Abstract
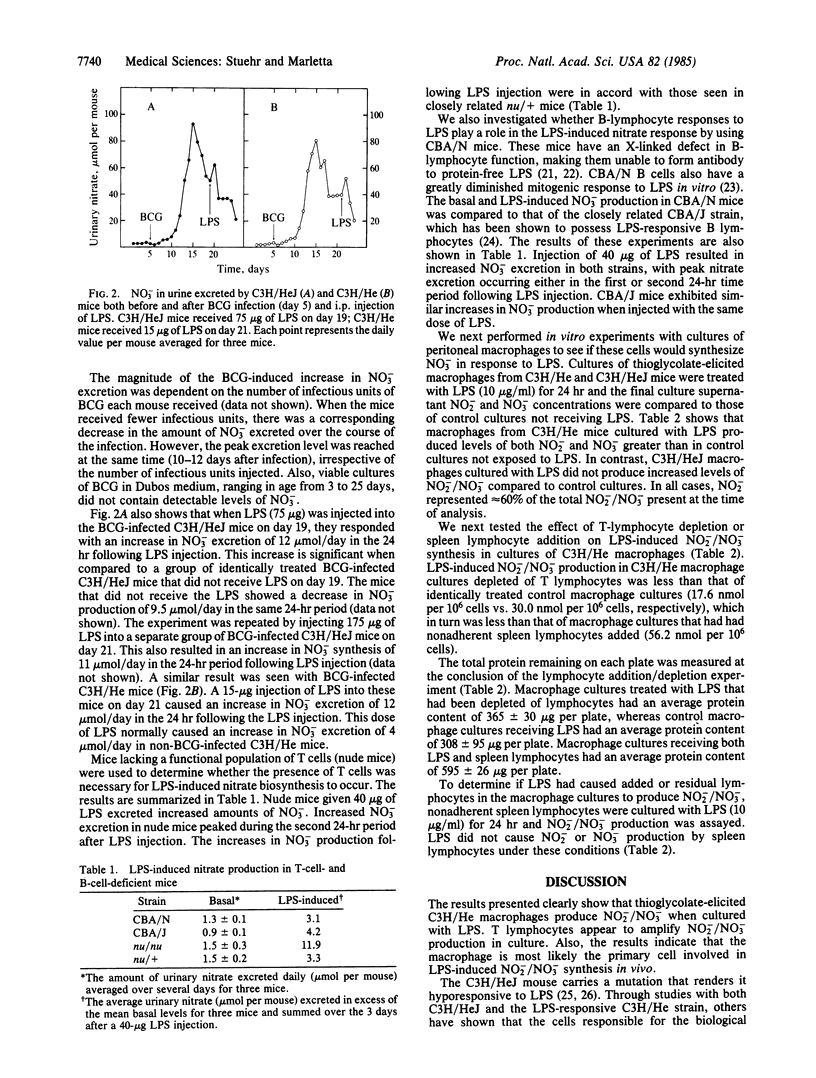
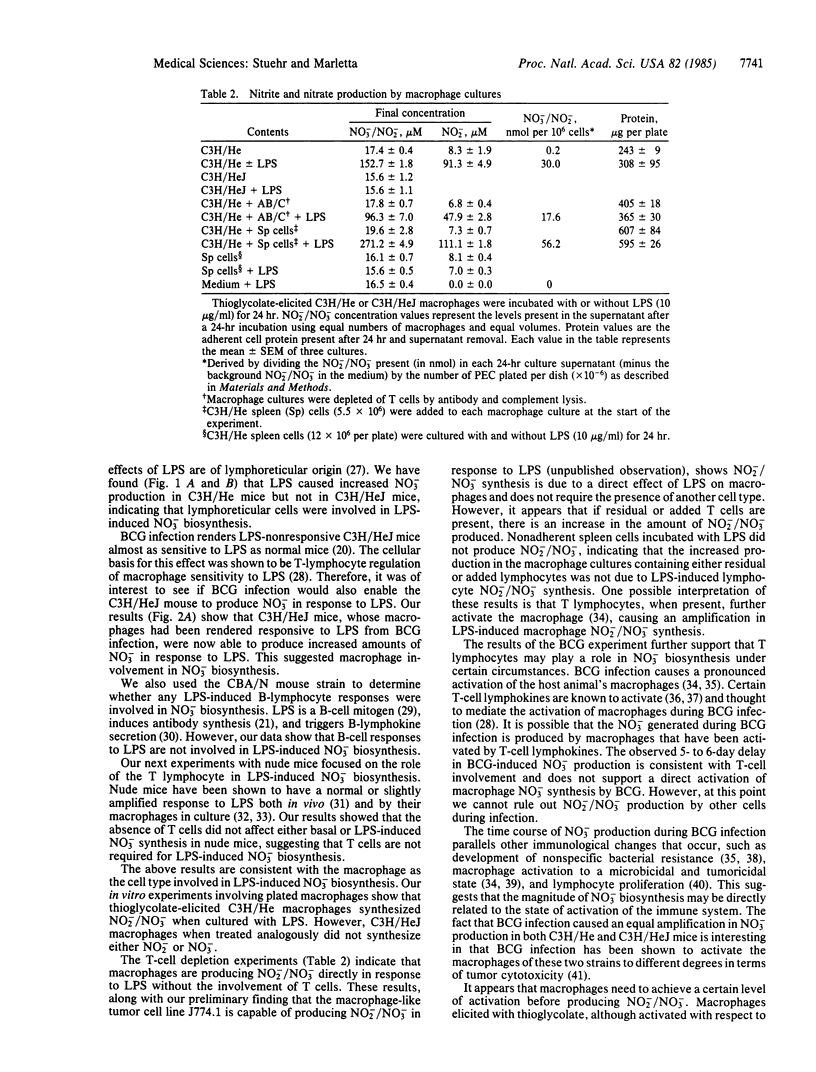
Escherichia coli lipopolysaccharide (LPS)-induced nitrate biosynthesis was studied in LPS-sensitive C3H/He and LPS-resistant C3H/HeJ mice. Intraperitoneal injection of 15 micrograms of LPS led to a temporary 5- to 6-fold increase in blood nitrate concentration in the C3H/He strain. Levels of nitrate excreted in the urine were also increased. In contrast, no increase was observed in the C3H/HeJ strain with LPS injections up to 175 micrograms. Furthermore, thioglycolate-elicited peritoneal macrophages from C3H/He, but not from C3H/HeJ mice, produced nitrite (60%) and nitrate (40%) when cultured with LPS (10 micrograms/ml). T-lymphocyte addition/depletion experiments showed the presence of T cells enhanced this response. However, LPS did not cause nitrite or nitrate production in cultures of spleen lymphocytes from either strain. LPS-induced nitrate synthesis was also observed with nude mice and CBA/N mice, indicating that neither functional T lymphocytes nor LPS-responsive B lymphocytes were required for the response in vivo. This was consistent with the in vitro results showing macrophages alone were competent. Mycobacterium bovis infection of C3H/He and C3H/HeJ mice resulted in a large increase in nitrate production over the course of the infection for both strains, suggesting T-lymphocyte-mediated activation of macrophages as a potent stimulus for nitrate biosynthesis. The synthesis of nitrite is significant in that it can directly participate in the endogenous formation of nitrosamines and may also be involved in some aspect of the chemistry of cytotoxicity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Hamilton T. A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- Berman B., Gigli I. Complement receptors on guinea pig epidermal Langerhans cells. J Immunol. 1980 Feb;124(2):685–690. [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogovski P., Bogovski S. Animal Species in which N-nitroso compounds induce cancer. Int J Cancer. 1981;27(4):471–474. doi: 10.1002/ijc.2910270408. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cheers C., Waller R. Activated macrophages in congenitally athymic "nude mice" and in lethally irradiate mice. J Immunol. 1975 Sep;115(3):844–847. [PubMed] [Google Scholar]
- Cohn Z. A. Activation of mononuclear phagocytes: fact, fancy, and future. J Immunol. 1978 Sep;121(3):813–816. [PubMed] [Google Scholar]
- Cuello C., Correa P., Haenszel W., Gordillo G., Brown C., Archer M., Tannenbaum S. Gastric cancer in Colombia. I. Cancer risk and suspect environmental agents. J Natl Cancer Inst. 1976 Nov;57(5):1015–1020. doi: 10.1093/jnci/57.5.1015. [DOI] [PubMed] [Google Scholar]
- Dull B. J., Hotchkiss J. H. Activated oxygen and mammalian nitrate biosynthesis. Carcinogenesis. 1984 Sep;5(9):1161–1164. doi: 10.1093/carcin/5.9.1161. [DOI] [PubMed] [Google Scholar]
- Glode L. M., Rosenstreich D. L. Genetic control of B cell activation by bacterial lipopolysaccharide is mediated by multiple distinct genes or alleles. J Immunol. 1976 Dec;117(6):2061–2066. [PubMed] [Google Scholar]
- Green L. C., Ruiz de Luzuriaga K., Wagner D. A., Rand W., Istfan N., Young V. R., Tannenbaum S. R. Nitrate biosynthesis in man. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7764–7768. doi: 10.1073/pnas.78.12.7764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982 Oct;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. A., Peters P. A., Civil R. H., Remington J. S. In vitro killing of schistosomula of Schistosoma mansoni by BCG and C. parvum-activated macrophages. J Immunol. 1979 May;122(5):1655–1657. doi: 10.2196/41502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer M. S., Ruco L. P., Boraschi D., Nacy C. A. Macrophage activation for tumor cytotoxicity: analysis of intermediary reactions. J Reticuloendothel Soc. 1979 Oct;26(4):403–415. [PubMed] [Google Scholar]
- Michalek S. M., Moore R. N., McGhee J. R., Rosenstreich D. L., Mergenhagen S. E. The primary role of lymphoreticular cells in the mediation of host responses to bacterial endotoxim. J Infect Dis. 1980 Jan;141(1):55–63. doi: 10.1093/infdis/141.1.55. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Wiebe M. E., Rubin B. Y. Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity. J Exp Med. 1983 Sep 1;158(3):670–689. doi: 10.1084/jem.158.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. T cell dependence of macrophage activation and mobilization during infection with Mycobacterium tuberculosis. Infect Immun. 1974 Jul;10(1):66–71. doi: 10.1128/iai.10.1.66-71.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Vogel S. N., Jacques A., Wahl L. M., Scher I., Mergenhagen S. E. Differential endotoxin sensitivity of lymphocytes and macrophages from mice with an X-linked defect in B cell maturation. J Immunol. 1978 Aug;121(2):685–690. [PubMed] [Google Scholar]
- Ruco L. P., Meltzer M. S. Defective tumoricidal capacity of macrophages from C3H/HeJ mice. J Immunol. 1978 Jan;120(1):329–334. [PubMed] [Google Scholar]
- Ryan J. L., Yohe W. B. Lymphocyte mediation of lipopolysaccharide-stimulated macrophage metabolism. J Immunol. 1981 Sep;127(3):912–916. [PubMed] [Google Scholar]
- SUTER E., KIRSANOW E. M. Hyperreactivity to endotoxin in mice infected with mycobacteria. Induction and elicitation of the reactions. Immunology. 1961 Oct;4:354–365. [PMC free article] [PubMed] [Google Scholar]
- Saul R. L., Archer M. C. Oxidation of ammonia and hydroxylamine to nitrate in the rat and in vitro. Carcinogenesis. 1984 Jan;5(1):77–81. doi: 10.1093/carcin/5.1.77. [DOI] [PubMed] [Google Scholar]
- Saul R. L., Kabir S. H., Cohen Z., Bruce W. R., Archer M. C. Reevaluation of nitrate and nitrite levels in the human intestine. Cancer Res. 1981 Jun;41(6):2280–2283. [PubMed] [Google Scholar]
- Senterfitt V. C., Shands J. W., Jr Endotoxin induced metabolic alterations in BCG infected (hyperreactive) mice. Proc Soc Exp Biol Med. 1978 Oct;159(1):69–74. doi: 10.3181/00379727-159-40286. [DOI] [PubMed] [Google Scholar]
- Sharp A. K., Colston M. J. The regulation of macrophage activity in congenitally athymic mice. Eur J Immunol. 1984 Jan;14(1):102–105. doi: 10.1002/eji.1830140119. [DOI] [PubMed] [Google Scholar]
- Shuval H. I., Gruener N. Epidemiological and toxicological aspects of nitrates and nitrites in the environment. Am J Public Health. 1972 Aug;62(8):1045–1052. doi: 10.2105/ajph.62.8.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum S. R., Fett D., Young V. R., Land P. D., Bruce W. R. Nitrite and nitrate are formed by endogenous synthesis in the human intestine. Science. 1978 Jun 30;200(4349):1487–1489. doi: 10.1126/science.663630. [DOI] [PubMed] [Google Scholar]
- Tannenbaum S. R., Moran D., Rand W., Cuello C., Correa P. Gastric cancer in Colombia. IV. Nitrite and other ions in gastric contents of residents from a high-risk region. J Natl Cancer Inst. 1979 Jan;62(1):9–12. [PubMed] [Google Scholar]
- Tannenbaum S. R., Sinskey A. J., Weisman M., Bishop W. Nitrite in human saliva. Its possible relationship to nitrosamine formation. J Natl Cancer Inst. 1974 Jul;53(1):79–84. [PubMed] [Google Scholar]
- Unanue E. R. The regulatory role of macrophages in antigenic stimulation. Part Two: symbiotic relationship between lymphocytes and macrophages. Adv Immunol. 1981;31:1–136. doi: 10.1016/s0065-2776(08)60919-0. [DOI] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- Vogel S. N., Weedon L. L., Wahl L. M., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. II. T cell modulation of macrophage sensitivity to LPS in vitro. Immunobiology. 1982 Feb;160(5):479–493. doi: 10.1016/S0171-2985(82)80010-7. [DOI] [PubMed] [Google Scholar]
- Wagner D. A., Young V. R., Tannenbaum S. R. Mammalian nitrate biosynthesis: incorporation of 15NH3 into nitrate is enhanced by endotoxin treatment. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4518–4521. doi: 10.1073/pnas.80.14.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaldívar R. Nitrate fertilizers as environmental pollutants: positive correlation between nitrates (NaNO3 and KNO3) used per unit area and stomach cancer mortality rates. Experientia. 1977 Feb 15;33(2):264–265. doi: 10.1007/BF02124102. [DOI] [PubMed] [Google Scholar]



