Abstract
Nanotechnology in drug delivery has been manifested into nanoparticles that can have unique properties both in vitro and in vivo, especially in targeted drug delivery to tumors. Numerous nanoparticle formulations have been designed and tested to great effect in small animal models, but the translation of the small animal results to clinical success has been limited. Successful translation requires revisiting the meaning of nanotechnology in drug delivery, understanding the limitations of nanoparticles, identifying the misconceptions pervasive in the field, and facing inconvenient truths. Nanoparticle approaches can have real impact in improving drug delivery by focusing on the problems at hand, such as enhancing their drug loading capacity, affinity to target cells, and spatiotemporal control of drug release.
It is debatable when nanotechnology, as we now know it, began. Perhaps, we can trace the beginnings to the invention of the scanning tunneling microscope1,2 in 1980, as it and the subsequently developed atomic force microscope3 enabled manipulation of individual atoms and molecules. The nanotechnology fever we are experiencing now began when the United States launched the National Nanotechnology Initiative,4 the world’s first program of its kind, in 2000. Since then, we have been bombarded by the dazzling images and cartoons of nanotechnology, such as nanorobots killing cancer cells resembling the plot of Fantastic Voyage. Tens of thousands of articles have been published on nanotechnology, and the press feed the public a steady diet of potential advances due to nanotechnology.
In this Perspective, the focus will be on drug-delivery aspects of nanotechnology, specifically, targeted drug delivery to tumors using nanoparticles. Nanoparticles designed for drug delivery have been called by many different names, including nanovehicles, nanocarriers, nanoconstructs, nanospheres, etc. Here, “nanoparticle” is used to represent all of these different formulations, including liposomes, polymer micelles, emulsion, and solid particles made of chitosan or poly(lactic-co-glycolic acid) (PLGA). Almost all papers on such nanoparticles end up with the same conclusion: nanotechnology has great potential for drug delivery. It is true. The question, then, is to ask what can be done to turn this potential into tangible outcomes, i.e., formulations that can benefit patients. It would be counterproductive only to talk about the potential for another decade. To achieve tangible outcomes, they first need to be defined. This, in turn, requires understanding the goals, which may depend on individuals.
Why Do Scientists Do What They Do?
Scientists and engineers do their work because they love what they do. If the goal of research on nanotechnology is just to make something nano, new, and more complicated, then the progress made in the last decade has clearly achieved the goal, at least in part. The ultimate goal of any research in drug delivery, however, must be to develop drug-delivery systems, nanoparticulate systems in this case, to prevent, to control, and to treat debilitating diseases. Most scientists working in the pharmaceutical and biotechnology sectors, as well as in academia, want to develop nanoparticle formulations that can deliver drugs more effectively to the target site for enhanced efficacy and reduced side effects.
There are many diseases that need to be addressed. Diabetes patients still have to poke their fingers to measure blood glucose levels and to inject necessary quantities of insulin multiple times a day. Can this be made easier through nanotechnology? Heart disease is the leading cause of death in the United States. Can nanotechnology lower the mortality rate? Alzheimer’s disease not only devastates the patients themselves, but also the patients’ family members and friends. Can this disease be identified early and be treated effectively via nanotechnology? Many prescription opioid drugs are widely abused. Can nanotechnology be used to develop abuse-deterrent formulations? Cancer claims millions of lives each year. Can this be prevented by nanotechnology? Unfortunately, nanotechnology, with all of its hype and unwarranted high expectations, has not yet produced anything significant to deal with these issues. It is common to see studies on nanotechnology that just make things more complicated while achieving less than what traditional non-nanotechnology can do. Each investigator needs to have a clear goal in what they are doing, rather than simply making things more nano.
What is Nanotechnology in Drug Delivery?
Of the many sub-areas in drug delivery, most nanotechnology research has been focused on targeted drug delivery to tumors. This specific area will be used to define a goal and to assess the progress of nanotechnology in the last decade.
Quite frequently, Doxil® and Abraxane® have been used as examples of nanotechnology-based drug-delivery systems, mainly because they are in the nanometer size range. The development of Doxil, a PEGylated liposome formulation, began in the early 1980s and was approved by the US Food and Drug Administration (FDA) in 1995.5 The main reason for approval was the equivalent efficacy and reduced cardiotoxicity or improved safety profiles as compared with free doxorubicin.5 The promise of nanotechnology in drug delivery is to deliver a drug selectively to the target site for enhanced efficacy with reduced side effects. In that sense, a portion of the nanotechnology promises were achieved. Liposomes have been known for 60 years6 and PEGylation for 40 years.7 Abraxane is a simple formulation based on oil/water (o/w) emulsion.8 Paclitaxel-dissolved methylene chloride is emulsified in albumin-dissolved aqueous solution to form an o/w emulsion, and subsequently homogenized to form nanodroplets. Albumin-coated paclitaxel nanoparticles are obtained by evaporating the solvent under reduced pressure. Albumin-coated nanocrystals can also be formed by simply adding albumin (as a surface modifier) to coarse drug crystals during milling9 or to the formed nanocrystals. The size of Doxil and Abraxane is certainly at the nanoscale, but neither of these formulations were inspired by modern nanotechnology. They were prepared by methods that were already widely practiced before the concept of modern nanotechnology evolved. Does any drug-delivery formulation become a nanotechnology system just because the size is at the nanoscale, regardless of how it is made? If that is the case, the current nanotechnology in drug-delivery systems is just a name change without any technological advances.
Understanding the Limitations of Nanotechnology
New generations of scientists need to focus on solving the problems that are indeed worthwhile. Tom Friedman, in his book That Used to Be Us,10 states, “One thing we know for sure: The path to a happy ending begins with the awareness that something is wrong, that changes are necessary, and that we the people have to be the agents of those changes” (p. 348). One problem now is how nanotechnology is perceived.11 The next generations of scientists will have to be the agents of these changes.
One of the changes to be made is to stop spreading inaccurate information. Findings made in this area, which may be true only under limited experimental conditions, are frequently inflated and overblown with the futuristic rhetoric by the press and the media. Such rhetoric may be necessary to attract the public’s attention to nanotechnology, and thus, more funding, but these statements create unintended side effects. Researchers may be forced to create fiction-like stories for funding, instead of proposing solutions to real problems. Many reviewers at funding agencies, without a clear understanding of the field, may demand something innovative over the existing technology. It will be extremely difficult, for example, to propose something better than making nanoparticles that “selectively lock onto only the cancer cells.”
History of Drug-Delivery Technologies
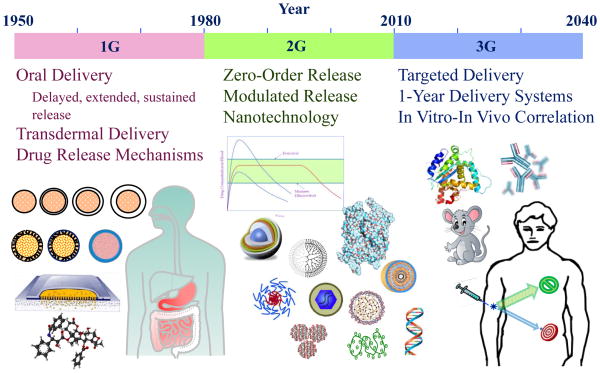
A brief overview of the history on controlled drug delivery provides some insight into how the current nanotechnology-based drug-delivery systems have evolved. As shown in Figure 1, the drug-delivery discipline is 60 years old. The first generation (1G) of drug-delivery systems was developed from the early 1950s to the end of the 1970s. During this time, the basic mechanisms of controlled drug release were established. Most of the drug-delivery formulations were for oral and transdermal administration, and, thus, the duration of drug release ranged from 12-hour (twice-a-day) oral formulations to 1-week transdermal formulations. Since then, numerous clinical products for oral delivery have been introduced to the market. The second generation (2G) from 1980 to 2010 was not as successful at introducing useful clinical systems. Extensive efforts were made to develop zero-order release techniques, which turned out not to be necessary. A dozen extended release depot formulations were developed, but this was minor compared with the thousands of oral controlled-release formulations available to patients. Other efforts on modulated (i.e. self-regulated) drug-delivery systems, e.g., glucose-dependent insulin delivery systems, have not been fruitful. This is mainly due to the difficulties associated with making an implantable closed system that has both glucose sensing and insulin release controlling abilities. At the turn of the 21st century, the National Nanotechnology Initiative initiated the current nanotechnology fever. During the fever, “new and innovative” often meant “nano and complicated.” The assumption was that the nanosized materials would have properties different and unachievable by microsized and larger materials. The assumption was thought to be reasonable and, thus, making something nano was all that was required at that time.
Figure 1.
Evolution of controlled drug-delivery systems. Adapted with permission from ref 12. Copyright 2013 Springer.
Convenient Misconceptions
In the area of targeted drug delivery, nanotechnology fever was fueled by an observation of the behavior of nanoparticles in tumors in mice, known as the enhanced permeation and retention (EPR) effect.13 The EPR effect is considered to be responsible for increased delivery of nanoparticles to targeted tumors in mouse experiments. This notion evolved into an idea that only nanoparticles have the EPR effect. Careful analysis of the original data, however, indicates that albumin and IgG are actually better in accumulating at the tumor site.14 It is also thought that PEGylated nanoparticles increase their blood circulation times, which in turn may enhance the EPR effect.15 Thus, it has been widely assumed that PEGylated nanoparticles having the EPR effect will result in an enhanced tumor-killing effect, and therefore, the problem of targeted drug delivery to tumors was partially solved. The reality is that these assumptions have produced numerous research articles, but have made no significant advances in translation into patient treatment.16 These convenient misconceptions have to face the inconvenient truth.
Inconvenient Truth
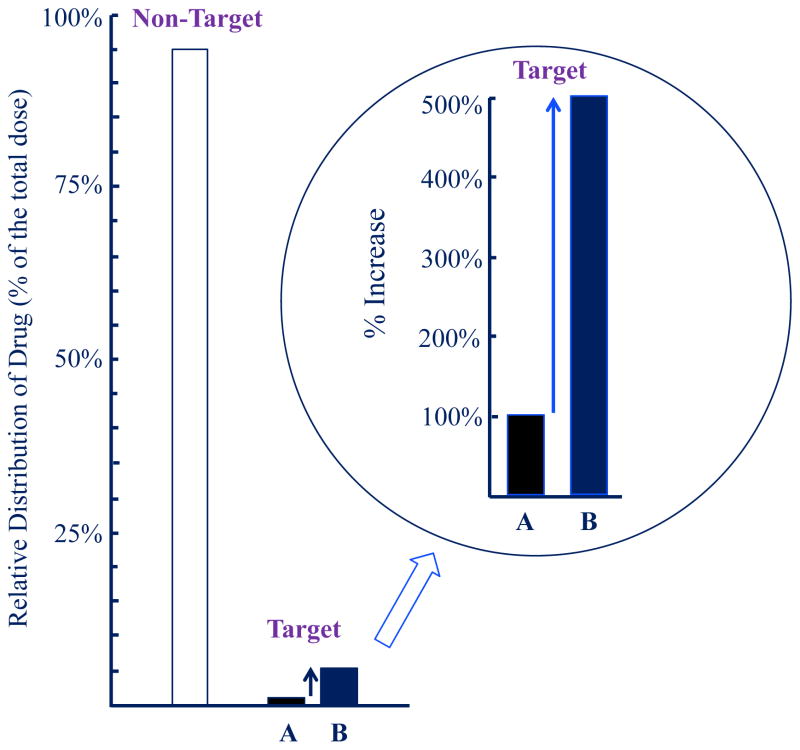
Nanoparticle formulations, as compared with solution formulations, increase the drug concentration around a tumor by 100–400% (Fig. 2 Circle). These increases are phenomenal by any measure. What is missing here, however, is the big picture showing the full story on drug delivery. It should be understood that >95% of the administered nanoparticles end up at sites other than the targeted tumor (Fig. 2); this fact has been largely overlooked.11 Clinical applications of Taxol®, Taxotere®, Abraxane®, and Genexol® show that the latter two nanoparticle formulations have similar performance to the first two, which based on solution formulations. The amount of a drug delivered to the target tumor may be about the same for different formulations. Taxol, Abraxane, and Genexol deliver paclitaxel, while Taxotere delivers docetaxel, a derivative of paclitaxel. Nanoparticles may provide an alternative way of making aqueous solution formulations for intravenous administration of poorly soluble drugs without using undesirable excipients, such as polysorbate 80 or cremophor EL.17 This is a great use of nanoparticle approaches. It is simply different than the widely believed notion that nanoparticles would be far superior to non-particulate solution formulations.
Figure 2.
Relative distribution of a drug at a target tumor site by (A) conventional solution formulation and (B) nanoparticulate formulation. The majority of the administered drug ends up at non-target sites, but the 5× more efficient delivery of the drug by nanoparticles can be exploited for maximizing drug efficacy. Adapted with permission from ref 12. Copyright 2013 Springer.
OUTLOOK AND FUTURE CHALLENGES
Turning the potential of nanoparticle systems into clinically useful formulations requires setting up clear, realistic goals. The challenges in targeted drug delivery using nanoparticles can be overcome through understanding the limitations of nanoparticle approaches and maximizing the existing capabilities of nanoparticle formulations.
Exploit the 5% Reaching the Target Tumor
Nanoparticles go to target tumors simply as a result of blood circulation. Thus, the percentage of the administered drug reaching the tumor is similar regardless of the formulation type. The nanoparticles remain around the tumor longer, because they do not diffuse back into the blood stream as easily as dissolved drug molecules. This results in more accumulation of the drug near the tumor site. Assuming 5% of the total administered nanoparticles can end up at the tumor site, one can make a nanoparticle system a clinically useful formulation. Currently, the drug loading in most nanoparticles is not high, usually around 10%. If the drug loading can be increased by a factor 5, it is the equivalent of delivering 25% of the total administered nanoparticles with 10% drug loading. For example, instead of loading a drug into liposomes or polymer micelles, one can use the drug nanocrystals themselves, which deliver 100% of the drug.18 The surface of the nanocrystals may need to be modified by polymers or proteins for enhancement of their affinity to cells or their stability.19 The percentage of the drug may decrease, but the majority of the total weight will be the drug. This approach, of course, delivers more drugs to other tissues, too, and this is where the reduced toxicity by nanoparticle approach is important. It is necessary to develop nanoparticle formulations having significantly reduced side effects by controlling the drug release depending on the environment. The drug delivery field can be advanced rapidly by making nanoparticles with high drug-loading capacity and the ability to control the drug release.
Once reaching the tumor site, nanoparticles need to be cleared from the site after releasing the loaded drug. If the empty nanoparticles remain at the same site due to the low clearance rate, they may present a physical barrier for delivery of additional nanoparticles that are freshly administered. Extravasated liposomes 90 nm in diameter were observed to remain near the blood vessels even after 1 week.20 The study of in vivo degradation of nanoparticles, made of PLGA (L:G=50:50, Mw=44,000 Da) sized 200 and 500 nm, indicates that more than 1/3 of the administered nanoparticles remain not degraded after 1 week.21 Thus, nanoparticles need to be designed to undergo timely clearance from or degradation at the target site. Currently, little attention has been paid to this property.
Entering the Tumor Cells
For a drug to be effective, it needs to enter the tumor cells. Thus, improving the cellular interaction, leading to cellular uptake, is another necessary innovation. In an attempt to maximize interactions with the cell, a new nanocage approach was developed. In this issue of ACS Nano, Professor In San Kim and his group describe a nanocage they designed that displays a high affinity to cell receptors.22 Specific peptides identified by phage display were genetically fused onto the surface of cage proteins. Symmetrical assembly of the cage proteins forms clusters of the peptides in bunches. The resulting peptide bunches on the nanocage synergistically increase the affinity of the peptide ligands, leading to substantial increases in therapeutic efficacy. If such a nanocage can be grafted to the surface of drug nanocrystals, the therapeutic effect will be enhanced considerably.
The high affinity of nanoparticles to the cell surface may have the added benefit of increasing the intratumoral distribution of the nanoparticles. Extravascular transport and, thus, the tumor-targeting efficiency of nanoparticles depends on the nature of targeting ligands attached to the nanoparticle surface.23 Receptor-mediated transcytosis can facilitate extravascular transport of nanoparticles, leading to enhanced nanoparticle delivery to solid tumors. It overcomes the barrier to efficient dispersion of nanoparticles in tumor interstitium. The efficient delivery of nanoparticles into the tumor interstitum exposes tumor cells to lethal doses and makes them less susceptible to the development of resistance. The presence of agonists on the nanoparticle surface may not improve the delivery from blood circulation to the target site, but can enhance subsequent extravascular transport.24
Dealing with Biological Issues
Even if nanoparticles are designed to have high affinity to the tumor cell surface, the actual interaction between the two occurs when the tumor cells express the receptors. It needs to be understood that not all tumor cells express receptors. More importantly, tumor cells may not have overexpressed receptors at the time of nanoparticle arrival. The heterogeneity in tumor cells themselves and temporal receptor overexpression are not an easily addressed problem.25 Thus, nanoparticles with the ability to control drug release depending on environmental conditions become even more important.
Time to be Realistic
A few clinical studies were done to test the newly developed nanoparticle formulations. For example, a thermo-sensitive liposome formulation, which showed excellent efficacy in mouse models,26,27 was tested for its efficacy in clinical studies. Patients were treated with heat before administration of the low temperature-sensitive liposome formulation. The result was not as good as expected and did not meet the goal of demonstrating evidence of clinical effectiveness.28 For this approach to be successful, it may require fine-tuning of the procedure to maximize the usefulness of the liposome properties. Apparently, the optimal condition found in small animal studies was not optimal in a clinical application. The differences in size and other variables between small animals and humans may require changes in the time and duration of heat exposure. Enormous resources required for clinical studies, however, prevent repeated clinical experiments. This necessitates development of improved animal and in vitro models that can provide better predictions on nanoparticle efficacy in humans. It is difficult, as well as unnecessary, to develop a single model that represents all aspects of human physiology. It will be more than sufficient if each model can predict one or more aspects of nanoparticle behavior in humans. Microfluidic devices can test the effectiveness of nanoparticles to extravasate from the blood vessel into the surrounding tissues and subsequent clearance from the site.29, 30 A three-dimensional tumor spheroid model can be used to examine how effectively nanoparticles interact with the cells on the surface and achieve intratumoral distribution.31
One thing that nanoparticle scientists need to realize is that clinical application of any formulation requires approval by the FDA or its equivalent overseas. The safety and efficacy of new formulations must be proven through controlled clinical studies. Pharmaceutical and biotechnology companies prefer using excipients that have already been used in clinical products approved by the FDA. In this way, there will be little concern about the safety and toxicity of the excipients themselves. This brings another constraint in developing clinically useful nanoparticle formulations. By understanding the many limitations and constraints in developing clinically useful formulations, nanoparticle scientists can have better perspective in their pursuit of finding the next generations of drug-delivery systems. Achieving “the next big thing” starts with being realistic now. There simply needs to be an understanding that overcoming the enormous difficulties involved in clinical applications of nanoparticles requires more than just rhetoric and pretty pictures.32
Suggested Pull Quotes.
In this Perspective, the focus will be on the drug delivery aspect of nanotechnology, in particular targeted drug delivery to tumors using nanoparticles.
The challenges in targeted drug delivery using nanoparticles can be overcome through understanding the limitations of nanoparticle approaches and maximizing the existing capabilities of nanoparticle formulations.
In this issue of ACS Nano, Professor In San Kim and his group describe a nanocage they designed that displays high affinity to cell receptors.
Acknowledgments
This work was supported by National Institute of Health through CA129287 and GM095879, and the Showalter Research Trust Fund.
Footnotes
Disclosure: Views expressed in this Perspective are those of the author and not necessarily the views of ACS.
REFERENCES AND NOTES
- 1.Binnig G, Rohrer H. Scanning Tunneling Microscope. 4,343,993. USP. 1980, 1982 Sep 12;
- 2.Binnig G, Rohrer H, Gerber C, Weibel E. Tunneling Through a Controllable Vacuum Gap. Appl PhysLett. 1982;40:178–180. [Google Scholar]
- 3.Binnig G, Quate CF, Gerber C. Atomic Force Microscope. Phys RevLett. 1986;56:930–933. doi: 10.1103/PhysRevLett.56.930. [DOI] [PubMed] [Google Scholar]
- 4.The White House Office of the Press Secretary. National Nanotechnology Initiative: Leading to the Next Industrial Revolution. http://clinton4.nara.gov/WH/New/html/20000121_4.html.
- 5.Barenholz Y. Doxil®— The First FDA-Approved Nano-Drug: Lessons Learned. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 6.Park K. Not All Liposomes are Created Equal. J Control Release. 2013;166:316. doi: 10.1016/j.jconrel.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman AS. The Origins and Evolution of “Controlled” Drug Delivery Systems. J Control Release. 2008;132:153–163. doi: 10.1016/j.jconrel.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 8.Desai NP, Soon-Shiong P, Trieu V. Compositions and Methods of Delivery of Pharmacological Agents. 7820788. US Patent. 2010 Oct 26;
- 9.Liversidge GG, Liversidge E, Sarpotdar PP. Surface Modified Anticancer Nanoparticles. US5399363. US Patent. 1995 Mar 21;:A.
- 10.Friedman TL, Mandelbaum M. That Used to Be Us: How America Fell Behind in the World It Invented and How We Can Come Back. Farrar, Straus and Giroux; New York, NY: 2011. [Google Scholar]
- 11.Bae YH, Park K. Targeted Drug Delivery to Tumors: Myths, Reality, and Possibility. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park K, Bae YH, Mrsny R. The Missing Components Today and the New Treatments Tomorrow. In: Bae YH, Mrsny R, Park K, editors. Cancer Targeted Drug Delivery: An Elusive Dream. Springer; New York: 2013. pp. 689–707. [Google Scholar]
- 13.Matsumura Y, Maeda H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent SMANCS. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 14.Kwon IK, Lee SC, Han B, Park K. Analysis on the Current Status of Targeted Drug Delivery to Tumors. J Control Release. 2012;164:108–114. doi: 10.1016/j.jconrel.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi J, Giasson S, Khalid MN, Delmas P, Allen C, Leroux JC. Long-Circulating Poly(ethylene glycol)-Coated Emulsions To Target Solid Tumors. Eur J PharmBiopharm. 2007;67:329–338. doi: 10.1016/j.ejpb.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 16.Venditto VJ, Szoka FC., Jr Cancer Nanomedicines: So Many Papers and so Few Drugs! Adv Drug Del Rev. 2013;65:80–88. doi: 10.1016/j.addr.2012.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kratz F, Warnecke A. Finding the Optimal Balance: Challenges of Improving Conventional Cancer Chemotherapy Using Suitable Combinations with Nano-Sized Drug Delivery Systems. J Control Release. 2012;164:221–235. doi: 10.1016/j.jconrel.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 18.Gao L, Liu G, Ma J, Wang X, Zhou L, Li X. Drug Nanocrystals: In Vivo Performances. J Control Release. 2012;160:418–430. doi: 10.1016/j.jconrel.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Nkansah P, Antipas A, Lu Y, Varma M, Rotter C, Rago B, El-Kattan A, Taylor G, Rubio M, Litchfield J. Development and Evaluation of Novel Solid Nanodispersion System for Oral Delivery of Poorly Water-Soluble Drugs. J Control Release. 2013;169:150–161. doi: 10.1016/j.jconrel.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 20.Yuan F, Leunig M, Huang SK, Berk DA, Papahadjopoulos D, Jain RK. Microvascular Permeability and Interstitial Penetration of Sterically Stabilized (Stealth) Liposomes in a Human Tumor Xenograft. Cancer Res. 1994;54:3352–3356. [PubMed] [Google Scholar]
- 21.Mohammad AK, Reineke JJ. Quantitative Detection of PLGA Nanoparticle Degradation in Tissues Following Intravenous Administration. MolPharm. 2013;10:2183–2189. doi: 10.1021/mp300559v. [DOI] [PubMed] [Google Scholar]
- 22.Jeon JO, Kim S, Choi E, Shin K, Cha K, So I-S, Kim S-J, Jun E, Kim D, Ahn HJ, et al. Designed Nanocage Displaying Ligand-Specific Peptide Bunches for High Affinity and Biological Activity. ACS Nano. 2013 doi: 10.1021/nn403184u. [DOI] [PubMed] [Google Scholar]
- 23.Lu W, Xiong C, Zhang R, Shi L, Huang M, Zhang G, Song S, Huang Q, Liu G, Li C. Receptor-Mediated Transcytosis: A Mechanism for Active Extravascular Transport of Nanoparticles in Solid Tumors. J Control Release. 2012;161:959–966. doi: 10.1016/j.jconrel.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K. Extravascular Transport of Nanoparticles in Solid Tumors. J Control Release. 2012;161:967. doi: 10.1016/j.jconrel.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 25.Denison TA, Bae YH. Tumor Heterogeneity and its Implication for Drug Delivery. J Control Release. 2012;164:187–191. doi: 10.1016/j.jconrel.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banno B, Ickenstein LM, Chiu GNC, Bally MB, Thewalt J, Brief E, Wasan EK. The Functional Roles of Poly(ethylene glycol)-Lipid and Lysolipid in the Drug Retention and Release from Lysolipid-Containing Thermosensitive Liposomes In Vitro and In Vivo. J PharmSci. 2010;99:2295–2308. doi: 10.1002/jps.21988. [DOI] [PubMed] [Google Scholar]
- 27.Landon CD, Park JY, Needham D, Dewhirst MW. Nanoscale Drug Delivery and Hyperthermia: The Materials Design and Preclinical and Clinical Testing of Low Temperature-Sensitive Liposomes Used in Combination with Mild Hyperthermia in the Treatment of Local Cancer. Open NanomedJ. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celcion-Corporation. Celsion Announces Results of Phase III HEAT Study of Thermodox® in Primary Liver Cancer. http://investor.celsion.com/releasedetail.cfm?ReleaseID=737033.
- 29.Hashimoto M, Tong R, Kohane DS. Microdevices for Nanomedicine. MolPharm. 2013;10:2127–2144. doi: 10.1021/mp300652m. [DOI] [PubMed] [Google Scholar]
- 30.Han B. Complex Transport Around Tumor: Need for Realistic In Vitro Tumor Transport Model. In: Bae YH, Mrsny R, Park K, editors. Cancer Targeted Drug Delivery: An Elusive Dream. Springer; New York: 2013. pp. 667–685. [Google Scholar]
- 31.Shin CS, Kwak B, Han B, Park K. Development of an In Vitro 3D Tumor Model To Study Therapeutic Efficiency of an Anticancer Drug. MolPharm. 2013;10:2167–2175. doi: 10.1021/mp300595a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park K. A Two-Step External Activation for Targeted Intracellular Delivery. J Control Release. 2012;161:150. [Google Scholar]