab, and pegaptanib. Retina. 2011;31:1028–1035. doi: 10.1097/IAE.0b013e318217ffde. [DOI] [PubMed] [Google Scholar]
- 28.Adelman RA, Zheng Q, Mayer HR. Persistent ocular hypertension following intravitreal bevacizumab and ranibizumab injections. J Ocul Pharmacol Ther. 2010;26:105–110. doi: 10.1089/jop.2009.0076. [DOI] [PubMed] [Google Scholar]
- 29.Jalil A, Fenerty C, Charles S. Intravitreal bevacizumab (Avastin) causing acute glaucoma: an unreported complication. Eye (Lond) 2007;21:1541. doi: 10.1038/sj.eye.6703018. [DOI] [PubMed] [Google Scholar]
- 30.Semoun O, Blumen-Ohana E, de Preobrajensky N, Nordmann JP. Acute angle-closure glaucoma complicating an intravitreal injection of bevacizumab. J Fr Ophtalmol. 2009;32:58. doi: 10.1016/j.jfo.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Woo SJ, Han JM, Ahn J, et al. Bilateral same-day intravitreal injections using a single vial and molecular bacterial screening for safety surveillance. Retina. 2012;32:667–671. doi: 10.1097/IAE.0b013e31822c296b. [DOI] [PubMed] [Google Scholar]
- 32.Rasier R, Artunay O, Yuzbasioglu E, et al. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (Lond) 2009;23:1714–1718. doi: 10.1038/eye.2008.360. [DOI] [PubMed] [Google Scholar]
- 33.Chung YR, Lee K, Cho EH, Lew HM. Blood pressure changes after intravitreal bevacizumab in patients grouped by ocular pathology. Eye (Lond) 2010;24:1320–1324. doi: 10.1038/eye.2010.22. [DOI] [PubMed] [Google Scholar]
- 34.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IVAN Study Investigators. Chakravarthy U, Harding SP, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. doi: 10.1016/j.ophtha.2012.04.015. [DOI] [PubMed] [Google Scholar]