Abstract
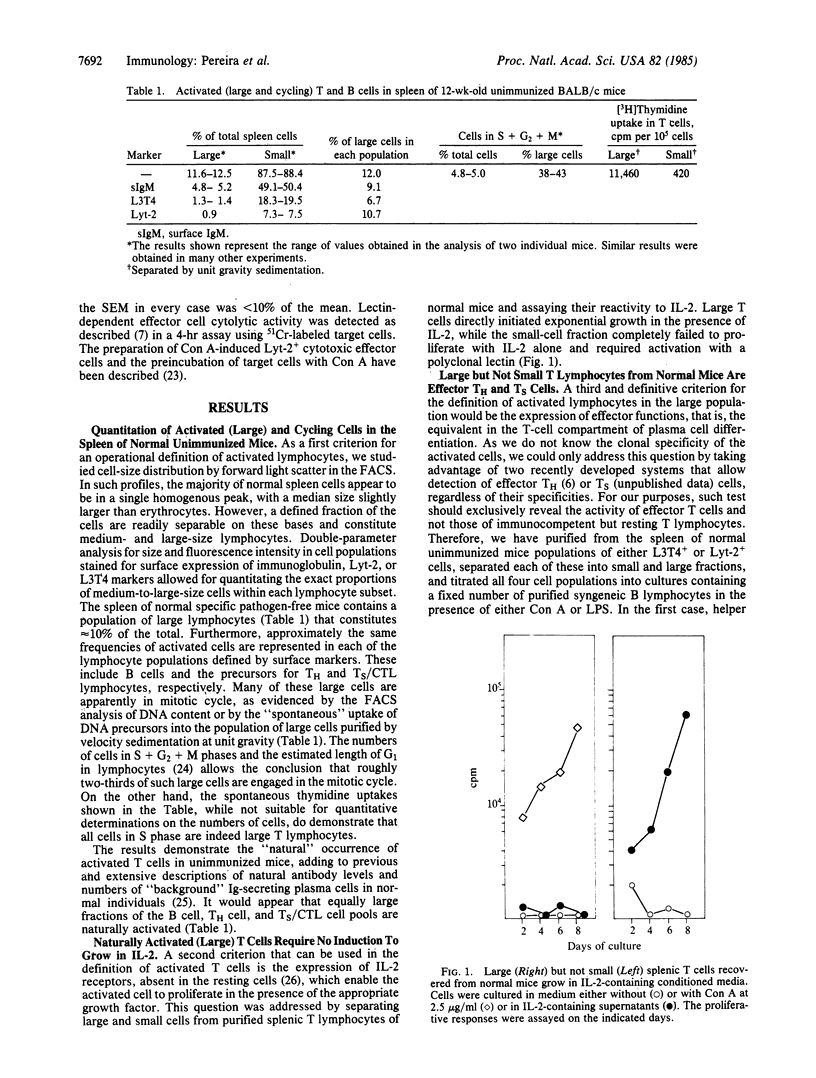
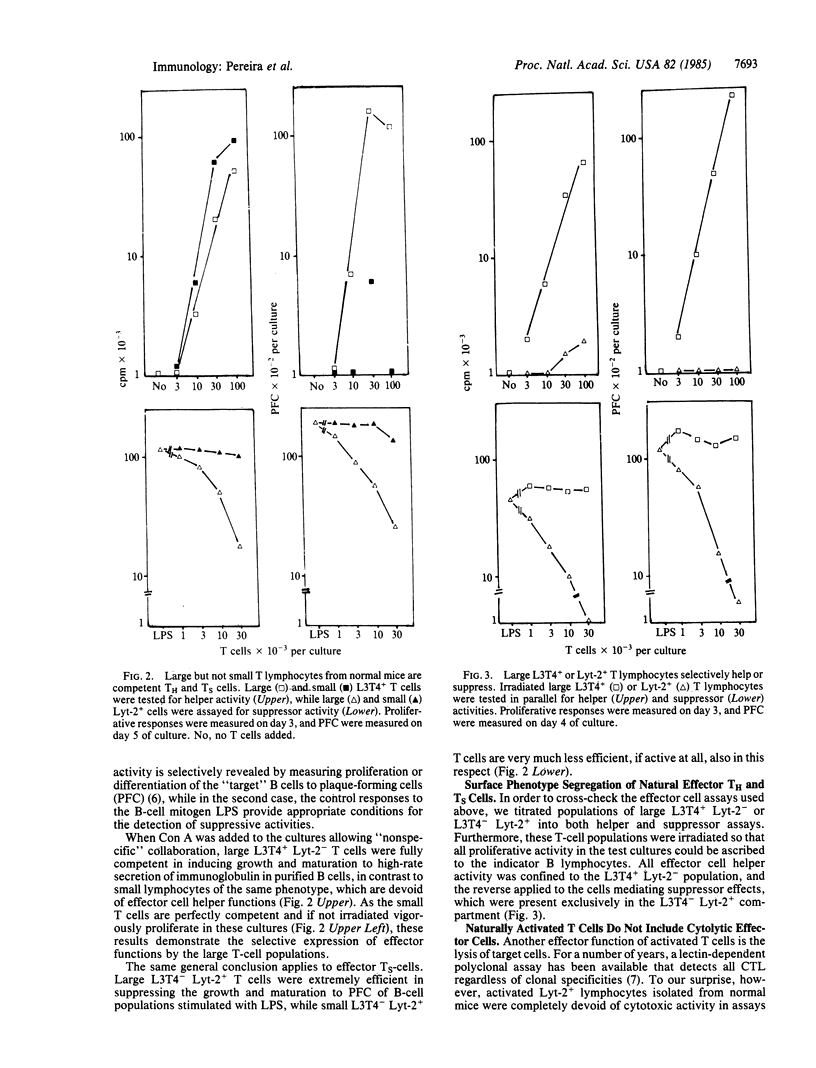
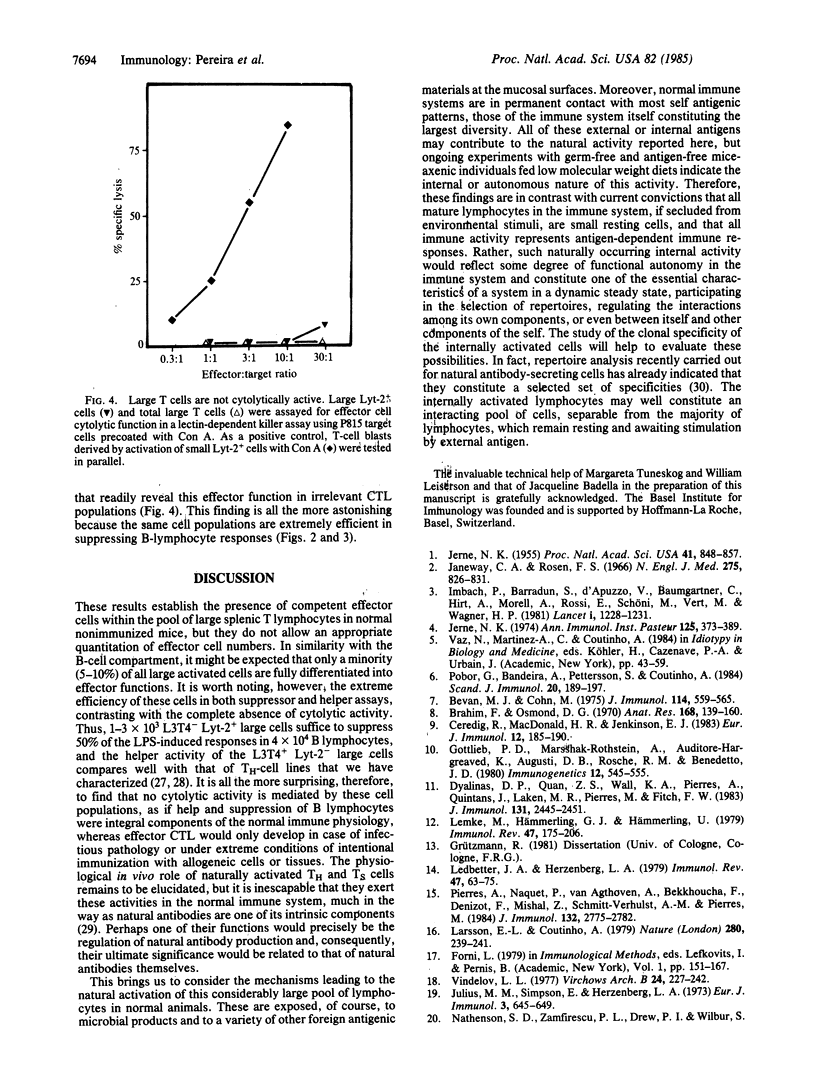
The "natural" T-cell activity in normal unimmunized mice was studied. By double-parameter fluorescence-activated cell sorter analysis, it was found that 5-10% of all splenic Lyt-2+ and L3T4+ lymphocytes are large, of which more than half are in mitotic cycle. In contrast with small resting cells of the same phenotype, activated (large) T cells isolated from normal mice are functional effector cells: L3T4+ large cells induce normal B lymphocytes into proliferation and antibody secretion, while large Lyt-2+ cells efficiently suppress B-lymphocyte responses. No effector cell cytolytic activity could be detected among naturally activated T cells. The significance of these findings for the internal activity in the normal immune system is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin A. A., Coutinho A. Specific T helper cells that activate B cells polyclonally. In vitro enrichment and cooperative function. J Exp Med. 1980 Mar 1;151(3):587–601. doi: 10.1084/jem.151.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. J., Cohn M. Cytotoxic effects of antigen- and mitogen-induced T cells on various targets. J Immunol. 1975 Feb;114(2 Pt 1):559–565. [PubMed] [Google Scholar]
- Brahim F., Osmond D. G. Migration of bone marrow lymphocytes demonstrated by selective bone marrow labeling with thymidine-H3. Anat Rec. 1970 Oct;168(2):139–159. doi: 10.1002/ar.1091680202. [DOI] [PubMed] [Google Scholar]
- Ceredig R., MacDonald H. R., Jenkinson E. J. Flow microfluorometric analysis of mouse thymus development in vivo and in vitro. Eur J Immunol. 1983 Mar;13(3):185–190. doi: 10.1002/eji.1830130302. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Gullberg M., Pobor G., Bandeira A., Larsson E. L., Coutinho A. Differential requirements for activation and growth of unprimed cytotoxic and helper T lymphocytes. Eur J Immunol. 1983 Sep;13(9):719–725. doi: 10.1002/eji.1830130906. [DOI] [PubMed] [Google Scholar]
- Holmberg D., Forsgren S., Ivars F., Coutinho A. Reactions among IgM antibodies derived from normal, neonatal mice. Eur J Immunol. 1984 May;14(5):435–441. doi: 10.1002/eji.1830140510. [DOI] [PubMed] [Google Scholar]
- Hooijkaas H., Benner R., Pleasants J. R., Wostmann B. S. Isotypes and specificities of immunoglobulins produced by germ-free mice fed chemically defined ultrafiltered "antigen-free" diet. Eur J Immunol. 1984 Dec;14(12):1127–1130. doi: 10.1002/eji.1830141212. [DOI] [PubMed] [Google Scholar]
- Imbach P., Barandun S., d'Apuzzo V., Baumgartner C., Hirt A., Morell A., Rossi E., Schöni M., Vest M., Wagner H. P. High-dose intravenous gammaglobulin for idiopathic thrombocytopenic purpura in childhood. Lancet. 1981 Jun 6;1(8232):1228–1231. doi: 10.1016/s0140-6736(81)92400-4. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Rosen F. S. The gamma globulins. IV. Therapeutic uses of gamma globulin. N Engl J Med. 1966 Oct 13;275(15):826–831. doi: 10.1056/NEJM196610132751508. [DOI] [PubMed] [Google Scholar]
- Jerne N. K. THE NATURAL-SELECTION THEORY OF ANTIBODY FORMATION. Proc Natl Acad Sci U S A. 1955 Nov 15;41(11):849–857. doi: 10.1073/pnas.41.11.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A. The role of mitogenic lectins in T-cell triggering. Nature. 1979 Jul 19;280(5719):239–241. doi: 10.1038/280239a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Miller R. G., Phillips R. A. Separation of cells by velocity sedimentation. J Cell Physiol. 1969 Jun;73(3):191–201. doi: 10.1002/jcp.1040730305. [DOI] [PubMed] [Google Scholar]
- Pardee A. B., Dubrow R., Hamlin J. L., Kletzien R. F. Animal cell cycle. Annu Rev Biochem. 1978;47:715–750. doi: 10.1146/annurev.bi.47.070178.003435. [DOI] [PubMed] [Google Scholar]
- Pierres A., Naquet P., Van Agthoven A., Bekkhoucha F., Denizot F., Mishal Z., Schmitt-Verhulst A. M., Pierres M. A rat anti-mouse T4 monoclonal antibody (H129.19) inhibits the proliferation of Ia-reactive T cell clones and delineates two phenotypically distinct (T4+, Lyt-2,3-, and T4-, Lyt-2,3+) subsets among anti-Ia cytolytic T cell clones. J Immunol. 1984 Jun;132(6):2775–2782. [PubMed] [Google Scholar]
- Pobor G., Bandeira A., Pettersson S., Coutinho A. A quantitative assay detecting small numbers of effector helper T cells, regardless of clonal specificity. Scand J Immunol. 1984 Sep;20(3):189–197. doi: 10.1111/j.1365-3083.1984.tb00992.x. [DOI] [PubMed] [Google Scholar]
- Pobor G., Pettersson S., Bandeira A., Martinez C., Coutinho A. Activation of helper T cells for B lymphocytes in primary mixed lymphocyte cultures. Scand J Immunol. 1983 Sep;18(3):207–215. doi: 10.1111/j.1365-3083.1983.tb00859.x. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindelov L. L. Flow microfluorometric analysis of nuclear DNA in cells from solid tumors and cell suspensions. A new method for rapid isolation and straining of nuclei. Virchows Arch B Cell Pathol. 1977 Aug 10;24(3):227–242. [PubMed] [Google Scholar]



