Abstract
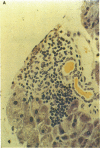
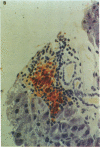
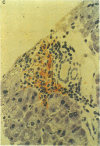
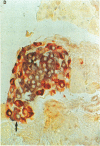
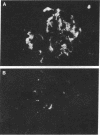
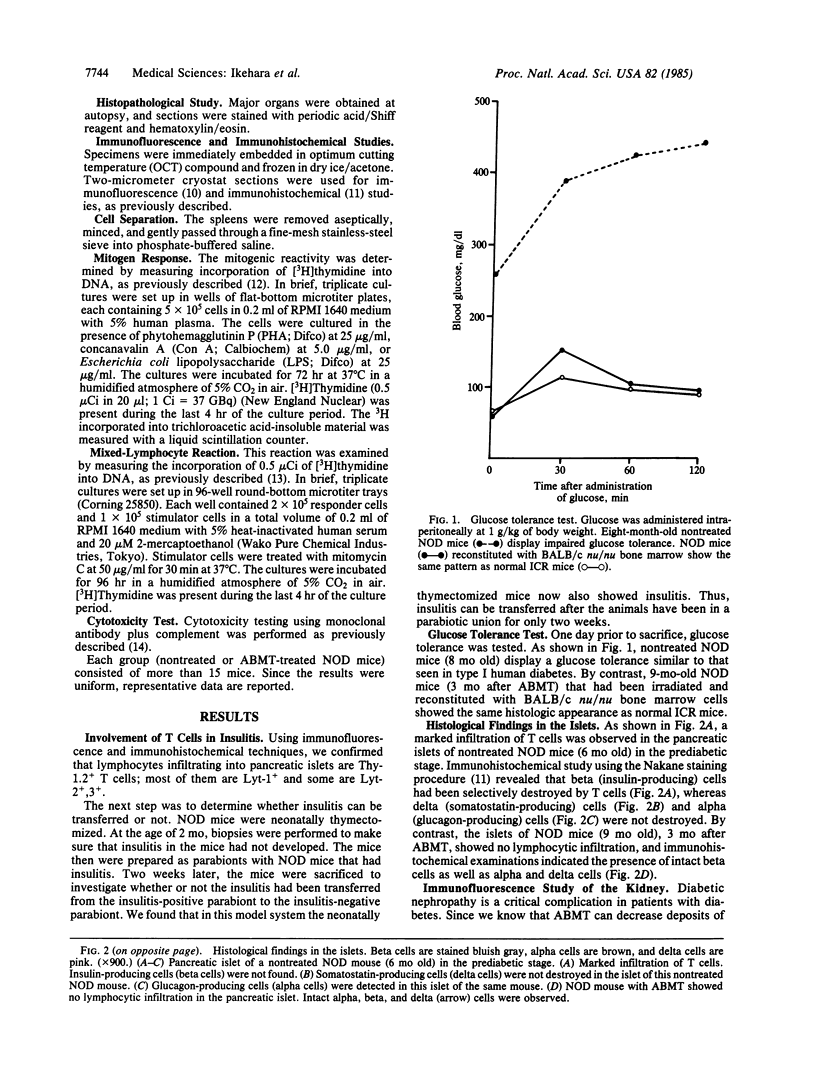
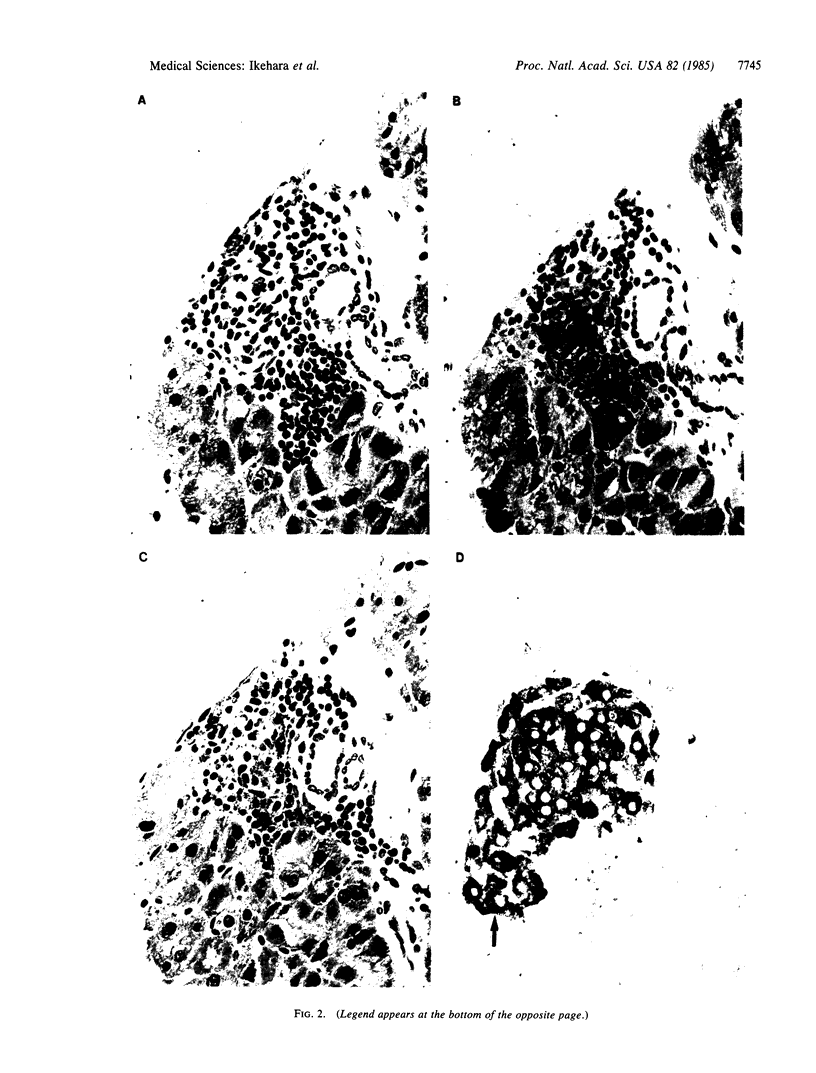
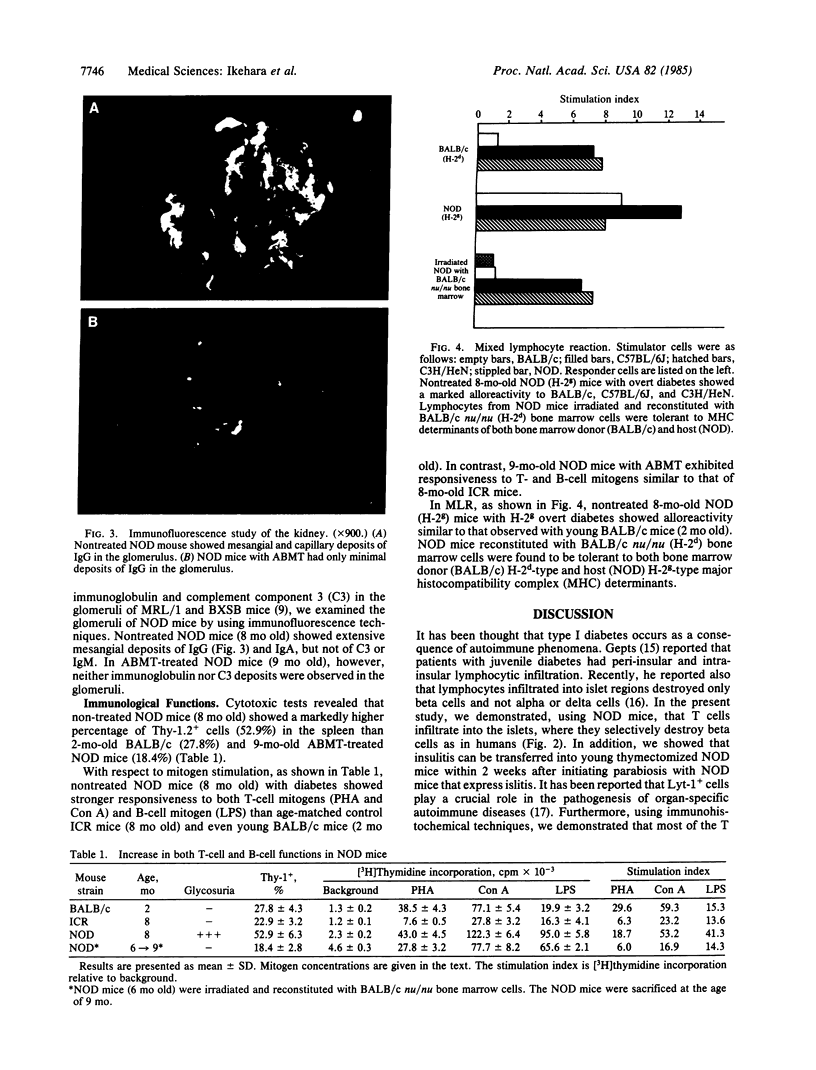
An animal model [the nonobese diabetic (NOD) mouse] for type I diabetes features a striking infiltration of T cells into the pancreatic islets. This infiltration selectively destroys beta cells. Most of the T cells are Lyt-1+, but some are Lyt-2+,3+. Transfer experiments using parabiosis revealed that insulitis can be transferred within 2 weeks after parabiosis to immunoincompetent thymectomized mice. When NOD mice (6 mo old) were irradiated and reconstituted with bone marrow cells from young BALB/c nu/nu mice (less than 2 mo old), the NOD mice exhibited neither insulitis nor overt diabetes. Deposits of immunoglobulin in mesangial areas of the glomeruli disappeared within 3 mo after bone marrow transplantation in such irradiated allogeneic bone marrow reconstituted mice. Assays for immunological functions, including mitogen response and mixed lymphocyte reaction, revealed that both T- and B-cell functions were increased in NOD mice with overt diabetes. NOD mice reconstituted with BALB/c nu/nu bone marrow cells displayed normal T- and B-cell functions. The newly developed T cells in the allogeneic bone marrow recipients are tolerant to cells with both donor- and host-type major histocompatibility complex determinants. These results suggest that bone marrow transplantation may ultimately be developed as a component of a strategy to be employed for treatment of type I diabetes in humans.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965 Oct;14(10):619–633. doi: 10.2337/diab.14.10.619. [DOI] [PubMed] [Google Scholar]
- Haspel M. V., Onodera T., Prabhakar B. S., Horita M., Suzuki H., Notkins A. L. Virus-induced autoimmunity: monoclonal antibodies that react with endocrine tissues. Science. 1983 Apr 15;220(4594):304–306. doi: 10.1126/science.6301002. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Good R. A., Modak M. J., Pahwa R. N. Adenosine 5'-triphosphate (ATP)-mediated stimulation and suppression of DNA synthesis in lymphoid cells. II. Suppressive effect of ATP on murine T-cell functions. Int J Immunopharmacol. 1983;5(6):567–573. doi: 10.1016/0192-0561(83)90050-4. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Good R. A., Nakamura T., Sekita K., Inoue S., Oo M. M., Muso E., Ogawa K., Hamashima Y. Rationale for bone marrow transplantation in the treatment of autoimmune diseases. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2483–2487. doi: 10.1073/pnas.82.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Pahwa R. N., Fernandes G., Hansen C. T., Good R. A. Functional T cells in athymic nude mice. Proc Natl Acad Sci U S A. 1984 Feb;81(3):886–888. doi: 10.1073/pnas.81.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S., Pahwa R. N., Lunzer D. G., Good R. A., Modak M. J. Adenosine-5'-triphosphate-(ATP) mediated stimulation and suppression of DNA synthesis in lymphoid cells. I. Characterization of ATP responsive cells in mouse lymphoid organs. J Immunol. 1981 Nov;127(5):1834–1838. [PubMed] [Google Scholar]
- Naji A., Silvers W. K., Barker C. F. Autoimmunity and type I (insulin-dependent) diabetes mellitus. Transplantation. 1983 Oct;36(4):355–361. doi: 10.1097/00007890-198310000-00001. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem. 1968 Sep;16(9):557–560. doi: 10.1177/16.9.557. [DOI] [PubMed] [Google Scholar]
- Nakhooda A. F., Like A. A., Chappel C. I., Murray F. T., Marliss E. B. The spontaneously diabetic Wistar rat. Metabolic and morphologic studies. Diabetes. 1977 Feb;26(2):100–112. doi: 10.2337/diab.26.2.100. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Takahashi T., Nishizuka Y. Study on cellular events in postthymectomy autoimmune oophoritis in mice. I. Requirement of Lyt-1 effector cells for oocytes damage after adoptive transfer. J Exp Med. 1982 Dec 1;156(6):1565–1576. doi: 10.1084/jem.156.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]