Abstract
Objective
To examine the association between second-trimester maternal serum 25-hydroxyvitamin D (25[OH]D) concentrations and risk of small for gestational age (SGA) in singleton, live births.
Methods
We assayed serum samples at 12 to 26 weeks of gestation for 25(OH)D in a sample of participants in a multicenter clinical trial of low-dose aspirin for the prevention of preeclampsia in high-risk women (n=792). Multivariable log-binomial regression models were used to assess the association between 25(OH)D and risk of SGA (birth weight less than the 10th percentile of gestational age) after adjustment for confounders including maternal prepregnancy obesity, race, treatment allocation, and risk group.
Results
Thirteen percent of infants were SGA at birth. Mean (SD) 25(OH)D concentrations were lower in women who delivered SGA (57.9 [29.9] nmol/L0 vs. non-SGA infants (64.8 [29.3] nmol/L, P=0.028). In adjusted models, 25(OH)D concentrations of 50-74 nmol/L and ≥75 nmol/L compared with <30 nmol/L were associated with 43% (95% confidence interval [CI] 0.33-0.99) and 54% (95% CI 0.24-0.87) reductions in risk of SGA, respectively. Race and maternal obesity each modified this association. White women with 25(OH)D ≥50 vs. <50 nmol/L had a 68% reduction in SGA risk (adjusted risk ratio [RR] 0.32, 95% CI 0.17-0.63) and nonobese women 25(OH)D ≥50 vs. <50 nmol/L had a 50% reduction in SGA risk (adjusted RR 0.50, 95% CI 0.31-0.82). There was no association between 25(OH)D and risk of SGA in black or obese mothers.
Conclusion
Maternal vitamin D status in the second trimester is associated with risk of SGA among all women, and in the subgroups of white and nonobese women.
Introduction
Fetal growth restriction, most often estimated by incidence of a birth weight that is small for gestational age (SGA), is a major public health issue across the globe (1, 2). Infants suffering from growth restriction are at higher risk of death and serious neonatal morbidities (3), and alarmingly, health risks continue into adulthood (4). SGA is associated with a range of maternal factors including nutritional status, obesity, age, smoking, and infection, although there are few effective interventions for prevention (5).
Vitamin D deficiency continues to be a public health issue in the US, particularly among women of reproductive age, and deficiency rates have been increasing (6). Vitamin D is unique among essential micronutrients as it can be produced by the body subcutaneously after exposure to ultraviolet-B (UVB) radiation. Vitamin D receptors have been identified in tissues throughout the body allowing for a plethora of hormonal roles for the biologically active 1,25-dihydroxyvitamin D (1,25(OH)2D). Maternal vitamin D deficiency is related to a range of poor pregnancy outcomes, including preterm birth, preeclampsia, and SGA (7). Vitamin D could potentially be related to fetal growth through calcium metabolism and bone growth (8), or altering placental function (9, 10).
Several observational studies have linked maternal 25(OH)D concentrations and risk of SGA in general obstetric populations (11-15). Little is known about this association in high-risk pregnancies where competing risk factors could augment or dampen the vitamin D-SGA relationship. As well, populations that are geographically- and racially-diverse are important in vitamin D research to observe a range of solar radiation exposure, cutaneous vitamin D production, and dietary intake. The objective of this study was to examine the association between maternal vitamin D status at 12 to 26 weeks of gestation and risk of SGA in a multicenter US cohort of women at high risk for preeclampsia who delivered singleton, live births.
Materials and Methods
This was an observational study that used data and blood samples from the High-Risk Aspirin Study, a randomized controlled trial of low-dose aspirin for the prevention of preeclampsia. The trial was conducted in women at high risk for preeclampsia in 12 medical centers across the US (1991-95) (16). Women who had prepregnancy, insulin-treated diabetes; chronic hypertension; preeclampsia in a previous pregnancy; or multifetal gestation were enrolled at 12 to 26 weeks of gestation. Further details of enrollment criteria are published (16). Exclusion criteria included planned delivery elsewhere, significant bleeding or bleeding disorders, aspirin allergy, current drug or alcohol abuse, renal failure, active hepatitis, uncontrollable hypertension, fetal anomalies incompatible with life, and fetal hydrops fetalis. After providing informed, written consent, women were randomized to receive 60 mg of aspirin or placebo daily until delivery or the development of preeclampsia. The trial found no effect of aspirin on the incidence of preeclampsia, preterm birth, or SGA (16). Data were collected at enrollment on women's medical histories and sociodemographics. Nonfasting blood samples were taken before randomization (i.e. prior to treatment) and infants were weighed at birth.
Of 1,851 eligible women with singleton pregnancies enrolled, 839 had a serum sample at ≤26 weeks available for vitamin D assessment. We excluded 29 stillbirths, 10 pregnancies missing birth weight, and eight missing prepregnancy BMI for a final analytic sample of 792 singleton live births. Our study used deidentified data and was approved by the University of Pittsburgh Institutional Review Board.
Circulating serum 25(OH)D represents vitamin D from oral intake and cutaneous production and is considered the best biomarker of vitamin D status (17). In a DEQAS (Vitamin D External Quality Assessment Scheme)-proficient laboratory, total serum 25(OH)D [25(OH)D2 + 25(OH)D3] was measured using liquid-chromatography-tandem mass spectrometry according to National Institute of Standards and Technology (NIST) specifications (18). This method has a 2 ng/mL lower limit of detection and no upper limit. The intra-assay coefficients of variation were 8.2% and 5.9% for 25(OH)D2 and 25(OH)D3, respectively. As there is no universally accepted definition of vitamin D deficiency during pregnancy (19, 20), we examined 25(OH)D by multiple cut-points from 25 to 80 nmol/L and used splines to investigate nonlinearity. We presented cut-points of 30 and 50 nmol/L per the IOM's definition of risk of vitamin D deficiency and inadequacy, respectfully (19), and 75 nmol/L per the Endocrine Society's definition of insufficiency (20).
We defined SGA as <10th percentile of birth weight for gestational age based on a fetal standard (21). We chose the fetal standard because we had a high incidence of preterm birth (26%), and SGA is underestimated in preterm births when birth weight standards are used (22, 23). Defining SGA based on a fetal standard has shown to better predict adverse outcomes (24-26). Gestational age was determined by ultrasound or date of last menstrual period in the absence of ultrasound.
Women were interviewed at baseline and self-reported pre-pregnancy weight, parity, marital status, smoking habits, age, and total years of schooling. Women self-reported predominant race which we classified as black (non-Hispanic black and Hispanic black) or white (non-Hispanic white, Hispanic white, and American Indian/Alaskan). Height was measured and date of blood draw was recorded. Data were also available on the season of blood draw [winter (December-February), spring (March-May), summer (June-August), or fall (September-November)], latitude of study site, and infant sex.
The distribution of 25(OH)D was approximately normal by visual inspection with a Kernel density plot, and we used the t-test to compare mean levels of 25(OH)D for SGA and non-SGA. We used log-binomial models to estimate risk ratios, risk differences, and their respective 95% confidence intervals for the association between 25(OH)D and SGA. We assessed non-linearity with restricted cubic splines and tested the spline terms with the Wald test. Biologic interaction was tested on the additive scale for prepregnancy BMI, race, gestational age at blood sample, season of blood sample, parity, and infant sex in full models with all potential confounders using the synergy index (27, 28). Statistical interaction terms were included in the regression model when biological interaction was present. Potential confounders (race, pre-pregnancy body mass index, height, gestational age at blood sampling, season at blood sampling, marital status, smoking, education, age, latitude, and infant sex) were identified using a theory-based causal graph (29). Confounding was considered present if variable removal from the full model changed the 25(OH)D and SGA association by >10%. None of the potential confounders met this criterion. We retained BMI and race out of convention and included treatment group, risk group (prepregnancy diabetes; chronic hypertension; previous preeclampsia), and study latitude to account for the design of the high risk aspirin trial.
In sensitivity analysis, we tested associations in conditional logistic regression models conditioned on study site, since the sample size did not provide adequate power to include 11 indicator variables for study site in final models.
Results
There were no differences in maternal 25(OH)D levels, birth weight, or SGA incidence in women excluded (n=47) compared to included (n=792; data not shown). Most women in the study were non-Hispanic black, 20-29 years old, parous, not married, non-smokers, and had a high school education (Table 1). In this high risk population, only a third of women were of normal weight (BMI <25 kg/m2), while a quarter were severely obese (BMI ≥35kg/m2). Thirteen percent of infants were SGA at birth. A higher proportion of SGA infants were born to smokers and women with less education (Table 1). Twenty-six percent of deliveries were <37 weeks. The mean (SD) of maternal 25(OH)D at study entry (i.e. before randomization) was 63.9 (29.5) nmol/L. Overall, 11.2%, 36.6%, and 67.9% of women had serum 25(OH)D concentrations of <30, <50, and <75 nmol/L, respectively.
Table 1. Characteristics of pregnant women at enrollment and their infants at birth in the High-Risk Aspirin Study (1991-1996).
| Total N=792 | Non-SGA n=689 | SGA n=103 | P | |
|---|---|---|---|---|
| Mothers | ||||
| Race | ||||
| Non-Hispanic White | 243 (30.7) | 216 (31.4) | 27 (26.2) | 0.30 |
| Non-Hispanic Black | 493 (62.5) | 422 (61.3) | 71 (68.9) | |
| Other* | 56 (7.1) | 51 (7.4) | 5 (4.9) | |
| Maternal age (years)† | ||||
| <20 | 96 (12.1) | 83 (12.1) | 13 (12.6) | 0.76 |
| 20-29 | 444 (56.1) | 384 (55.7) | 60 (58.3) | |
| >=30 | 246 (31.1) | 216 (31.4) | 30 (29.1) | |
| Parity | ||||
| Nulliparous | 175 (22.1) | 538 (78.1) | 79 (76.7) | 0.75 |
| Parous | 617 (77.9) | 151 (21.9) | 24 (23.3) | |
| Marital status | ||||
| Married | 289 (36.5) | 433 (62.8) | 70 (68.0) | 0.31 |
| Not married | 503 (63.5) | 256 (37.2) | 33 (32.0) | |
| Smoking at study entry | ||||
| Smoker | 133 (16.8) | 582 (84.5) | 77 (74.8) | 0.01 |
| Nonsmoker | 659 (83.2) | 107 (15.5) | 26 (25.2) | |
| Prepregnancy BMI (kg/m2) | ||||
| <25 | 259 (32.7) | 224 (32.5) | 35 (34.0) | 0.33 |
| 25-29.9 | 185 (23.4) | 155 (22.5) | 30 (29.1) | |
| 30-34.9 | 155 (19.6) | 140 (20.3) | 15 (14.6) | |
| ≥35 | 193 (24.4) | 170 (24.7) | 23 (22.3) | |
| Education† | ||||
| < High school | 217 (27.4) | 195 (28.3) | 22 (21.4) | 0.02 |
| High school graduate | 356 (45.0) | 301 (43.7) | 55 (53.4) | |
| Some college | 160 (20.2) | 144 (20.9) | 16 (15.5) | |
| College graduate | 58 (7.3) | 49 (7.11) | 9 (8.74) | |
| Gestational age at enrollment, weeks‡ | 19.5 ± 3.9 | 19.6 ± 4.0 | 19.4 ± 3.9 | 0.61 |
| Season of blood sampling | ||||
| Winter (December-February) | 186 (23.5) | 162 (23.5) | 24 (23.3) | 1.00 |
| Spring (March-May) | 202 (25.5) | 175 (25.4) | 27 (26.2) | |
| Summer (June-August) | 185 (23.4) | 161 (23.4) | 24 (23.3) | |
| Fall (September-November) | 219 (27.7) | 191 (27.7) | 28 (27.2) | |
| Latitude of study site | ||||
| ≥40 degrees North | 173 (21.8) | 149 (21.6) | 24 (23.3 | 0.89 |
| 37-39 degrees North | 133 (16.8) | 115 (16.7) | 18 (17.5) | |
| 35-36 degrees North | 281 (35.5) | 248 (36.0) | 33 (32.0) | |
| ≤35 degrees North | 205 (25.9) | 177 (25.7) | 28 (27.2) | |
| Risk group in Aspirin trial | 0.21 | |||
| Prepregnancy diabetes | 184 (23.2) | 167 (24.2) | 17 (16.5) | |
| Chronic Hypertension | 322 (40.7) | 275 (39.9) | 47 (45.6) | |
| Previous preeclampsia | 286 (36.1) | 247 (35.9) | 39 (37.9) | |
| Infants | ||||
| Birth weight, g | 3143 ± 723 | 3284 ± 636 | 2203 ± 549 | <0.01 |
| Length, cm | 49.2 ± 4.0 | 49.8 ± 3.6 | 45.5 ± 4.5 | <0.01 |
| Head circumference, cm | 33.7 ± 2.4 | 34.0 ± 2.2 | 31.3 ± 2.5 | <0.01 |
| Infant sex | <0.01 | |||
| Male | 417 (52.7) | 377 (54.7) | 40 (38.8) | |
| Female | 375 (47.4) | 312 (45.3) | 63 (61.2) | |
| Gestational age at birth, weeks | 37.5 ± 2.6 | 37.7 ± 2.5 | 36.6 ± 3.1 | <0.01 |
| Preterm (<37 weeks) | 208 (26.3) | 166 (24.1) | 42 (40.8) | <0.01 |
| Term (≥37 weeks) | 584 (73.7) | 523 (75.9) | 61 (59.2) | |
| Low birth weight status | <0.01 | |||
| <2500 g | 128 (16.2) | 62 (9.0) | 66 (64.1) | |
| ≥2500 g | 664 (83.8) | 627 (91.0) | 37 (35.9) | |
Data are n(%) or mean±standard deviation unless otherwise specified.
SGA, small-for-gestational-age; BMI, body mass index.
n=53 Hispanic white, n=1 Hispanic black, n=2 American Indian/Alaskan
Six missing values for age; one missing value for education.
range 12-26 weeks.
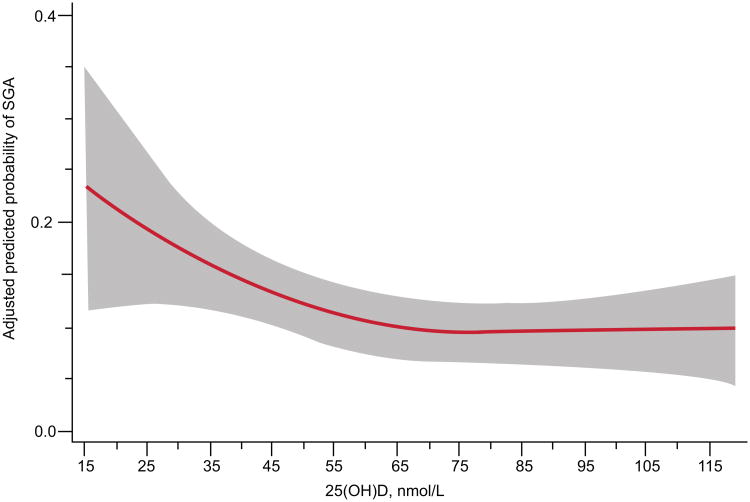
In unadjusted analyses, mean (SD) 25(OH)D concentrations were lower in women who delivered SGA infants compared to women delivering non-SGA infants (57.9 (29.9) vs. 64.8 (29.3) nmol/L, P=0.028). We observed a nonlinear relationship between 25(OH)D and risk of SGA (P<0.04; Figure 1). After adjustment for race, pregravid BMI, latitude, treatment group, and risk group in restricted cubic spline models, the risk of SGA declined as 25(OH)D concentrations increased up to about 50 nmol/L and leveled off thereafter. In categorical analysis, 25(OH)D concentrations of 50-74 nmol/L and ≥75 nmol/L compared to <30 nmol/L were associated with 43% and 54% reductions in risk of SGA and 8.4 and 10.7 fewer cases of SGA per 100 births, respectively (Table 2). Additional adjustment for gestational age at blood sampling, season at blood sampling, height, marital status, smoking, education, age, and infant sex did not meaningfully change the findings, nor did use of conditional logistic models conditioned on site (data not shown).
Figure 1.
Adjusted risk (solid line) of small-for-gestational-age birth by maternal serum 25-hydroxyvitamin D (25(OH)D) at 12–26 weeks of gestation in 792 women at high risk for preeclampsia (P=0.02). Gray shading is 95% confidence intervals. 25(OH)D was modeled by restricted cubic splines with three knots (28.8, 60.0, and 103.3) at percentiles 10%, 50%, and 90% in a log-binomial model adjusted for latitude, obesity status, race, treatment group, and risk group. 25(OH)D values less than 15 nmol/L (n=9) and more than 120 nmol/L (n=34) were omitted from the figure.
Table 2. Maternal vitamin D status and risk of small for gestational age in women at high risk for preeclampsia.
| Serum 25(OH)D categories | Non-SGA | SGA | Adjusted risk ratio* | 95% CI | Adjusted risk difference*,† | 95% CI |
|---|---|---|---|---|---|---|
|
| ||||||
| n (incidence %) | ||||||
| Overall | ||||||
| <30 nmol/L | 71 (79.8) | 18 (20.2) | Reference | Reference | ||
| 30-49 nmol/L | 171 (85.1) | 30 (14.9) | 0.71 | 0.42 - 1.19 | -5.8 | -15.0-3.4 |
| 50-74 nmol/L | 218 (87.9) | 30 (12.1) | 0.57 | 0.33 - 0.99 | -8.4 | -17.7-0.9 |
| ≥75 nmol/L | 229 (90.2) | 25 (9.8) | 0.46 | 0.24 - 0.87 | -10.8 | -20.6- -0.9 |
| <50 nmol/L‡ | 242 (83.5) | 48 (16.6) | Reference | Reference | ||
| ≥50 nmol/L | 447 (89.0) | 55 (11.0) | 0.66 | 0.43 - 1.01 | -5.3 | -11.0-0.36 |
| Maternal race§ | ||||||
| Whites‡,║ | ||||||
| <50 nmol/L | 37 (75.5) | 12 (24.5) | Reference | Reference | ||
| ≥50 nmol/L | 229 (92.0) | 20 (8.0) | 0.32 | 0.17 - 0.63 | -15.5 | -27.7- -3.3 |
| Blacks | ||||||
| <50 nmol/L | 205 (85.1) | 36 (14.9) | Reference | Reference | ||
| ≥50 nmol/L | 218 (86.2) | 35 (13.8) | 0.86 | 0.55 - 1.34 | -2.1 | -8.2-4.1 |
| Maternal obesity status§ ¶ | ||||||
| Nonobese‡ | ||||||
| <50 nmol/L | 101 (76.5) | 31 (23.5) | Reference | Reference | ||
| ≥50 nmol/L | 278 (89.1) | 34 (10.9) | 0.50 | 0.31 - 0.82 | -11.1 | -19.7- -2.5 |
| Obese | ||||||
| <50 nmol/L | 141 (89.2) | 17 (10.8) | Reference | Reference | ||
| ≥50 nmol/L | 169 (89.0) | 21 (11.1) | 1.09 | 0.58 - 2.04 | 0.8 | -5.5-7.1 |
25(OH)D, 25-hydroxyvitamin D; SGA, small-for-gestational-age; CI, confidence interval.
Adjusted for latitude, obesity status, race, treatment group, and risk group.
Per 100 live births.
P=0.001 for difference in incidence of SGA by 25(OH)D categories.
Synergy index for additive interaction = 0.43 (95% CI: 0.22, 0.64) by race and 0.50 (95% CI: 0.28, 0.71) by obese status
n=243 non-Hispanic white, n=53 Hispanic white, n=2 American Indian/Alaskan.
Nonobese = BMI <30 kg/m2; Obese = BMI ≥30 kg/m2.
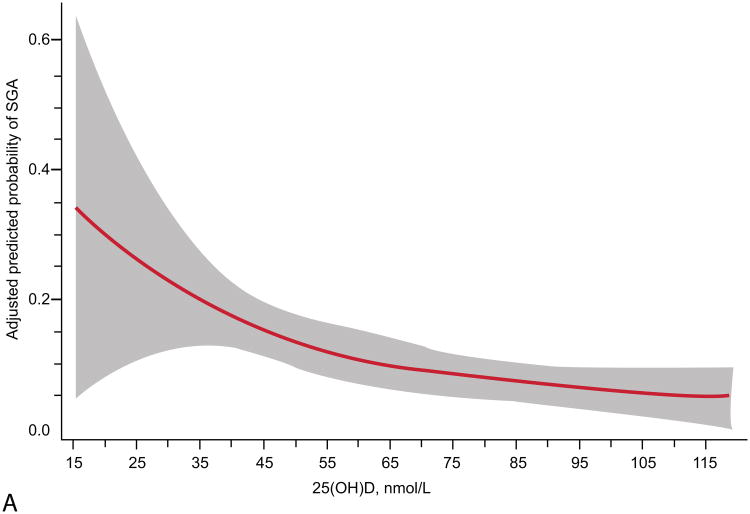
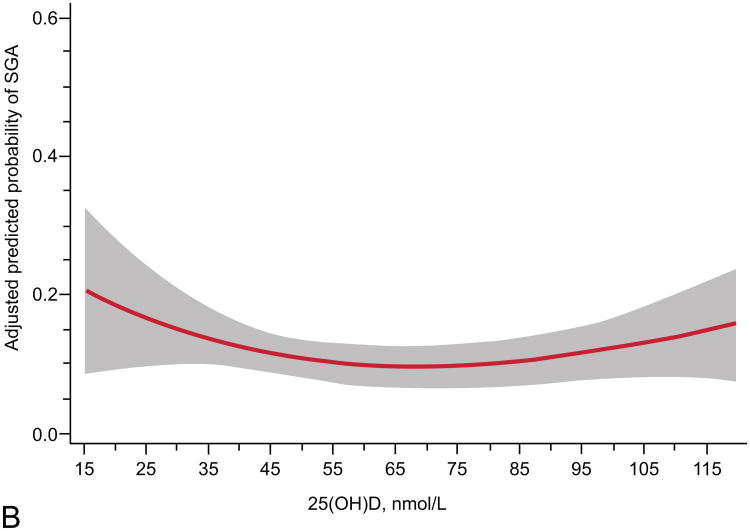
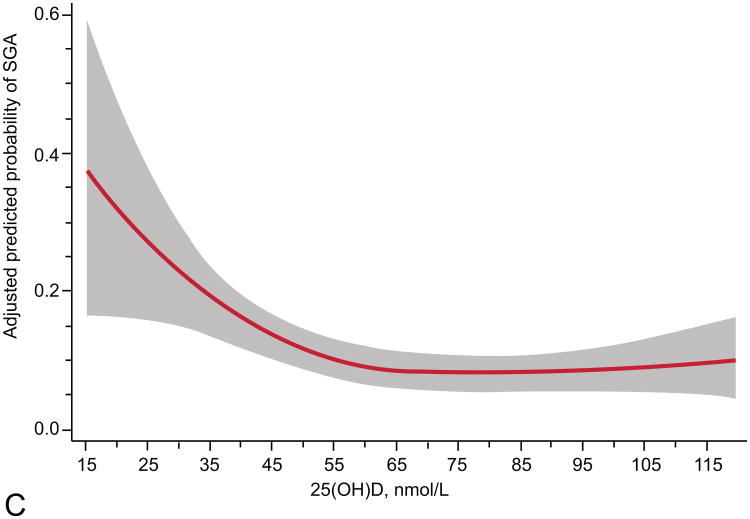
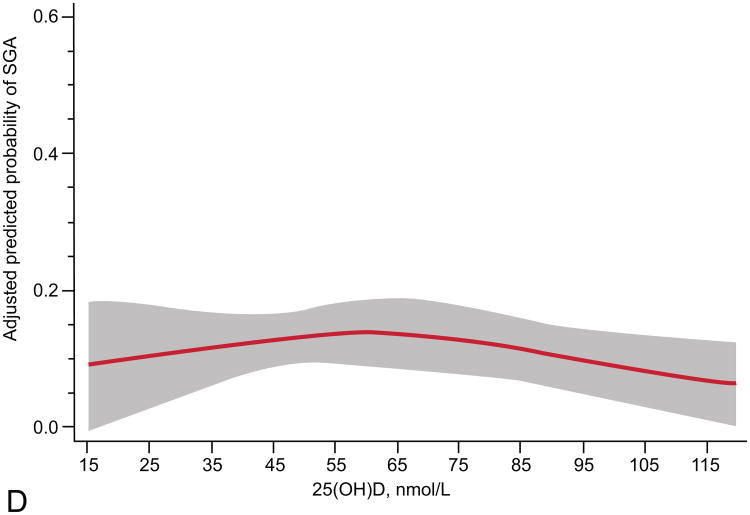
The relationship of maternal vitamin D status and risk of SGA was modified by race and obesity status (Table 2). Prevalence of 25(OH)D <50 nmol/L differed by race (16% in whites vs. 49% in blacks, P<0.001) and obesity (30% in nonobese vs. 45% in obese, P<0.001). We observed a different curvilinear relationship between 25(OH)D and risk of SGA in white compared with black mothers and nonobese compared with obese mothers. White women and nonobese women had higher risks of SGA with lower maternal 25(OH)D status (P<0.01; Figure 2). Further, in adjusted models white women and nonobese women with 25(OH)D ≥50 compared to <50 nmol/L had reduced risks of delivering an infant who was SGA (Table 2). There was no association between 25(OH)D and risk of SGA in black or obese mothers. There was only moderate overlap of white mothers and nonobese mothers. Sixty-two percent (n=184) of white women and 53% (n=260) of black women were not obese. There were an insufficient number of SGA cases to stratify results into four race/obesity groups. Associations were not modified by gestational age at study entry, parity, or infant sex.
Figure 2.
Adjusted risk (solid line) of small-for-gestational-age birth by maternal serum 25-hydroxyvitamin D (25(OH)D) at 12 – 26 weeks of gestation in (A) 298 white women (P less than 0.01, includes American Indian/Alaskan, n=2), (B) 494 black women (P=0.09), (C) 444 non-obese women (P=0.01, body mass index (BMI) less than 30 kg/m2), and (D) 348 obese women (P=0.39; BMI greater than 30 kg/m2) all at high risk for preeclampsia. Gray shading is 95% confidence intervals. 25(OH)D was modeled by restricted cubic splines with three knots (28.8, 60.0, and 103.3) at percentiles 10%, 50%, and 90% in a log-binomial model adjusted for latitude, obesity status, race, treatment group, and risk group. 25(OH)D values less than 15 nmol/L (n=9) and greater than 120 nmol/L (n=34) were omitted from the figure.
Discussion
In this multi-center study of women at high risk for preeclampsia, we found that maternal second trimester vitamin D status was inversely associated with risk of SGA in singleton pregnancies. The association seemed to be driven by white mothers and nonobese mothers. In both of these groups, 25(OH)D <50 nmol/L was associated with an increased risk of SGA after adjustment for confounders.
There are few randomized trials of maternal vitamin D supplementation, and we identified two that studied pathologic infant growth (30, 31). In 126 Asian women living in Britain, daily 1000 IU in the third trimester compared to placebo reduced risk of SGA by 13%, although this difference was not statistically significant (0.05 < P < 0.10) (30). In another British population (n=179), no effect of daily 800 IU or a large single dose of 200,000 IU at 27 weeks compared to no treatment was observed (31). This trial was not placebo controlled. A recent Cochrane review reported that there is limited evidence to assess the impact of vitamin D supplementation on SGA (32).
In observational studies, maternal vitamin D deficiency has been associated with risk of SGA in several general obstetric populations across the US and Europe (11-15). The overall association is similar to the magnitude of effect we observed in this high risk population, with 25(OH)D concentrations of 25-37.5 nmol/L appearing to be an important cutoff for increased risk. Two smaller studies, one in a general obstetric population (33) and one in women at high risk for preeclampsia (34), found no association between SGA and vitamin D status but were likely underpowered with only 46 and 13 cases of SGA, respectively.
Our results of an association between low 25(OH)D and SGA in white mothers but no association among black mothers is consistent with one previous study (12). Two other studies examined an interaction by maternal race and reported no black-white differences (11, 13). Although more research is needed to examine whether 25(OH)D has a different association with SGA by maternal race, it is possible that differences in genetic variability play a role (12, 35). We did not have a large enough sample size to study the interaction between vitamin D and polymorphisms in genes related to its metabolism, but such studies would be fascinating. Some have speculated that the lower range of 25(OH)D values in blacks may limit the ability to observe associations. The distribution of 25(OH)D was left-shifted in blacks compared to whites in this study, but the range was still wide with approximately 50% of black women having values above 50 nmol/L. Thus it does not seem that the range or distribution was a limitation in this case. Others have posed that blacks may not be impacted by low vitamin D status or may have different adaptive responses compared to whites (36). For example, black women with vitamin D deficiency have a decreased osteoporosis risk and increase in bone mass compared with whites with vitamin D deficiency, indicating there may be a lower threshold for parathyroid hormone induced bone turnover in blacks (36).
We are unaware of any previous vitamin D-fetal growth study to have assessed differences by prepregnancy BMI. However, our findings are consistent with a growing body of observational data suggesting that nutritional exposures may be protective against adverse pregnancy and birth outcomes such as SGA birth only among lean women (37). Obese mothers have altered metabolism and are at risk of multiple micronutrient deficiencies, including vitamin D deficiency (38). It is possible that higher doses of nutrients are needed in obese women to have effects similar to lean women (37).
The biologic mechanisms that may connect maternal vitamin D status to fetal growth remains elusive. A plausible mechanism for the impact of maternal vitamin D on fetal growth is placental vascularization, which has received considerable attention in its association with fetal growth (39-41). Several observational studies have connected poor vitamin D status with higher risk of preeclampsia (7, 42), which, like fetal growth restriction, has placental origins related to angiogenesis and uterine blood flow (39, 43). Mice raised on vitamin D deficient diets have placentas with narrower fetal vessels in the placental labyrinth compared to mice fed vitamin D sufficient diets, indicating dysregulated vascularization (44). We have demonstrated an inverse relationship between maternal 25(OH)D and risk of placental vascular lesions in pregnancies with male fetuses (10) and others have documented associations between vitamin D and biomarkers of angiogenesis (45-47). More basic science research is needed in this area as well as studies with multiple measurements of fetal growth and placental vascularization.
We did not have information on vitamin D dietary intake, supplement use, or sun exposure to inform the determinants of 25(OH)D in this population. Our study was limited by one measurement of 25(OH)D between 12 and 26 weeks of gestation. Because others have shown that exposure to vitamin D is differentially associated with SGA depending on trimester of sampling (11), measurements of 25(OH)D across gestation would have been ideal to allow us to study other critical windows outside this range. Although the choice of a weight standard to classify SGA is controversial, we chose a fetal weight standard to better capture SGA in preterm births since we had over a quarter of subject born at <37 weeks of gestation.
It is unclear how our findings in high-risk mothers translate to a general obstetric population. However, the vitamin D-SGA association we observed was similar to that reported in other general obstetrics populations, which may demonstrate broad importance of maternal vitamin D status even in pregnancies with many competing risk factors for poor fetal growth. We were not able to study Hispanic women as a separate race/ethnicity group due to small sample size (n=54), notwithstanding, our study benefits from a having a well-characterized and geographically- and racially-diverse population and use of gold standard methodology for assessing 25(OH)D.
Our cohort study adds to the body of evidence suggesting that maternal vitamin D status in pregnancy is related to fetal growth. Much of the supporting literature is based on observational studies, and only randomized controlled trials can determine whether this association is causal. Until data from trials are available, obstetricians should follow the ACOG guidelines for vitamin D screening and supplementation during pregnancy (38).
Acknowledgments
Supported by the National Institutes of Health grant HD056999 (PI: Dr. Bodnar).
The authors thank Mark Klebanoff for his assistance with analysis methods and Jill Diesel and Katharyn Baca for their assistance with data preparations.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Presented as a scientific poster at the Experimental Biology Meeting in Boston, MA on April 22, 2013.
Contributor Information
Alison D. Gernand, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA.
Hyagriv N. Simhan, Division of Maternal-Fetal Medicine, Magee-Womens Hospital and Department of Obstetrics, Gynecology and Reproductive Sciences, School of Medicine, University of Pittsburgh, Pittsburgh, PA.
Steve Caritis, Department of Obstetrics, Gynecology and Reproductive Sciences and Department of Pediatrics, School of Medicine, University of Pittsburgh, Pittsburgh, PA
Lisa M. Bodnar, Departments of Epidemiology and Obstetrics, Gynecology and Reproductive Sciences, University of Pittsburgh Graduate School of Public Health and School of Medicine, Pittsburgh, PA.
References
- 1.de Onis M, Blossner M, Villar J. Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr. 1998 Jan;52(1):S5–15. [PubMed] [Google Scholar]
- 2.Morisaki N, Esplin MS, Varner MW, Henry E, Oken E. Declines in birth weight and fetal growth independent of gestational length. Obstet Gynecol. 2013 Jan;121(1):51–8. doi: 10.1097/aog.0b013e318278d014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999 Apr 22;340(16):1234–8. doi: 10.1056/NEJM199904223401603. [DOI] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008 Jul 3;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer MS. Intrauterine growth and gestational duration determinants. Pediatrics. 1987 Oct;80(4):502–11. [PubMed] [Google Scholar]
- 6.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. Vitamin D status: United States, 2001-2006. NCHS Data Brief. 2011 Mar;(59):1–8. [PubMed] [Google Scholar]
- 7.Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169. doi: 10.1136/bmj.f1169. [DOI] [PubMed] [Google Scholar]
- 8.Specker BL. Does vitamin D during pregnancy impact offspring growth and bone? Proc Nutr Soc. 2012 Feb;71(1):38–45. doi: 10.1017/S0029665111003053. [DOI] [PubMed] [Google Scholar]
- 9.Thorne-Lyman A, Fawzi WW. Vitamin D during pregnancy and maternal, neonatal and infant health outcomes: a systematic review and meta-analysis. Paediatr Perinat Epidemiol. 2012 Jul;26(1):75–90. doi: 10.1111/j.1365-3016.2012.01283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gernand AD, Bodnar LM, Klebanoff MA, Parks WT, Simhan HN. Maternal serum 25-hydroxyvitamin D and placental vascular pathology in a multicenter US cohort. Am J Clin Nutr. 2013 Jun 26; doi: 10.3945/ajcn.112.055426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gernand AD, Simhan HN, Klebanoff MA, Bodnar LM. Maternal serum 25-hydroxyvitamin D and measures of newborn and placental weight in a U.S. multicenter cohort study. J Clin Endocrinol Metab. 2013 Jan;98(1):398–404. doi: 10.1210/jc.2012-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodnar LM, Catov JM, Zmuda JM, Cooper ME, Parrott MS, Roberts JM, et al. Maternal serum 25-hydroxyvitamin D concentrations are associated with small-for-gestational age births in white women. J Nutr. 2010 May;140(5):999–1006. doi: 10.3945/jn.109.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burris HH, Rifas-Shiman SL, Camargo CA, Jr, Litonjua AA, Huh SY, Rich-Edwards JW, et al. Plasma 25-hydroxyvitamin D during pregnancy and small-for-gestational age in black and white infants. Ann Epidemiol. 2012 Aug;22(8):581–6. doi: 10.1016/j.annepidem.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ertl R, Yu CK, Samaha R, Akolekar R, Nicolaides KH. Maternal serum vitamin D at 11-13 weeks in pregnancies delivering small for gestational age neonates. Fetal Diagn Ther. 2012;31(2):103–8. doi: 10.1159/000333810. [DOI] [PubMed] [Google Scholar]
- 15.Leffelaar ER, Vrijkotte TG, van Eijsden M. Maternal early pregnancy vitamin D status in relation to fetal and neonatal growth: results of the multi-ethnic Amsterdam Born Children and their Development cohort. Br J Nutr. 2010 Jul;104(1):108–17. doi: 10.1017/S000711451000022X. [DOI] [PubMed] [Google Scholar]
- 16.Caritis S, Sibai B, Hauth J, Lindheimer MD, Klebanoff M, Thom E, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998 Mar 12;338(11):701–5. doi: 10.1056/NEJM199803123381101. [DOI] [PubMed] [Google Scholar]
- 17.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr. 2009 Jun;89(6):1997S–2008S. doi: 10.3945/ajcn.2009.27230D. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab. 2005 Jun;90(6):3215–24. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 19.IOM. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 20.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–30. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 21.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991 Oct;181(1):129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 22.Ott WJ. Intrauterine growth retardation and preterm delivery. Am J Obstet Gynecol. 1993 Jun;168(6 Pt 1):1710–5. doi: 10.1016/0002-9378(93)90681-8. discussion 5-7. [DOI] [PubMed] [Google Scholar]
- 23.Hutcheon JA, Platt RW. The missing data problem in birth weight percentiles and thresholds for “small-for-gestational-age”. Am J Epidemiol. 2008 Apr 1;167(7):786–92. doi: 10.1093/aje/kwm327. [DOI] [PubMed] [Google Scholar]
- 24.Zaw W, Gagnon R, da Silva O. The risks of adverse neonatal outcome among preterm small for gestational age infants according to neonatal versus fetal growth standards. Pediatrics. 2003 Jun;111(6 Pt 1):1273–7. doi: 10.1542/peds.111.6.1273. [DOI] [PubMed] [Google Scholar]
- 25.Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001 Apr;184(5):946–53. doi: 10.1067/mob.2001.111719. [DOI] [PubMed] [Google Scholar]
- 26.Cooke RW. Conventional birth weight standards obscure fetal growth restriction in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2007 May;92(3):F189–92. doi: 10.1136/adc.2005.089698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skrondal A. Interaction as departure from additivity in case-control studies: a cautionary note. Am J Epidemiol. 2003 Aug 1;158(3):251–8. doi: 10.1093/aje/kwg113. [DOI] [PubMed] [Google Scholar]
- 28.Rothman KJ. The estimation of synergy or antagonism. Am J Epidemiol. 1976 May;103(5):506–11. doi: 10.1093/oxfordjournals.aje.a112252. [DOI] [PubMed] [Google Scholar]
- 29.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999 Jan;10(1):37–48. [PubMed] [Google Scholar]
- 30.Brooke OG, Brown IR, Bone CD, Carter ND, Cleeve HJ, Maxwell JD, et al. Vitamin D supplements in pregnant Asian women: effects on calcium status and fetal growth. Br Med J. 1980 Mar 15;280(6216):751–4. doi: 10.1136/bmj.280.6216.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu CK, Sykes L, Sethi M, Teoh TG, Robinson S. Vitamin D deficiency and supplementation during pregnancy. Clin Endocrinol (Oxf) 2009 May;70(5):685–90. doi: 10.1111/j.1365-2265.2008.03403.x. [DOI] [PubMed] [Google Scholar]
- 32.De-Regil LM, Palacios C, Ansary A, Kulier R, Pena-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2012;2:CD008873. doi: 10.1002/14651858.CD008873.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandez-Alonso AM, Dionis-Sanchez EC, Chedraui P, Gonzalez-Salmeron MD, Perez-Lopez FR. First-trimester maternal serum 25-hydroxyvitamin D(3) status and pregnancy outcome. Int J Gynaecol Obstet. 2012 Jan;116(1):6–9. doi: 10.1016/j.ijgo.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 34.Shand AW, Nassar N, Von Dadelszen P, Innis SM, Green TJ. Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia. BJOG. 2010 Dec;117(13):1593–8. doi: 10.1111/j.1471-0528.2010.02742.x. [DOI] [PubMed] [Google Scholar]
- 35.Swamy GK, Garrett ME, Miranda ML, Ashley-Koch AE. Maternal vitamin D receptor genetic variation contributes to infant birthweight among black mothers. Am J Med Genet A. 2011 Jun;155A(6):1264–71. doi: 10.1002/ajmg.a.33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cosman F, Nieves J, Dempster D, Lindsay R. Vitamin D economy in blacks. J Bone Miner Res. 2007 Dec;22(2):V34–8. doi: 10.1359/jbmr.07s220. [DOI] [PubMed] [Google Scholar]
- 37.Bodnar LM, Parrott MS. Intervention strategies to improve outcome in obese pregnancies: micronutrients and dietary supplements. In: Gillman MW, Poston L, editors. Maternal Obesity. Cambridge, UK: Cambridge University Press; 2012. [Google Scholar]
- 38.ACOG Committee Opinion No. 495: Vitamin D: Screening and supplementation during pregnancy. Obstet Gynecol. 2011 Jul;118(1):197–8. doi: 10.1097/AOG.0b013e318227f06b. [DOI] [PubMed] [Google Scholar]
- 39.Staff AC, Benton SJ, von Dadelszen P, Roberts JM, Taylor RN, Powers RW, et al. Redefining preeclampsia using placenta-derived biomarkers. Hypertension. 2013 May;61(5):932–42. doi: 10.1161/HYPERTENSIONAHA.111.00250. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Merialdi M, Platt LD, Kramer MS. Defining normal and abnormal fetal growth: promises and challenges. Am J Obstet Gynecol. 2010 Jun;202(6):522–8. doi: 10.1016/j.ajog.2009.10.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turan S, Miller J, Baschat AA. Integrated testing and management in fetal growth restriction. Semin Perinatol. 2008 Jun;32(3):194–200. doi: 10.1053/j.semperi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Christesen HT, Falkenberg T, Lamont RF, Jorgensen JS. The impact of vitamin D on pregnancy: a systematic review. Acta Obstet Gynecol Scand. 2012 Dec;91(12):1357–67. doi: 10.1111/aogs.12000. [DOI] [PubMed] [Google Scholar]
- 43.Bhattacharyya SK, Kundu S, Kabiraj SP. Prediction of preeclampsia by midtrimester uterine artery Doppler velocimetry in high-risk and low-risk women. J Obstet Gynaecol India. 2012 Jun;62(3):297–300. doi: 10.1007/s13224-012-0219-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu NQ, Ouyang Y, Bulut Y, Lagishetty V, Chan SY, Hollis BW, et al. Dietary Vitamin D Restriction in Pregnant Female Mice Is Associated With Maternal Hypertension and Altered Placental and Fetal Development. Endocrinology. 2013 May 15; doi: 10.1210/en.2012-2270. [DOI] [PubMed] [Google Scholar]
- 45.Wei SQ, Audibert F, Luo ZC, Nuyt AM, Masse B, Julien P, et al. Maternal plasma 25-hydroxyvitamin D levels, angiogenic factors, and preeclampsia. Am J Obstet Gynecol. 2013 May;208(5):390. doi: 10.1016/j.ajog.2013.03.025. e1-6. [DOI] [PubMed] [Google Scholar]
- 46.Grundmann M, Haidar M, Placzko S, Niendorf R, Darashchonak N, Hubel CA, et al. Vitamin D improves the angiogenic properties of endothelial progenitor cells. Am J Physiol Cell Physiol. 2012 Nov;303(9):C954–62. doi: 10.1152/ajpcell.00030.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Levine MJ, Teegarden D. 1alpha,25-dihydroxycholecalciferol increases the expression of vascular endothelial growth factor in C3H10T1/2 mouse embryo fibroblasts. J Nutr. 2004 Sep;134(9):2244–50. doi: 10.1093/jn/134.9.2244. [DOI] [PubMed] [Google Scholar]