Abstract
Background
The World Health Organization guidelines recommend cotrimoxazole prophylactic treatment (CPT) for all HIV-exposed infants from age 6 weeks to the cessation of breastfeeding and the exclusion of HIV infection. There are limited data about the effects of CPT among this population of infants. We examined the effects of CPT on adverse health outcomes among HIV-exposed infants during the first 36 weeks of life by using data from the Breastfeeding, Antiretrovirals, and Nutrition (BAN) study, a large clinical trial of antiretroviral drugs given to the mother or infant for prevention of HIV transmission during breastfeeding.
Methods
For the analysis, we assigned a status of CPT-exposed to infants who were participating in the study after the CPT program started. We estimated unadjusted and adjusted hazard ratios (HRs) for the effect of CPT status on time to incident malaria, severe illness or death, anemia, and weight-for-age Z score < −2.0. Participation in the study was limited to focus exclusively on HIV-exposed, uninfected infants.
Results
The HR for the effect of CPT on incident malaria was 0.35 (95% confidence interval [CI]: 0.21, 0.57) during the first 10 weeks of CPT exposure, and 0.93 (95% CI: 0.67, 1.29) for the remaining 20 weeks. CPT was not associated with the other outcomes examined.
Conclusions
CPT offered temporary protection against malaria among HIV-exposed, uninfected infants. However, CPT offered no protection against anemia, low weight for age, or the collapsed outcome of severe illness or death.
Keywords: HIV, malaria, cotrimoxazole, infants
More than 90% of new HIV infections among children are transmitted during pregnancy, birth, or breastfeeding.1 During the past decade, increasing access to perinatal interventions to prevent mother-to-child transmission of HIV has resulted in a growing number of infants who are exposed to HIV, but remain uninfected. These HIV-exposed, uninfected (HEU) infants face ongoing risk of acquiring HIV through breastfeeding. In addition, these infants may be at increased risk of acquiring other infections from close contact with immunodeficient members of their household who may be colonized with diverse pathogens.2–4 Malaria and HIV infections overlap geographically across much of sub-Saharan Africa, further illustrating the ongoing threat to the health of HIV-exposed children.5 Children younger than five years old generally experience the highest rate of malaria, and these children are especially vulnerable to infection during the first two years of life. The peak age of infection is inversely related to the intensity of malaria transmission—infants are more affected in high transmission areas.6
The World Health Organization (WHO) recommends daily cotrimoxazole prophylactic treatment (CPT) for infants born to HIV-infected mothers, from 6 weeks to the cessation of breastfeeding and the exclusion of infant HIV infection.7 Because of the difficulty and cost of diagnosing HIV-exposed infants early in many resource-limited settings, the WHO CPT guideline serves to protect infants who have undiagnosed HIV infection from other opportunistic infections. This WHO recommendation is based on the results of randomized controlled trials (RCTs) and non-experimental studies that demonstrated decreased incidence of severe events, hospitalizations, and death among HIV-infected adults and children.8–13 Specifically, the use of CPT among HIV-infected adults and children has been associated with reductions in the incidence of malaria.8, 14, 15
Very limited data are available to demonstrate the beneficial effects of CPT among HEU infants.16, 17 Even though malaria is the primary cause of death among African children younger than 5 years,18 there are no data about the effectiveness of CPT to reduce the incidence of malaria among HEU infants during the first few months of life. A better understanding of the effects of this widely recommended regimen on the incidence of poor health outcomes is important among an extremely vulnerable population. In the present analysis, we examine the effects of CPT on adverse health outcomes among HEU infants during the first 36 weeks of life.
METHODS
Study Design and Population
All children were enrolled in the randomized and controlled Breastfeeding, Antiretrovirals and Nutrition (BAN) trial, which took place at 4 clinics in Lilongwe, Malawi, during 2004–2009.19 The BAN study design and primary findings have been reported elsewhere and have shown that the use of either maternal antiretroviral drugs or infant nevirapine for 28 weeks was effective in reducing HIV transmission during breastfeeding.19, 20 HIV-infected, pregnant women who were 14 years or older, naïve to antiretroviral therapy (ART, and had reached 30 weeks or less of gestation were eligible for enrollment, if they had hemoglobin levels higher than 7 g/dL, CD4 cell counts of 250 cells/μL or higher (≥200 cells/μL before July 24, 2006), normal liver function tests (ALT < 2.5x the upper limit of normal), and no serious pregnancy complications.
If mother-infant pairs met secondary eligibility criteria, they were randomized within a week of birth to a 2-group maternal nutritional intervention and to a 3-group antiretroviral intervention consisting of a 3-drug antiretroviral regimen for the mother (maternal-regimen group), a daily dose of nevirapine for the infant (infant-regimen group), or neither (control antiretroviral group). The interventions began after delivery and were continued until the cessation of breastfeeding, but no longer than 28 weeks. Infants found to be HIV-infected at birth or in the first 2 weeks of life were disenrolled from the BAN study and referred for care. Infants who tested positive for HIV infection later than 2 weeks of life, which was the primary endpoint of the BAN study, were discontinued from the intervention but not disenrolled from the study, and were encouraged to continue attending regular study visits.
Mother-infant pairs were seen for visits at delivery and at 1, 2, 4, 6, 8, 12, 18, 21, 24, 28, 32, 36, 42 and 48 weeks postpartum. Data capturing anthropometrics, vital signs, illnesses and hospitalizations since the last visit, current symptoms, and physical exam findings were collected at all follow-up visits. Blood was collected at 2, 4, 6, 12, 18, 24, 28, 36 and 48 week visits. Participants were advised to return to the clinic between visits to receive treatment if the woman or child was ill.
In accordance with the Malawi Ministry of Health and Population Guidelines and WHO guidelines on cotrimoxazole prophylaxis,7 CPT was initiated in the BAN study for eligible women and infants in 2006; prior to 2006, the WHO and UNAIDS had not published CPT guidelines for resource-limited settings. Starting on 13 June 2006, CPT (240 mg once daily) was provided to all infants in the BAN study beginning at 6 weeks of age. Infants, who stopped breastfeeding by 28 weeks of age and were also HIV-uninfected at 28 weeks, continued CPT until age 36 weeks. Infants who did not wean by 28 weeks continued CPT until weaning occurred and HIV infection was ruled out. CPT for children who were HIV-infected was intended to be life-long and was provided for the duration of participation in the BAN study. CPT was also initiated at the same time for mothers who had a CD4 cell count less than 500 cells/μL, as measured during pregnancy or at 24 weeks post partum.
For the purpose of this analysis, we excluded infants who did not present for a visit between 6–8 weeks of age and who did not have at least one follow-up visit after that time. Infants diagnosed with HIV infection at or before the 6 week visit were not included in the analysis.
Ethical review
The BAN study protocol was approved by the Malawi National Health Science Research Committee and the institutional review boards at the University of North Carolina at Chapel Hill and the U.S. Centers for Disease Control and Prevention.
Statistical Analysis
All statistical analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC). Descriptive analyses included calculation of frequencies and medians of exposures, outcomes and covariables. Categorical proportions were compared using chi-square test and continuous variables were assessed using the Wilcoxon rank-sum test.
We estimated unadjusted and adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the effect of CPT status on time to (1) first infection with malaria, (2) first severe illness or death, (3) anemia, or (4) low weight for age. For each of these four outcomes, we began with separate bivariate models containing only CPT and the outcome of interest. Multivariable models were then constructed including covariables associated with CPT or the outcome of interest. We examined the proportional hazard assumption graphically using log-log plots and by adding interactions with time to the model. If the assumption was violated, it was relaxed by fitting interactions with categorical or continuous time.21 We explored rainy season, antiretroviral study arm, infant age and first pregnancy as modifiers of the association between CPT and the outcomes of interest.22 Covariables found to be important effect measure modifiers were included in the starting multivariable model through an interaction term with CPT exposure status. To construct final models, we used a manual, backward elimination, change-in-estimate strategy. Potential confounders were removed from the preliminary full model in order of p-value magnitude (covariables with the highest p-values were removed first). If the CPT-outcome association changed by less than 10% overall or in any stratum of an interacting variable, a given covariable was not retained.23
For time-to-event analyses, children who tested positive for HIV were censored at the last visit at which they tested negative. Infants were also censored at death, maternal death, or loss to follow-up.
Definitions
The unanticipated change in CPT guidelines and their implementation 2 years into the BAN study created a natural experiment, with a CPT-unexposed period followed by a CPT-exposed period. Therefore, for the purpose of this analysis, exposure to CPT was based on the 2006 time-point at which standardized CPT was implemented in the BAN study. To minimize misclassification of CPT, inclusion in our analysis was restricted according to 3 criteria. First, in order to account for any lag time between the decision to administer CPT and the routine implementation of this practice, person-time from infants presenting for a visit between 6–8 weeks of age between 13 June 2006 (the date the first infant was started on CPT) and 15 August 2006 was not included in these analyses. Second, infants were only included if they presented between 6–8 weeks of age, to ensure that all infants were started on CPT within a 2 week age window. Finally, to avoid mixed exposure, analyses only included children who were either never exposed to CPT (fully unexposed, age 36 weeks by June 13, 2006), or children who were exposed to CPT from 6–8 weeks of age onward (fully exposed, age 6 weeks on or after August 15, 2006).
Malaria was defined by the first episode of infection after 6 weeks of age and was diagnosed as a positive blood smear from a child arriving with symptoms of malaria. We excluded children who had a diagnosis of malaria before 6 weeks. Severe illness was defined by the first event after 6 weeks of diarrhea, malaria, meningitis, pneumonia, or serious febrile illness that was fatal or life-threatening, required inpatient hospitalization, or resulted in persistent or significant disability or incapacity. Anemia was defined by a hemoglobin level below 7 g/dl from 6 to 8 weeks, or below 9 g/dl after 8 weeks of age, according to toxicity tables (revised March 2006) from the Division of AIDS at the National Institute of Allergy and Infectious Diseases (NIAID). Malnutrition was classified on the basis of weight-for-age Z scores (WAZ) that were calculated by using the January 2011 WHO Child Growth Standards macros (version 3.2.2).24 An infant was considered undernourished if the WAZ was less than −2.0 after 6 weeks of age. The rainy season was defined as the period from November to March and analyzed as a time-varying covariable. In addition, the rainy season was evaluated as an effect measure modifier and a confounder in the analysis for each outcome.
RESULTS
Baseline Characteristics
After excluding 387 infants with mixed CPT exposures and 19 infants who were diagnosed with HIV at or before the 6-week visit, 1,522 mother-infant pairs, representing approximately two-thirds of the BAN cohort, were eligible for analysis (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B207). Mothers of infants exposed to CPT were slightly older (26 vs. 25 years) and more likely to be married (92.6% vs. 89.1%). Mothers of CPT-exposed infants had an insignificantly higher CD4 cell count (441.0 vs. 431.0 cells/μL). Accounting for censoring at HIV infection, death, loss to follow-up, or the 36-week visit, the median duration of follow-up from the 6-week visit was 188 and 189 days among the CPT-exposed and unexposed groups, respectively.
Effect of CPT on Time to Adverse Health Outcomes
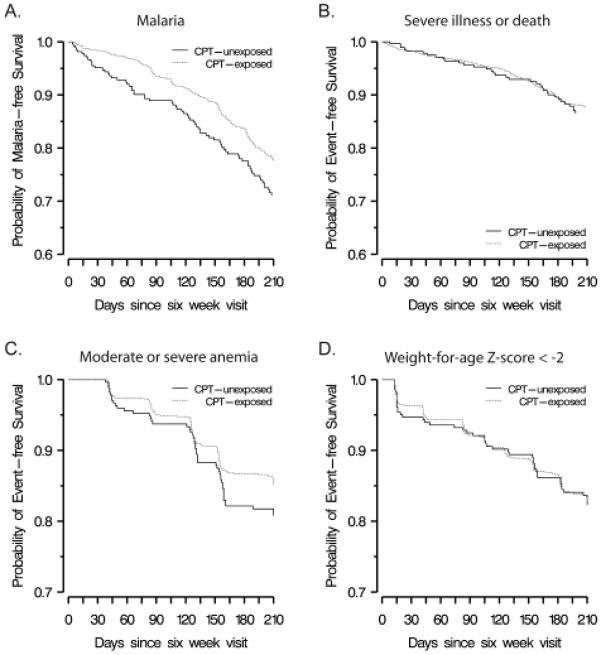
Thirty-four infants had malaria before 6 weeks of age and were not included in the time-to-event analysis examining the association between CPT and incident malaria. We observed 311 cases of infant malaria prior to 36 weeks of age (and prior to censoring due to HIV infection, death, or loss to follow-up) (Table 1) (Figure 1). The unadjusted HR for the effect of CPT exposure on time to incident malaria was 0.71 (95% CI: 0.55, 0.93). As evidenced by the log-log plot and the statistical significance of a continuous interaction term with time in the multivariable model, the effect of CPT appeared to change over time. Therefore, a categorical time interaction term at 70 days after initiation of CPT, corresponding to 16 weeks of age, was included in the model. This time-stratified analysis yielded an HR of 0.35 (95% CI: 0.21, 0.57) for the effect of CPT on incident malaria from 6 to 16 weeks and an HR of 0.94 (95% CI: 0.68, 1.30) from more than 16 to 36 weeks of age. Additional covariables did not meet the a priori criteria set for inclusion in the final model, and, thus, our final model for the outcome of malaria included only CPT and a categorical interaction term at 70 days after initiation of CPT.
TABLE 1.
Frequency of Outcomes of Interest from 6 to 36 Weeks of Age in CPT-Exposed and Unexposed Infants in Lilongwe, Malawi, During 2004–2009.
| Outcome | CPT-unexposed infants* (n = 283) | CPT-exposed infants* (n = 1,239) | Total (N = 1,522) | P-value |
|---|---|---|---|---|
| Malaria (any severity) | 70 (24.7%) | 241 (19.5%) | 311 (20.4%) | 0.03 |
| Anemia | 49 (17.3%) | 166 (13.4%) | 215 (14.1%) | 0.08 |
| Severe illness or death | 34 (12.0%) | 135 (10.9%) | 169 (11.1%) | 0.59 |
| Diarrhea | 11 (3.9%) | 35 (2.8%) | 46 (3.0%) | |
| Malaria(severe) | 10 (3.5%) | 14 (1.1%) | 24 (1.6%) | |
| Meningitis | 1 (0.4%) | 9 (0.7%) | 10 (0.7%) | |
| Pneumonia | 5 (1.8%) | 51 (4.1%) | 56 (3.7%) | |
| Febrile illness | 6 (2.1%) | 22 (1.8%) | 28 (1.8%) | |
| Vomiting | 0 (0.0%) | 2 (0.2%) | 2 (0.1%) | |
| Death | 1 (0.4%) | 2 (0.2%) | 3 (0.2%) | |
| Low weight for age (Z score < -2) | 46 (16.3%) | 192 (15.6%) | 238 (15.6%) | 0.75 |
Infants were considered CPT-unexposed if > 36 weeks of age by June 13, 2006; infants were considered CPT-exposed if they reached 6 weeks of age on or after August 15, 2006.
P-value based on chi-square test comparing CPT-exposed and CPT-unexposed groups. CPT = cotrimoxazole preventive therapy
FIGURE 1.
Kaplan-Meier curves illustrating the probability of event-free survival for (A) malaria, (B) severe illness or death, (C) moderate or severe anemia, and (D) weight-for-age Z score < -2. Red represents infants exposed to CPT; black represents infants unexposed to CPT.
We observed 169 severe illnesses or deaths among eligible infants (Table 1) (Figure 1). Pneumonia was the most common (33.1% of all events), followed by diarrhea (27.2%). The unadjusted HR for the effect of CPT exposure on time to severe illness was 0.92 (95% CI: 0.63, 1.34). None of the covariables met our criteria for effect measure modification or confounding of the effect of CPT on this outcome.
Anemia was documented in 49 CPT-unexposed infants and 166 CPT-exposed infants (Table 1) (Figure 1). The unadjusted HR for the effect of CPT exposure on time to anemia was 0.78 (95% CI: 0.58, 1.05). No covariable met our criteria for inclusion in the final model.
Low WAZ occurred in 46 CPT-unexposed and 192 CPT-exposed infants (Table 1) (Figure 1). The HR for the effect of CPT exposure on time to low weight for age was 0.97 (95% CI: 0.72, 1.34). With adjustment for baseline weight (at 6 weeks of age), the direction of the association was reversed (adjusted HR 1.18, 95% CI: 0.83, 1.67) but continued to be weak and not statistically significant. No other covariable met our criteria for inclusion in the final model.
Regarding possible CPT-related toxicities, CPT was not associated with more frequent severe cases of rash or neutropenia, however, there was a single case of asymptomatic severe anemia which was designated as possibly due to cotrimoxazole.20
DISCUSSION
Our results indicate that CPT may provide temporary protection against malaria in HEU infants during early infancy, but CPT provides no protection against anemia, low weight for age, and severe illness or death. Previous studies have shown that CPT protects against malaria in HIV-infected adults and children10, 14 and older HIV-uninfected children (aged 5–15 years).12 In one RCT, in which all infants were also provided with insecticide-treated bed nets (ITNs), HIV-exposed infants who continued CPT until 2 years of age, after cessation of breastfeeding and exclusion of HIV infection, had a 39% reduction in malaria incidence compared with infants who stopped CPT after cessation of breastfeeding at a median age of 10 months.25 We explored an earlier age period and found that CPT was associated with a 65% reduction in malaria episodes from 6 to 16 weeks (adjusted HR of 0.35 (95% CI: 0.21, 0.57)), but we did not observe a protective effect after 16 weeks of age.
The effect of CPT among infants, particularly during the first months of life, may be complex. Malaria is more commonly seen after the first 2–3 months of life, when maternal antibodies wane,26 with incidence generally peaking within the first 2 years, especially in areas of high transmission.26, 27 Maternal HIV infection has been demonstrated to negatively affect the transfer of some maternal antibodies28, 29 and is believed to affect the development of the immune system in utero.30, 31 These factors may cause HIV-exposed infants to be more susceptible to malaria and other infections during the first few months of life, when passive immunity usually provides protection, and when the infant’s immune system is still developing. Alternatively, there may be a synergistic interaction between CPT and existing maternal antibodies during the first few months of life, resulting in additional protection against malaria for the infant.
CPT was not significantly associated with reduced likelihood of grade 3 or 4 anemia, although we observed a protective trend among these Malawian infants. Observed associations between CPT and anemia in the literature are mixed. Protection against anemia was observed among healthy children (aged 5–15 years) receiving CPT, which may be secondary to the observed protection from malaria.12 In HIV-infected adults with a CD4 cell count of at least 500 cells, CPT had no effect on hemoglobin.32 The most applicable data come from a recent study of the effects of nevirapine and CPT in infants, where no increased risk of grade 3 or greater anemia was found.33
In the current study, CPT did not protect against severe illness, death, or low weight for age. In contrast, other studies have reported that CPT protects against death and respiratory infections among HIV-infected children,34 respiratory infections among HIV-infected infants,16 and gastrointestinal illness among older healthy children.12 We did not observe an effect of CPT on severe illness and death, but because of the small number of events, we were unable to assess associations between CPT and specific infections. Repeated measures analysis of the effects of cotrimoxazole on specific serious adverse events using the full BAN cohort is currently underway and will help to provide further insight into the effects of CPT on specific health outcomes, rather than the collapsed outcomes examined here. Exclusive breastfeeding, particularly in the first 6 months, is associated with protection from malnutrition and infectious disease among infants.35 Also, because of the lipid-based nutrient supplement provided to infants after cessation of breastfeeding in the BAN study, our analysis population may exhibit less variation in nutritional outcomes than what might be observed outside of this clinical trial setting. Therefore, it is possible that the protective effects of CPT against these outcomes would become more apparent in the context of replacement or mixed feeding. Benefits of CPT may also be more apparent when assessing all morbidity, including mild infections. However, a recent study of breastfeeding HEU children showed no protection of CPT against lower respiratory tract infection, with a trend toward increased incidence of diarrhea, though the association did not reach statistical significance (incidence rate ratio of 1.38 (95% CI: 0.98, 1.94)).36
Our analyses have important limitations that are similar to other observational studies. First, exposure status was based on presumed exposure by using the 2006 date that CPT was initiated in the BAN Study. A review of a random sample of patient pharmacy records showed good adherence to the CPT guidelines (data not shown). However, it is possible that some children classified as exposed may not have taken cotrimoxazole, which could bias our results. If this is the case, our observed estimates are likely weaker than the true magnitude of the effect of CPT on the outcomes of interest.
Second, unmeasured changes among the population during the more than 5 years of observation (2004–2009) may have coincided with the rollout of CPT (mid-2006). Temporal changes in disease incidence occurring during the study period cannot be separated from the effects of CPT. For example, starting in March 2007, ITNs were distributed to 3,500 HIV-infected pregnant women in Lilongwe, including some of the women participating in the BAN study. ITN use was unmeasured. If malaria decreased because of the use of ITNs or other factors, the protective effect in the first 16 weeks of life suggested by our analysis may be an artifact of ITN use or other changes in disease incidence unrelated to CPT. In addition, it is possible that the simultaneous initiation of CPT in BAN mothers with a CD4 cell count of less than 500 cells/μL could have contributed to the protective effect against malaria demonstrated in our analyses.3 It is also possible that the small number of CPT-unexposed infants may have limited our ability to detect differences between groups. As noted earlier, suboptimal adherence likely occurred in our study because we did not monitor adherence to CPT. However, suboptimal adherence also would occur outside of a study setting, and our study may thus approximate the normal conditions of varied adherence observed in routine rollout of CPT, which strengthens the generalizability of our findings. A final limitation was that malaria smears were not independently reviewed by a second person.
The primary rationale for CPT among HIV-exposed infants is to reduce death and disease from opportunistic infections among HIV-infected infants whose HIV statuses have not yet been determined. Following current WHO guidelines, it is no longer ethical not to administer CPT to HIV-exposed infants. Thus, the conduct of an RCT to examine the effect of CPT in HEU infants is not possible. Observational studies, such as this one, may be the only means of providing valuable insight into the effects of CPT among HEU infants. The incidence of infant HIV infection is declining with the use of effective preventive interventions. In addition, infants with HIV infection are diagnosed more quickly due to widely available HIV testing, allowing for more timely interventions to prevent opportunistic infections. Further study of the role of infant CPT in the current context of pediatric HIV should be conducted to ensure the best use of limited resources to protect the health of this vulnerable population.
Acknowledgments
Support: The BAN Study was supported by grants from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention (SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01), the National Institute of Allergy and Infectious Diseases (NIAID P30-AI50410), the University of North Carolina Center for AIDS Research (P30-AI50410), and the NIH Fogarty AIDS International Training and Research Program (DHHS/NIH/FIC 2-D43 Tw01039-06). The antiretrovirals used in the BAN Study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer-Ingelheim, Roche Pharmaceuticals and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation Call to Action and International Leadership Awards, UNICEF, World Food Programme, Malawi Ministry of Health, Johnson and Johnson, and USAID. The non-government funders had no role in the design, implementation, analysis and interpretation of the data.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors have no other funding or conflicts of interest to disclose.
References
- 1.UNAIDS. AIDS epidemic update. 2009 Available at http://data.unaids.org/pub/Report/2009/JC1700_Epi_Update_2009_en.pdf.
- 2.Cotton MF, Schaaf HS, Lottering G, Weber HL, Coetzee J, Nachman S. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis. 2008;12:225–7. [PubMed] [Google Scholar]
- 3.Mermin J, Lule J, Ekwaru JP, Downing R, Hughes P, Bunnell R, et al. Cotrimoxazole prophylaxis by HIV-infected persons in Uganda reduces morbidity and mortality among HIV-uninfected family members. Aids. 2005;19:1035–42. doi: 10.1097/01.aids.0000174449.32756.c7. [DOI] [PubMed] [Google Scholar]
- 4.Mofenson LM, Oleske J, Serchuck L, Van Dyke R, Wilfert C. Treating opportunistic infections among HIV-exposed and infected children: recommendations from CDC, the National Institutes of Health, and the Infectious Diseases Society of America. Clin Infect Dis. 2005;40 (Suppl 1):S1–84. doi: 10.1086/427295. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. World Malaria Report. Geneva: WHO; 2009. [Google Scholar]
- 6.Okiro EA, Al-Taiar A, Reyburn H, Idro R, Berkley JA, Snow RW. Age patterns of severe paediatric malaria and their relationship to Plasmodium falciparum transmission intensity. Malar J. 2009;8:4. doi: 10.1186/1475-2875-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. Guidelines on co-trimoxazole prophylaxis for HIV-related infections among children, adolescents and adults in resource-limited settings. Geneva: 2006. [Google Scholar]
- 8.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, et al. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d’Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–8. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 9.Mermin J, Lule J, Ekwaru JP, Malamba S, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis on morbidity, mortality, CD4-cell count, and viral load in HIV infection in rural Uganda. Lancet. 2004;364:1428–34. doi: 10.1016/S0140-6736(04)17225-5. [DOI] [PubMed] [Google Scholar]
- 10.Campbell J, Moore D, Degerman R, Kaharuza F, Were W, Muramuzi E, et al. HIV-infected Ugandans on HAART with CD4 Counts >200 Cells/mm3 Who Discontinue Cotrimoxazole Have Increased Risk of Malaria and Diarrhea. Vol. 2009. CROI; Montreal: 2009. [DOI] [PubMed] [Google Scholar]
- 11.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, et al. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d’Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–75. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 12.Thera MA, Sehdev PS, Coulibaly D, Traore K, Garba MN, Cissoko Y, et al. Impact of trimethoprim-sulfamethoxazole prophylaxis on falciparum malaria infection and disease. J Infect Dis. 2005;192:1823–9. doi: 10.1086/498249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zar HJ, Dechaboon A, Hanslo D, Apolles P, Magnus KG, Hussey G. Pneumocystis carinii pneumonia in South African children infected with human immunodeficiency virus. Pediatr Infect Dis J. 2000;19:603–7. doi: 10.1097/00006454-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Mermin J, Ekwaru JP, Liechty CA, Were W, Downing R, Ransom R, et al. Effect of co-trimoxazole prophylaxis, antiretroviral therapy, and insecticide-treated bednets on the frequency of malaria in HIV-1-infected adults in Uganda: a prospective cohort study. Lancet. 2006;367:1256–61. doi: 10.1016/S0140-6736(06)68541-3. [DOI] [PubMed] [Google Scholar]
- 15.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, Kekitiinwa A, et al. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. Aids. 2007;21:2059–66. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 16.Coutsoudis A, Pillay K, Spooner E, Coovadia HM, Pembrey L, Newell ML. Routinely available cotrimoxazole prophylaxis and occurrence of respiratory and diarrhoeal morbidity in infants born to HIV-infected mothers in South Africa. S Afr Med J. 2005;95:339–45. [PubMed] [Google Scholar]
- 17.Gill CJ, Mwanakasale V, Fox MP, Chilengi R, Tembo M, Nsofwa M, et al. Effect of presumptive co-trimoxazole prophylaxis on pneumococcal colonization rates, seroepidemiology and antibiotic resistance in Zambian infants: a longitudinal cohort study. Bull World Health Organ. 2008;86:929–38. doi: 10.2471/BLT.07.049668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. World Malaria Report 2008. Geneva: WHO; 2008. [Google Scholar]
- 19.van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 362:2271–81. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allison PD. Survival Analysis Using SAS. Cary, N.C: SAS Institute, Inc; 1995. [Google Scholar]
- 22.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven; 1998. [Google Scholar]
- 23.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–36. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. Child Growth Standards. [Accessed December 17, 2011];WHO Anthro (version 3.2.2, January 2011) and macros. 2011 Available at: http://www.who.int/childgrowth/software/en/
- 25.Sandison TG, Homsy J, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, et al. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snow RW, Nahlen B, Palmer A, Donnelly CA, Gupta S, Marsh K. Risk of severe malaria among African infants: direct evidence of clinical protection during early infancy. J Infect Dis. 1998;177:819–22. doi: 10.1086/517818. [DOI] [PubMed] [Google Scholar]
- 27.Reyburn H, Mbatia R, Drakeley C, Bruce J, Carneiro I, Olomi R, et al. Association of transmission intensity and age with clinical manifestations and case fatality of severe Plasmodium falciparum malaria. Jama. 2005;293:1461–70. doi: 10.1001/jama.293.12.1461. [DOI] [PubMed] [Google Scholar]
- 28.de Moraes-Pinto MI, Almeida AC, Kenj G, Filgueiras TE, Tobias W, Santos AM, et al. Placental transfer and maternally acquired neonatal IgG immunity in human immunodeficiency virus infection. J Infect Dis. 1996;173:1077–84. doi: 10.1093/infdis/173.5.1077. [DOI] [PubMed] [Google Scholar]
- 29.Farquhar C, Nduati R, Haigwood N, Sutton W, Mbori-Ngacha D, Richardson B, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–7. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chougnet C, Kovacs A, Baker R, Mueller BU, Luban NL, Liewehr DJ, et al. Influence of human immunodeficiency virus-infected maternal environment on development of infant interleukin-12 production. J Infect Dis. 2000;181:1590–7. doi: 10.1086/315458. [DOI] [PubMed] [Google Scholar]
- 31.Clerici M, Saresella M, Colombo F, Fossati S, Sala N, Bricalli D, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 32.Watera C, Todd J, Mutonyi G, Miiro G, Mpendo J, Hughes P, et al. Effects of cotrimoxazole on hematologic parameters in HIV-infected adults in a community-based clinic in Entebbe, Uganda. J Acquir Immune Defic Syndr. 2007;46:369–71. doi: 10.1097/QAI.0b013e3181170c47. [DOI] [PubMed] [Google Scholar]
- 33.Aizire J, Fowler MG, Wang J, Shetty AK, Stranix-Chibanda L, Kamateeka M, et al. Extended prophylaxis with nevirapine and cotrimoxazole among HIV-exposed uninfected infants is well tolerated. AIDS. 2012;26:325–33. doi: 10.1097/QAD.0b013e32834e892c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chintu C, Bhat GJ, Walker AS, Mulenga V, Sinyinza F, Lishimpi K, et al. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- 35.WHO collaborative Study Team on the Role of Breastfeeding on Prevention of Infant Mortality. Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355:1517–24. [PubMed] [Google Scholar]
- 36.Coutsoudis A, Kindra G, Esterhuizen T. Impact of cotrimoxazole prophylaxis on the health of breast-fed, HIV-exposed, HIV-negative infants in a resource-limited setting. AIDS. 25:1797–9. doi: 10.1097/QAD.0b013e32834ad699. [DOI] [PubMed] [Google Scholar]