Abstract
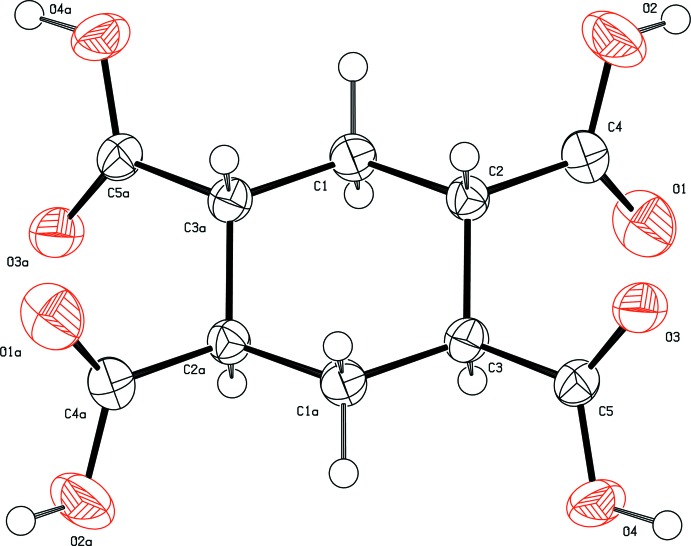
The title compound, C10H12O8, a prospective raw material for colourless polyimides which are applied to electronic and microelectronic devices, lies about an inversion centre and the cyclohexane ring adopts a chair conformation. Two crystallographycally independent carboxylic acid groups on adjacent C atoms are in equatorial positions, resulting in a mutually trans conformation. In the crystal, O—H⋯O hydrogen bonds around an inversion centre and a threefold rotoinversion axis, respectively, form an inversion dimer with an R 2 2(8) motif and a trimer with an R 3 3(12) motif.
Related literature
For background to polyimides, see: Ando et al. (2010 ▶); Hasegawa et al. (2007 ▶, 2013 ▶); Hasegawa & Horie (2001 ▶). For related structures, see: Uchida et al. (2003 ▶, 2012 ▶).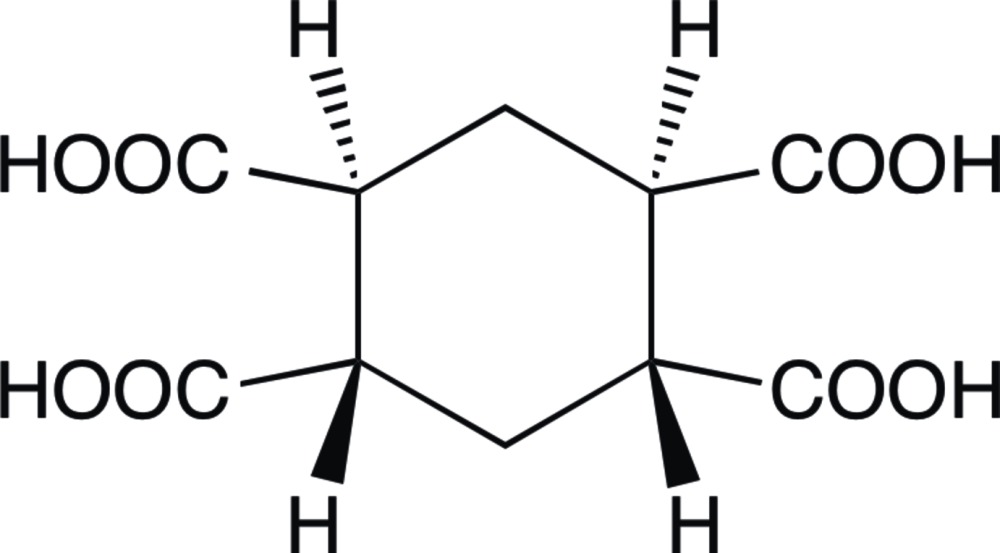
Experimental
Crystal data
C10H12O8
M r = 260.20
Trigonal,
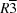
a = 17.6970 (6) Å
c = 9.5455 (6) Å
V = 2589.0 (2) Å3
Z = 9
Mo Kα radiation
μ = 0.13 mm−1
T = 298 K
0.33 × 0.26 × 0.26 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.891, T max = 0.966
6439 measured reflections
1653 independent reflections
1388 reflections with I > 2σ(I)
R int = 0.019
Refinement
R[F 2 > 2σ(F 2)] = 0.041
wR(F 2) = 0.119
S = 1.06
1653 reflections
90 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.38 e Å−3
Δρmin = −0.21 e Å−3
Data collection: APEX2 (Bruker, 2007 ▶); cell refinement: SAINT-Plus (Bruker, 2007 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL2013 (Sheldrick, 2008 ▶); molecular graphics: ORTEPIII (Burnett & Johnson, 1996 ▶) and PLATON (Spek, 2009 ▶); software used to prepare material for publication: SHELXL2013.
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813033795/is5327sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813033795/is5327Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536813033795/is5327Isup3.mol
Supporting information file. DOI: 10.1107/S1600536813033795/is5327Isup4.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H4⋯O1i | 0.81 (3) | 1.93 (3) | 2.705 (2) | 160 (3) |
| O4—H5⋯O3ii | 0.94 (3) | 1.70 (3) | 2.632 (1) | 176 (3) |
Symmetry codes: (i) 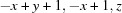 ; (ii)
; (ii) 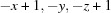 .
.
supplementary crystallographic information
1. Comment
Aromatic polyimides (PI) are one of the most important heat-resistant polymeric materials in various electronic applications for their reliable combined properties: considerably high glass transition temperatures (Tg), non-flammability, and good dielectric and mechanical properties (Ando et al., 2010). However, intensive coloration of conventional PI films, which arises from charge-transfer (CT) interactions (Hasegawa & Horie, 2001), often disturbs their applications as optical materials. A recent strong demand is to replace inorganic glass substrates in flat panel displays (300–700 µm thick) by plastic substrates (< 100 µm thick), thereby the displays become drastically light and flexible. However, it is difficult to obtain the substrate materials simultaneously possessing excellent combined properties, i.e., optical transparency, heat resistance, dimensional stability against thermal cycles undergoing in the device fabrication process, flexibility, and processability. The most effective strategy for completely erasing the coloration is to inhibit the CT interactions by using non-aromatic (cycloaliphatic) monomers either in tetracarboxylic dianhydride or diamine components. For this purpose, we previously investigated the steric structures of hydrogenated pyromellitic dianhydride isomers, i.e., 1S,2R,4S,5R-cyclohexanetetracarboxylic dianhydride (H-PMDA) (Uchida et al., 2003) and 1R,2S,4S,5R-cyclohexanetetracarboxylic dianhydride (H"-PMDA) (Uchida et al., 2012). H"-PMDA showed much higher reactivity with diamines than H-PMDA and provided highly flexible colourless PI films with significantly improved solution-processability while keeping very high Tgs (Hasegawa et al., 2007, 2013). The results are based on a peculiar steric structure of H"-PMDA. Unfortunately, neither H-PMDA nor H"-PMDA led to PI films with low coefficients of thermal expansion (CTE) required for the excellent dimensional stability, probably owing to their non-linear/non-planar steric structures. An additional H-PMDA isomer, i.e., 1S,2S,4R,5R-cyclohexanetetracarboxylic dianhydride (H'-PMDA) can be expected to derive a novel low-CTE colourless PI system. The present work reports a crystal structure of a hydrolyzed compound of H'-PMDA.
2. Experimental
The title compound, (I), was synthesized as follows. Pyromellitic dianhydride was first hydrolyzed with a NaOH aqueous solution. The pyromellitic acid tetrasodium salt formed was hydrogenated in a high-pressure hydrogen atmosphere at 160 °C in the presence of a ruthenium catalyst. After hydrogenation was completed, the solution was additionally heated at a precisely controlled temperature for several hours, and cooled to room temperature. The solution was neutralized by slowly adding conc. HCl. The white precipitate formed was collected by filtration, recrystallized from water, and dried in vacuum at 80 °C for 5 h to obtain crystals of (I) suitable for X-ray analysis.
3. Refinement
All H atoms were observable in a difference Fourier map. H atoms on O atoms were refined freely [O—H = 0.81 (3) and 0.94 (3) Å]. Other H atoms were placed in calculated positions with C—H = 0.97–0.98 Å, and allowed to ride on their carrier atoms, with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of the title compound, showing displacement ellipsoids at the 50% probability level. H atoms are represented by circles of arbitrary size.
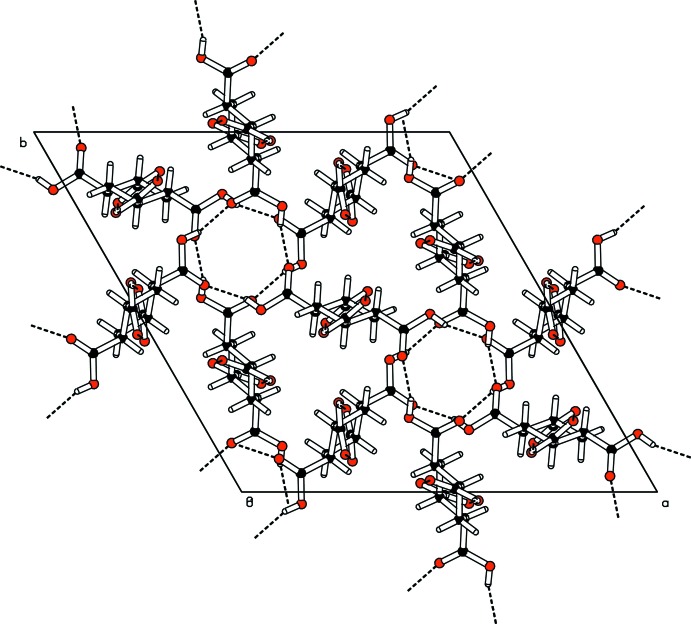
Fig. 2.
The packing of the title compound, viewed down the c axis,
Crystal data
| C10H12O8 | Dx = 1.502 Mg m−3 |
| Mr = 260.20 | Mo Kα radiation, λ = 0.71073 Å |
| Trigonal, R3 | Cell parameters from 2340 reflections |
| a = 17.6970 (6) Å | θ = 2.3–30.0° |
| c = 9.5455 (6) Å | µ = 0.13 mm−1 |
| V = 2589.0 (2) Å3 | T = 298 K |
| Z = 9 | Block, colourless |
| F(000) = 1224 | 0.33 × 0.26 × 0.26 mm |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 1388 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.019 |
| φ and ω scans | θmax = 30.0°, θmin = 2.3° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −24→21 |
| Tmin = 0.891, Tmax = 0.966 | k = −20→24 |
| 6439 measured reflections | l = −13→11 |
| 1653 independent reflections |
Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: mixed |
| R[F2 > 2σ(F2)] = 0.041 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.119 | w = 1/[σ2(Fo2) + (0.0607P)2 + 1.9122P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.06 | (Δ/σ)max < 0.001 |
| 1653 reflections | Δρmax = 0.38 e Å−3 |
| 90 parameters | Δρmin = −0.21 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | 0.51496 (8) | 0.07671 (7) | −0.08154 (11) | 0.0279 (3) | |
| H1A | 0.4929 | 0.1116 | −0.1242 | 0.034* | |
| H1B | 0.5770 | 0.1045 | −0.1011 | 0.034* | |
| C2 | 0.50063 (7) | 0.07293 (7) | 0.07787 (11) | 0.0233 (2) | |
| H2 | 0.4383 | 0.0484 | 0.0969 | 0.028* | |
| C3 | 0.53165 (7) | 0.01523 (7) | 0.14585 (11) | 0.0242 (2) | |
| H3 | 0.5944 | 0.0414 | 0.1285 | 0.029* | |
| C4 | 0.55013 (8) | 0.16503 (7) | 0.13456 (12) | 0.0283 (3) | |
| C5 | 0.51702 (8) | 0.00882 (8) | 0.30236 (12) | 0.0267 (3) | |
| O1 | 0.62253 (6) | 0.19739 (7) | 0.18327 (13) | 0.0471 (3) | |
| H4 | 0.5352 (17) | 0.2558 (18) | 0.151 (3) | 0.083 (8)* | |
| O2 | 0.50596 (8) | 0.20615 (8) | 0.12244 (15) | 0.0543 (3) | |
| O3 | 0.46919 (7) | 0.03210 (7) | 0.35746 (9) | 0.0380 (3) | |
| O4 | 0.55716 (7) | −0.02417 (7) | 0.37025 (10) | 0.0400 (3) | |
| H5 | 0.5485 (19) | −0.0245 (19) | 0.467 (3) | 0.110 (10)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0389 (6) | 0.0247 (5) | 0.0218 (5) | 0.0171 (5) | 0.0021 (4) | 0.0033 (4) |
| C2 | 0.0259 (5) | 0.0227 (5) | 0.0214 (5) | 0.0123 (4) | 0.0009 (4) | 0.0002 (4) |
| C3 | 0.0274 (5) | 0.0268 (5) | 0.0198 (5) | 0.0147 (4) | 0.0014 (4) | 0.0015 (4) |
| C4 | 0.0309 (6) | 0.0246 (5) | 0.0267 (5) | 0.0119 (5) | 0.0041 (4) | 0.0008 (4) |
| C5 | 0.0310 (6) | 0.0282 (5) | 0.0214 (5) | 0.0153 (5) | −0.0010 (4) | 0.0009 (4) |
| O1 | 0.0302 (5) | 0.0342 (5) | 0.0672 (8) | 0.0088 (4) | −0.0056 (5) | −0.0095 (5) |
| O2 | 0.0559 (7) | 0.0333 (5) | 0.0820 (9) | 0.0285 (5) | −0.0212 (6) | −0.0187 (5) |
| O3 | 0.0517 (6) | 0.0540 (6) | 0.0237 (4) | 0.0380 (5) | 0.0042 (4) | 0.0031 (4) |
| O4 | 0.0531 (6) | 0.0611 (7) | 0.0238 (4) | 0.0420 (6) | 0.0015 (4) | 0.0059 (4) |
Geometric parameters (Å, º)
| C1—C3i | 1.5370 (16) | C3—H3 | 0.9800 |
| C1—C2 | 1.5386 (15) | C4—O1 | 1.2049 (16) |
| C1—H1A | 0.9700 | C4—O2 | 1.3126 (16) |
| C1—H1B | 0.9700 | C5—O3 | 1.2295 (15) |
| C2—C4 | 1.5130 (15) | C5—O4 | 1.2961 (14) |
| C2—C3 | 1.5251 (15) | O2—H4 | 0.81 (3) |
| C2—H2 | 0.9800 | O4—H5 | 0.94 (3) |
| C3—C5 | 1.5108 (15) | ||
| C3i—C1—C2 | 111.06 (9) | C5—C3—C1i | 109.53 (9) |
| C3i—C1—H1A | 109.4 | C2—C3—C1i | 110.89 (9) |
| C2—C1—H1A | 109.4 | C5—C3—H3 | 108.3 |
| C3i—C1—H1B | 109.4 | C2—C3—H3 | 108.3 |
| C2—C1—H1B | 109.4 | C1i—C3—H3 | 108.3 |
| H1A—C1—H1B | 108.0 | O1—C4—O2 | 123.84 (12) |
| C4—C2—C3 | 111.15 (9) | O1—C4—C2 | 123.74 (11) |
| C4—C2—C1 | 108.23 (9) | O2—C4—C2 | 112.41 (11) |
| C3—C2—C1 | 110.07 (9) | O3—C5—O4 | 124.14 (11) |
| C4—C2—H2 | 109.1 | O3—C5—C3 | 121.25 (10) |
| C3—C2—H2 | 109.1 | O4—C5—C3 | 114.60 (10) |
| C1—C2—H2 | 109.1 | C4—O2—H4 | 109.5 (18) |
| C5—C3—C2 | 111.43 (9) | C5—O4—H5 | 111.8 (17) |
| C3i—C1—C2—C4 | 178.45 (9) | C1—C2—C4—O1 | −95.39 (14) |
| C3i—C1—C2—C3 | 56.81 (13) | C3—C2—C4—O2 | −155.53 (11) |
| C4—C2—C3—C5 | 61.11 (12) | C1—C2—C4—O2 | 83.49 (13) |
| C1—C2—C3—C5 | −179.00 (9) | C2—C3—C5—O3 | 14.47 (16) |
| C4—C2—C3—C1i | −176.60 (9) | C1i—C3—C5—O3 | −108.61 (13) |
| C1—C2—C3—C1i | −56.70 (13) | C2—C3—C5—O4 | −166.81 (10) |
| C3—C2—C4—O1 | 25.59 (16) | C1i—C3—C5—O4 | 70.12 (13) |
Symmetry code: (i) −x+1, −y, −z.
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H4···O1ii | 0.81 (3) | 1.93 (3) | 2.705 (2) | 160 (3) |
| O4—H5···O3iii | 0.94 (3) | 1.70 (3) | 2.632 (1) | 176 (3) |
Symmetry codes: (ii) −x+y+1, −x+1, z; (iii) −x+1, −y, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS5327).
References
- Ando, S., Ueda, M., Kakimoto, M., Kochi, M., Takeichi, T., Hasegawa, M. & Yokota, R. (2010). In The Latest Polyimides: Fundamentals and Applications, 2nd ed. Tokyo: NTS.
- Bruker (2007). APEX2 and SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Burnett, M. N. & Johnson, C. K. (1996). ORTEPIII Report ORNL-6895. Oak Ridge National Laboratory, Tennessee, USA.
- Hasegawa, M., Hirano, D., Fujii, M., Haga, M., Takezawa, E., Yamaguchi, S., Ishikawa, A. & Kagayama, T. (2013). J. Polym. Sci. Part A, 51, 575–592.
- Hasegawa, M. & Horie, K. (2001). Prog. Polym. Sci. 26, 259–335.
- Hasegawa, M., Horiuchi, M. & Wada, Y. (2007). High Perform. Polym. 19, 175–193.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Uchida, A., Hasegawa, M. & Manami, H. (2003). Acta Cryst. C59, o435–o438. [DOI] [PubMed]
- Uchida, A., Hasegawa, M., Takezawa, E., Yamaguchi, S., Ishikawa, A. & Kagayama, T. (2012). Acta Cryst. E68, o579. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813033795/is5327sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813033795/is5327Isup2.hkl
Supporting information file. DOI: 10.1107/S1600536813033795/is5327Isup3.mol
Supporting information file. DOI: 10.1107/S1600536813033795/is5327Isup4.cml
Additional supporting information: crystallographic information; 3D view; checkCIF report