Abstract
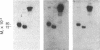
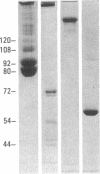
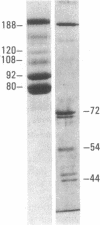
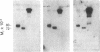
Epitopes for 22 alloantibodies that inhibit factor VIII procoagulant protein (FVIII) from multitransfused individuals with severe hemophilia A and three autoantibodies from nonhemophilic individuals appeared to be restricted to two specific regions of the FVIII molecule. Immunoblotting of purified FVIII and purified thrombin-degraded FVIII, followed by reaction with inhibitor plasma samples, monoclonal anti-human IgG3 and IgG4 antibodies, and radiolabeled affinity-purified rabbit anti-mouse IgG, revealed that inhibitor epitopes could be localized to the Mr 72,000 and Mr 44,000 thrombin fragments of FVIII. These two chains are located at the carboxyl terminus and near the amino terminus of the FVIII molecule, respectively. The pattern of reactivity of the inhibitor alloantibodies could be divided into three types: 10 reacted with the Mr 72,000 chain, 3 reacted with the Mr 44,000 chain, and 9 reacted with both of these chains. Among the 3 inhibitor autoantibodies, 1 of each type was found. Ten normal plasmas, as well as 14 plasmas from multitransfused individuals with severe hemophilia A and no inhibitor, were not reactive with the FVIII immunoblots. However, one multitransfused individual with severe hemophilia A and no detectable inhibitor revealed the presence of an antibody reactive with the middle section of the FVIII molecule. The existence of FVIII inhibitor epitopes on both the Mr 72,000 and Mr 44,000 chains raises the possibility that these epitopes might be further restricted to regions of homology between the two chains. These data suggest the possibility of designing inhibitor blocking polypeptides for use as therapeutic agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Church W. R., Jernigan R. L., Toole J., Hewick R. M., Knopf J., Knutson G. J., Nesheim M. E., Mann K. G., Fass D. N. Coagulation factors V and VIII and ceruloplasmin constitute a family of structurally related proteins. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6934–6937. doi: 10.1073/pnas.81.22.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croissant M. P., Zuzel M., Allain J. P. Heterogeneity of autoantibodies to factor VIII: differences in specificity for apparently distinct antigenic determinants of factor VIII coagulant protein. Blood. 1983 Jul;62(1):133–140. [PubMed] [Google Scholar]
- Davies B. L., Furlong R. A., Peake I. R. Studies on the relationship between factor VIII related antigen (VIIIRAg) and factor VIII clotting antigen (VIIICAg) by immunoelectrophoresis and autoradiography using 125I anti VIIICAg. Thromb Res. 1981 Apr 1;22(1-2):87–96. doi: 10.1016/0049-3848(81)90311-x. [DOI] [PubMed] [Google Scholar]
- Fass D. N., Knutson G. J., Katzmann J. A. Monoclonal antibodies to porcine factor VIII coagulant and their use in the isolation of active coagulant protein. Blood. 1982 Mar;59(3):594–600. [PubMed] [Google Scholar]
- Fulcher C. A., Gardiner J. E., Griffin J. H., Zimmerman T. S. Proteolytic inactivation of human factor VIII procoagulant protein by activated human protein C and its analogy with factor V. Blood. 1984 Feb;63(2):486–489. [PubMed] [Google Scholar]
- Fulcher C. A., Roberts J. R., Holland L. Z., Zimmerman T. S. Human factor VIII procoagulant protein. Monoclonal antibodies define precursor-product relationships and functional epitopes. J Clin Invest. 1985 Jul;76(1):117–124. doi: 10.1172/JCI111933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher C. A., Roberts J. R., Zimmerman T. S. Thrombin proteolysis of purified factor viii procoagulant protein: correlation of activation with generation of a specific polypeptide. Blood. 1983 Apr;61(4):807–811. [PubMed] [Google Scholar]
- Fulcher C. A., Zimmerman T. S. Characterization of the human factor VIII procoagulant protein with a heterologous precipitating antibody. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1648–1652. doi: 10.1073/pnas.79.5.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawryl M. S., Hoyer L. W. Immunologic studies of antihemophilic factor (AHF, VIII:C). VI. Characterization of antigenic determinants using human antibodies. Clin Immunol Immunopathol. 1982 May;23(2):517–526. doi: 10.1016/0090-1229(82)90135-0. [DOI] [PubMed] [Google Scholar]
- Gawryl M. S., Hoyer L. W. Inactivation of factor VIII coagulant activity by two different types of human antibodies. Blood. 1982 Nov;60(5):1103–1109. [PubMed] [Google Scholar]
- Hoyer L. W., Gawryl M. S., de la Fuente B. Immunochemical characterization of factor VIII inhibitors. Prog Clin Biol Res. 1984;150:73–85. [PubMed] [Google Scholar]
- Kasper C. K. Management of inhibitors to factor VIII. Prog Hematol. 1981;12:143–163. [PubMed] [Google Scholar]
- Knutson G. J., Fass D. N. Porcine factor VIII:C prepared by affinity interaction with von Willebrand factor and heterologous antibodies: sodium dodecyl sulfate polyacrylamide gel analysis. Blood. 1982 Mar;59(3):615–624. [PubMed] [Google Scholar]
- Letter: A more uniform measurement of factor VIII inhibitors. Thromb Diath Haemorrh. 1975 Dec 15;34(3):869–872. [PubMed] [Google Scholar]
- Lollar P., Knutson G. J., Fass D. N. Stabilization of thrombin-activated porcine factor VIII:C by factor IXa phospholipid. Blood. 1984 Jun;63(6):1303–1308. [PubMed] [Google Scholar]
- Roberts H. R., Cromartie R. Overview of inhibitors to factor VIII and IX. Prog Clin Biol Res. 1984;150:1–18. [PubMed] [Google Scholar]
- Suzuki K., Dahlbäck B., Stenflo J. Thrombin-catalyzed activation of human coagulation factor V. J Biol Chem. 1982 Jun 10;257(11):6556–6564. [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Davie E. W. Preparation and properties of bovine factor VIII (antihemophilic factor). Biochemistry. 1980 Feb 5;19(3):401–410. doi: 10.1021/bi00544a001. [DOI] [PubMed] [Google Scholar]
- Vehar G. A., Keyt B., Eaton D., Rodriguez H., O'Brien D. P., Rotblat F., Oppermann H., Keck R., Wood W. I., Harkins R. N. Structure of human factor VIII. Nature. 1984 Nov 22;312(5992):337–342. doi: 10.1038/312337a0. [DOI] [PubMed] [Google Scholar]