Abstract
In the title molecule, C7H7Br2N, the C—Br vectors of the bromomethyl groups extend to opposite sides of the pyridine ring and are oriented nearly perpendicular to its plane. In the crystal, the molecules related by a c-glide-plane operation are arranged into stacks along the c axis, with centroid–centroid distances between neighboring aromatic rings of 3.778 (2) Å. A short Br⋯Br contact of 3.6025 (11) Å is observed within a pair of inversion-related molecules.
Related literature
For the isomorphous crystal structure of 2,6-bis(chloromethyl)pyridine, see: Betz et al. (2011 ▶). For the synthesis of the title compound, see: Dioury et al. (2009 ▶).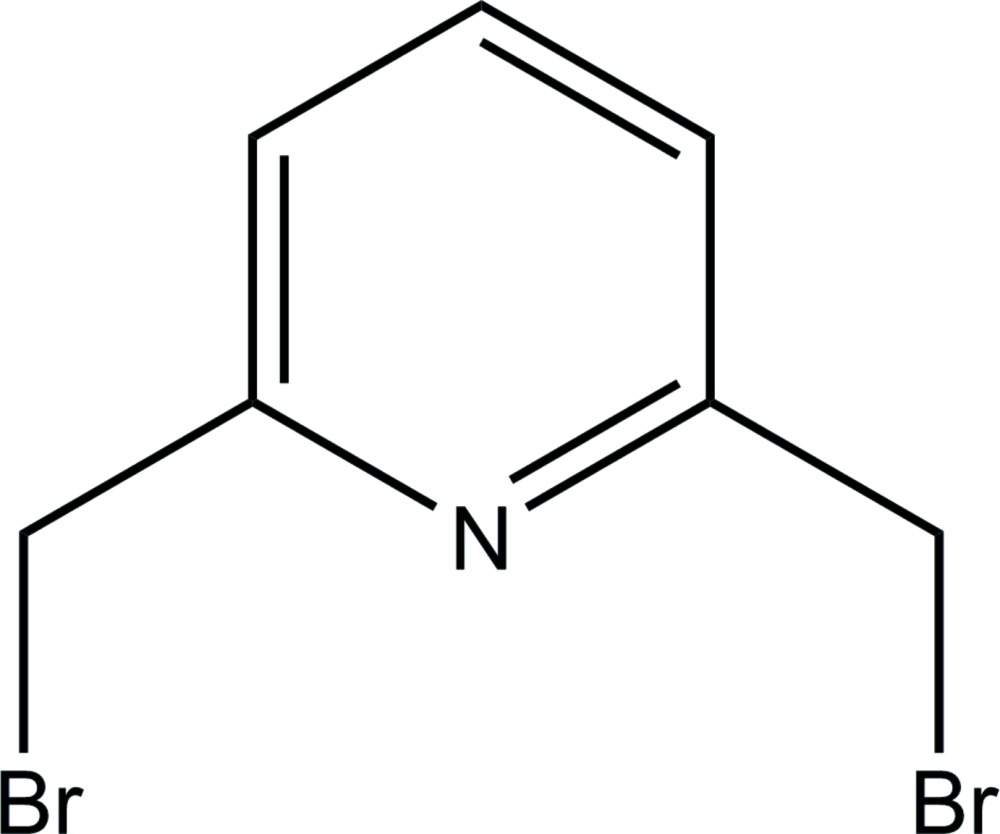
Experimental
Crystal data
C7H7Br2N
M r = 264.96
Monoclinic,
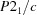
a = 9.2955 (19) Å
b = 12.980 (3) Å
c = 7.5288 (15) Å
β = 110.75 (3)°
V = 849.5 (3) Å3
Z = 4
Mo Kα radiation
μ = 9.47 mm−1
T = 293 K
0.28 × 0.26 × 0.12 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SORTAV; Blessing, 1995 ▶) T min = 0.088, T max = 0.321
10219 measured reflections
2480 independent reflections
1923 reflections with I > 2σ(I)
R int = 0.048
Refinement
R[F 2 > 2σ(F 2)] = 0.044
wR(F 2) = 0.119
S = 1.04
2480 reflections
92 parameters
H-atom parameters constrained
Δρmax = 0.99 e Å−3
Δρmin = −1.06 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: DENZO/SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO/SCALEPACK; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: Mercury (Macrae et al., 2006 ▶) and XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: enCIFer (Allen et al., 2004 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536813032364/gk2596sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813032364/gk2596Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536813032364/gk2596Isup3.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
We thank Dr Michel Giorgi (Spectropole, Aix Marseille University) for helpful advice. This research was supported by the bilateral Moldo–French project entitled ‘Nickel and iron complexes as models of active centre of hydrogenases’ 13.820.08.01/FrF; nr. 170783.
supplementary crystallographic information
1. Comment
2,6-Bis(bromomethyl)pyridine is a well known organic compound which serves as a precursor in various organic reactions leading to formation of a large range of pyridine derivatives. Additionally, the presence of N-donor atom enables coordination of metal ions by this molecule. Due to conformational flexibility of the bromomethyl arms, the title compound has been used as a starting material for the synthesis of macrocycles (Dioury et al., 2009).
Recently, the crystal structure of the Cl analogue of the title compound - 2,6-bis(chloromethyl)pyridine - has been reported and the two compounds form an isomorphous pair.
In the crystal, π-π stacking interaction between aromatic rings of molecules forming stacks along the c axis are observed. The distance between the centroids of the stacked pyridine rings is 3.778 (2) Å (symmetry code: x, 1/2 - y, -1/2 + z).
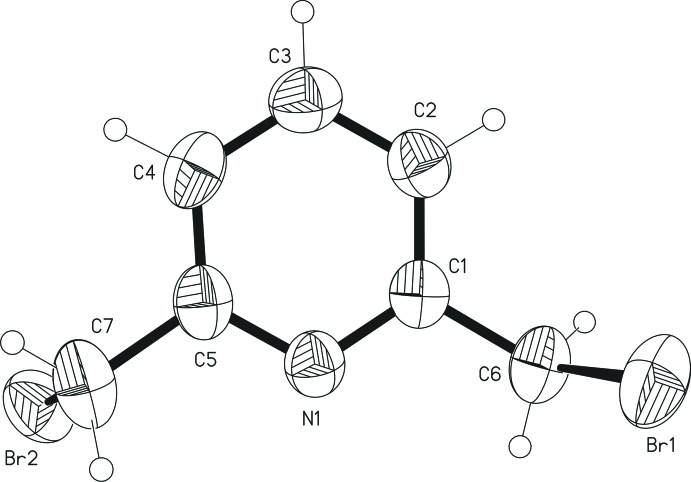
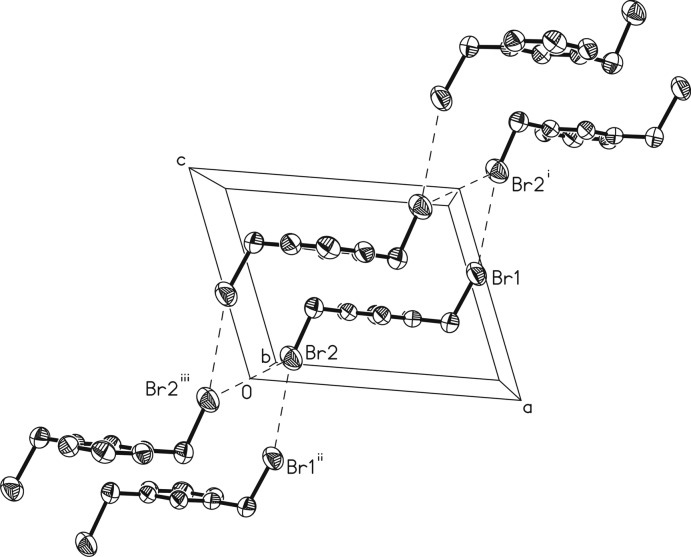
The bromine atoms are situated at both sides of the pyridine plane and bromomethyl arms are pointing at approximately opposite directions. In the crystal, there are two different Br···Br contacts of type I. The contact longer than the sum of van der Waals radii [Br2···Br1i 3.7051 (11) Å; symmetry code: (i) 1 + x, y, 1 + z] links the molecules into infinite chains along [1 0 1] whereas the other one [Br2···Br2iii 3.6025 (11) Å; symmetry code: (iii) -x, -y, -z] is formed between neighbouring chains.
2. Experimental
The compund was obtained according to previously reported procedure from the 2,6-bis(hydroxymethyl)pyridine (Dioury et al., 2009). Crystals suitable for the X-ray diffraction study were obtained by recrystallization from diethylether at room temperature.
3. Refinement
The H atoms were positioned geometrically and refined using a riding model with C—H = 0.95–0.99 Å and with Uiso(H) = 1.2 times Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound. Displacement ellipsoids are drawn at the 50% probability level. H atoms are presented as small spheres of arbitrary radius.
Fig. 2.
Intermolecular Br···Br contacts (dashed lines) in the title compound. Symmetry codes: (i) 1 + x, y, 1 + z; (ii) -1 + x, y, -1 + z; (iii) -x, -y, -z. H atoms have been omitted for clarity.
Crystal data
| C7H7Br2N | F(000) = 504 |
| Mr = 264.96 | Dx = 2.072 Mg m−3 |
| Monoclinic, P21/c | Melting point = 358–360 K |
| Hall symbol: -P 2ybc | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.2955 (19) Å | Cell parameters from 10219 reflections |
| b = 12.980 (3) Å | θ = 2.8–30.4° |
| c = 7.5288 (15) Å | µ = 9.47 mm−1 |
| β = 110.75 (3)° | T = 293 K |
| V = 849.5 (3) Å3 | Platelet, colourless |
| Z = 4 | 0.28 × 0.26 × 0.12 mm |
Data collection
| Nonius KappaCCD diffractometer | 2480 independent reflections |
| Radiation source: fine-focus sealed tube | 1923 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.048 |
| ω and Phi scan | θmax = 30.4°, θmin = 2.8° |
| Absorption correction: multi-scan (SORTAV; Blessing, 1995) | h = −11→13 |
| Tmin = 0.088, Tmax = 0.321 | k = −17→14 |
| 10219 measured reflections | l = −10→8 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.044 | H-atom parameters constrained |
| wR(F2) = 0.119 | w = 1/[σ2(Fo2) + (0.0467P)2 + 1.3504P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 2480 reflections | Δρmax = 0.99 e Å−3 |
| 92 parameters | Δρmin = −1.06 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.036 (3) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.97970 (5) | 0.13488 (4) | 0.59690 (7) | 0.0698 (2) | |
| Br2 | 0.17000 (5) | 0.07677 (4) | 0.10894 (7) | 0.0696 (2) | |
| N1 | 0.5656 (3) | 0.1307 (2) | 0.3525 (4) | 0.0461 (6) | |
| C1 | 0.6810 (4) | 0.1899 (3) | 0.3462 (5) | 0.0447 (7) | |
| C2 | 0.6751 (4) | 0.2963 (3) | 0.3423 (5) | 0.0509 (8) | |
| H2 | 0.7580 | 0.3347 | 0.3371 | 0.061* | |
| C3 | 0.5438 (5) | 0.3448 (3) | 0.3464 (6) | 0.0560 (9) | |
| H3 | 0.5369 | 0.4162 | 0.3454 | 0.067* | |
| C4 | 0.4230 (4) | 0.2844 (3) | 0.3521 (5) | 0.0535 (9) | |
| H4 | 0.3329 | 0.3148 | 0.3536 | 0.064* | |
| C5 | 0.4380 (4) | 0.1781 (3) | 0.3554 (5) | 0.0479 (8) | |
| C6 | 0.8218 (4) | 0.1350 (4) | 0.3439 (6) | 0.0559 (9) | |
| H6A | 0.8610 | 0.1688 | 0.2555 | 0.067* | |
| H6B | 0.7957 | 0.0646 | 0.3015 | 0.067* | |
| C7 | 0.3117 (5) | 0.1099 (4) | 0.3646 (6) | 0.0646 (11) | |
| H7A | 0.2560 | 0.1441 | 0.4350 | 0.078* | |
| H7B | 0.3552 | 0.0468 | 0.4310 | 0.078* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0465 (3) | 0.0867 (4) | 0.0672 (3) | 0.01422 (19) | 0.00885 (19) | −0.0045 (2) |
| Br2 | 0.0558 (3) | 0.0711 (3) | 0.0710 (3) | −0.01709 (19) | 0.0091 (2) | 0.0004 (2) |
| N1 | 0.0412 (15) | 0.0523 (16) | 0.0429 (15) | 0.0000 (12) | 0.0127 (12) | 0.0034 (12) |
| C1 | 0.0419 (16) | 0.0556 (19) | 0.0357 (15) | 0.0006 (14) | 0.0127 (13) | 0.0011 (13) |
| C2 | 0.0466 (18) | 0.056 (2) | 0.0481 (19) | −0.0040 (15) | 0.0141 (15) | 0.0016 (15) |
| C3 | 0.057 (2) | 0.052 (2) | 0.052 (2) | 0.0039 (16) | 0.0115 (17) | −0.0016 (16) |
| C4 | 0.0417 (17) | 0.070 (2) | 0.0460 (18) | 0.0105 (16) | 0.0117 (14) | −0.0023 (16) |
| C5 | 0.0382 (16) | 0.065 (2) | 0.0374 (16) | −0.0015 (14) | 0.0100 (13) | 0.0030 (15) |
| C6 | 0.0458 (19) | 0.072 (3) | 0.052 (2) | 0.0069 (17) | 0.0199 (16) | 0.0008 (17) |
| C7 | 0.048 (2) | 0.092 (3) | 0.055 (2) | −0.010 (2) | 0.0190 (18) | 0.008 (2) |
Geometric parameters (Å, º)
| Br1—C6 | 1.950 (4) | C3—H3 | 0.9300 |
| Br2—C7 | 1.956 (4) | C4—C5 | 1.387 (6) |
| N1—C1 | 1.334 (4) | C4—H4 | 0.9300 |
| N1—C5 | 1.343 (5) | C5—C7 | 1.491 (5) |
| C1—C2 | 1.382 (6) | C6—H6A | 0.9700 |
| C1—C6 | 1.496 (5) | C6—H6B | 0.9700 |
| C2—C3 | 1.383 (6) | C7—H7A | 0.9700 |
| C2—H2 | 0.9300 | C7—H7B | 0.9700 |
| C3—C4 | 1.382 (6) | ||
| C1—N1—C5 | 117.5 (3) | N1—C5—C7 | 116.3 (4) |
| N1—C1—C2 | 123.4 (3) | C4—C5—C7 | 121.1 (4) |
| N1—C1—C6 | 116.3 (3) | C1—C6—Br1 | 110.4 (3) |
| C2—C1—C6 | 120.3 (4) | C1—C6—H6A | 109.6 |
| C1—C2—C3 | 118.8 (4) | Br1—C6—H6A | 109.6 |
| C1—C2—H2 | 120.6 | C1—C6—H6B | 109.6 |
| C3—C2—H2 | 120.6 | Br1—C6—H6B | 109.6 |
| C4—C3—C2 | 118.4 (4) | H6A—C6—H6B | 108.1 |
| C4—C3—H3 | 120.8 | C5—C7—Br2 | 110.6 (3) |
| C2—C3—H3 | 120.8 | C5—C7—H7A | 109.5 |
| C3—C4—C5 | 119.2 (3) | Br2—C7—H7A | 109.5 |
| C3—C4—H4 | 120.4 | C5—C7—H7B | 109.5 |
| C5—C4—H4 | 120.4 | Br2—C7—H7B | 109.5 |
| N1—C5—C4 | 122.6 (3) | H7A—C7—H7B | 108.1 |
| C5—N1—C1—C2 | 0.0 (5) | C1—N1—C5—C7 | −179.4 (3) |
| C5—N1—C1—C6 | 179.7 (3) | C3—C4—C5—N1 | −0.3 (6) |
| N1—C1—C2—C3 | 0.4 (6) | C3—C4—C5—C7 | 179.0 (4) |
| C6—C1—C2—C3 | −179.4 (3) | N1—C1—C6—Br1 | −100.1 (3) |
| C1—C2—C3—C4 | −0.7 (6) | C2—C1—C6—Br1 | 79.7 (4) |
| C2—C3—C4—C5 | 0.6 (6) | N1—C5—C7—Br2 | −91.3 (4) |
| C1—N1—C5—C4 | 0.0 (5) | C4—C5—C7—Br2 | 89.4 (4) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GK2596).
References
- Allen, F. H., Johnson, O., Shields, G. P., Smith, B. R. & Towler, M. (2004). J. Appl. Cryst. 37, 335–338.
- Betz, R., Gerber, T. & Schalekamp, H. (2011). Acta Cryst. E67, o1577. [DOI] [PMC free article] [PubMed]
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. [DOI] [PubMed]
- Dioury, F., Ferroud, C., Guy, A. & Port, M. (2009). Tetrahedron, 65, 7573–7579.
- Macrae, C. F., Edgington, P. R., McCabe, P., Pidcock, E., Shields, G. P., Taylor, R., Towler, M. & van de Streek, J. (2006). J. Appl. Cryst. 39, 453–457.
- Nonius (1998). COLLECT Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S1600536813032364/gk2596sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813032364/gk2596Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536813032364/gk2596Isup3.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report