Mucosal Barriers to Pathogen Transmission
Exogenous viral, bacterial, fungal and parasitic pathogens typically encounter several barriers to infection of their requisite host. In humans, these barriers are most commonly encountered at mucosal surfaces. These surfaces cover the eyes, lacrimal glands and tear ducts, the gastrointestinal tract, the bronchioalveolar surfaces, the mammary glands and ducts and the urogenital tracts of both men and women. When encountering transmission barriers, most pathogens must initially survive in a unique and specialized local mucosal microenvironment prior to or during transit across the mucosal epithelial barrier; others make this environment their permanent niche During co-evolution with their host, successful pathogens have acquired mechanisms that allow survival in these microenvironments, even if only for brief periods of time. For example, the common gastrointestinal bacterium, Heliobacter pylori, expresses colonization factors, such as outer membrane adhesins, that allow the bacteria to overcome the inhibitory effects of gastrointestinal luminal acidity and mucus-secretion while attaching to the epithelial cells lining the mucosal surface [1]. Pulmonary anaerobes encounter relatively high oxygen content and a surfactant lipoprotein layer within the small bronchioles and pulmonary alveoli [2]. Heterosexually transmitted pathogens, including Chlamydia trachomatis (C. trachomatis), Neisseria gonorrheae (N. gonorrheae), herpes simplex viruses types 1 and 2 (HSV-1 and HSV-2) and the human immunodeficiency virus type-1 (HIV-1) are typically delivered into the female genital tract in the presence of semen. The effects of semen on sexually transmitted infections (STIs) are complex and include characteristics that enhance and that block transmission. For instance, the alkalinity of semen effectively neutralizes the relative acidity of the healthy lower genital tract of females, but semen contains antibacterial factors [3–4] and virus-enhancing [5] peptides that create mixed and pathogen-specific effects on heterosexual STI transmission. Within the healthy lower female genital tract, most surfaces are covered with exudates (vaginal) and/or layers of cervical mucus that trap and dilute pathogens, inhibiting their access to the underlying epithelial surface [6].
If a pathogen survives the intraluminal microenvironment at mucosal surfaces to reach the epithelia, most must next breech this sometimes formidable impediment to transmission. The epithelial barrier varies dramatically among mucosal sites and this variability is closely related to STI transmission rates. Beginning distally and ascending within the human female reproductive tract, the vulva and vagina and ectocervix are lined by multilayered, stratified and keratinized epithelia, the cervical transformation zone by an increasingly thin, stratified and transitional epithelium and the endocervical crypts by a single layer of non-keratinized epithelial cells [7]. Recent investigations modeling sexual transmission in non-human primate (NHP) models via atraumatic vaginal exposure to simian immunodeficiency virus (SIV) show that viral transmission occurs most readily across the single-layered endocervix [8–9]. Similar vulnerabilities to retroviral transmission are seen in another single-cell layered mucosal surface—the rectum. Studies in NHP models have confirmed epidemiologic data demonstrating that transmission of HIV across the rectum after receptive anal intercourse is higher than after receptive vaginal intercourse [10]. Although this effect has been logically attributed to the relative vulnerability of the single-celled rectal epithelium to the micro- and macro-trauma incumbent in receptive anal intercourse, transmission characteristics in the female genital tract after atraumatic vaginal exposure (as above) suggest that other factors are likely involved. Data showing short and long-term decreases in female-to-male HIV sexual transmission with male circumcision [11–12] also point to changes in epithelial characteristics as important in HIV transmission.
Mucosal pathogens that have survived the luminal microenvirnment and have breached the mucosal epithelia must surmount detection and elimination by epithelial innate and adaptive immune responses. These responses are mediated by the largest and most wide-spread component of all human lymphoid tissue, the mucosa-associated lymphoid tissues (MALT; Figure 1). MALT is comprised of intraepithelial and subepithelial immune cell populations and local, small, isolated lymph nodes. The lymphoid cell populations in MALT are sometimes organized into lymphoid follicles within the subepithelial lamina propria, most readily exemplified by the Peyer’s patches of the gastrointestinal tract. MALT has been variously categorized into several regional and functional subsets, including bronchial-associated lymphoid tissues (BALT), mammary-associated lymphoid tissues, genitourinary-associated lymphoid tissues, and gut-associated lymphoid tissues (GALT). Oropharyngeal-associated lymphoid tissues (the parotid gland, parotid and salivary glands and tonsils) are part of GALT but are frequently discussed as a distinct entity. Likewise, nasopharyngeal-associated lymphoid tissues (NALT) are frequently separately acknowledged although they share anatomic and functional attributes with both oropharyngeal-associated lymphoid tissues and BALT. Detection of exogenous pathogens by MALT-associated innate immune responses involves the host complement system and host macrophages, dendritic cells, monocytes, neutrophils and eosinophils [13]. Cell-mediated innate immune pathogen recognition utilizes Toll-like receptors (TLRs), nucleotide-binding and oligomerization domain (NOD)-like receptors and helicases [14]. The mucosal bacterial pathogens Salmonella enterica, Escherichia coli and several Brucella species employ molecular mimicry to evade host immune signaling and the innate immune recognition and clearance mediated by these cell surface receptors [14]. Other MALT-associated immune components straddle innate and adaptive immune functionality, including natural killer (NK) and natural killer T (NKT) cells. Successful pathogens finally encounter the adaptive components of MALT, including B and T cells, which feed into and interact with systemic immunity. Several pathogens have evolved mechanisms to subvert, oftentimes only temporarily, innate and adaptive immune recognition. For example, intracellular pathogens, including several DNA viruses, can block presentation of virus-derived antigens to host immune cells via major histocompatibility (MHC) class I molecules [15–16]. Extracellular pathogens are more likely to target antigen presentation by MHC class II molecules.
Figure 1. Mucosal Immune Sites.
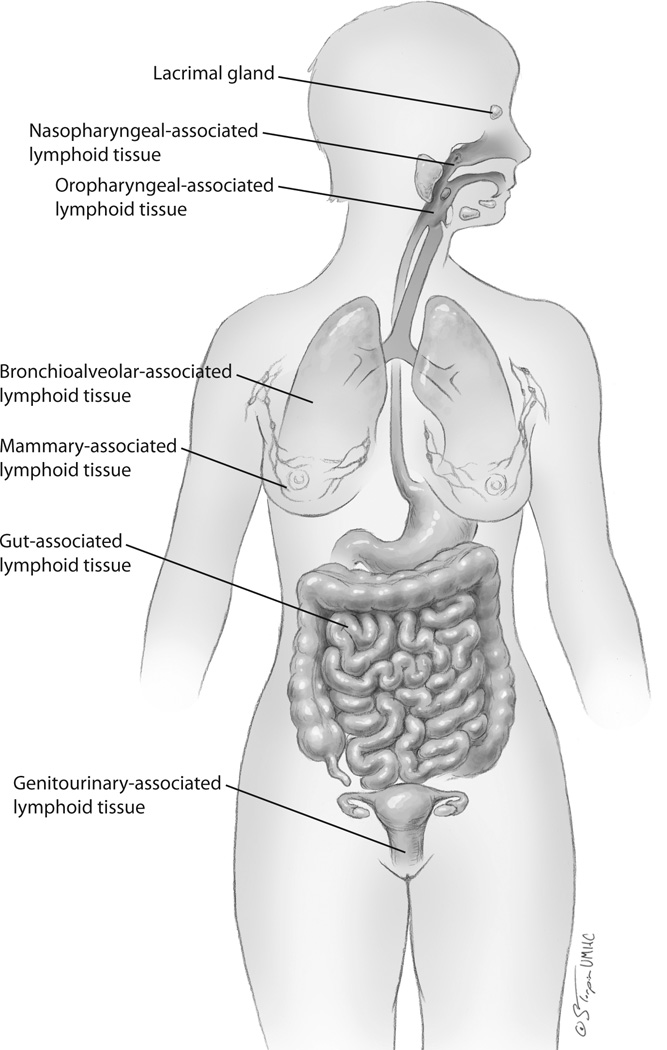
The immune cells populating mucosal sites create the largest immune barrier in the human body. Innate immune responses are particularly prevalent at mucosal immune sites although adaptive responses are also represented. The vast majority of exogenous pathogens first encounter host immune defenses at mucosal sites. Mucosal immune tissues are anatomically subdivided. One paradigm divides these tissues into: 1) nasopharyngeal-associated lymphoid tissues (NALT), 2) oropharyngeal-associated lymphoid tissues, 3) bronchioalveolar-associated lymphoid tissues, 4) mammary-associated lymphoid tissues, 5) gut (gastrointestinal)-associated lymphoid tissues and 6) genitourinary-associated lymphoid tissues. The lacrimal gland and eye also exert mucosa-associated immune reactivities.
Despite the extensive mechanisms employed by human pathogens to invade their host, the complementary and redundant protective mechanisms present at mucosal epithelial sites are remarkably effective. For the human immunodeficiency virus, these protective mechanisms couple with the relative fragility of the virus outside its host to keep transmission rates very low. In a study in Uganda, male-to-female HIV-1 transmission was estimated to be between 1 in 122 and 1 in 1400 coital acts, depending on stage of disease in the infected transmitting male partner [17]. A study conducted in Kenya revealed transmission rates of 1 in 159 vaginal coital acts between HIV positive women and their HIV-negative male partners [18]. Transmission rates to the receptive partner in men who have sex with men (MSM) varies along with sexual practice preferences, but was demonstrated to be approximately 1 in 70 acts of insertive anal intercourse in a study in Sydney, Australia [19]. While these numbers come from widely differing populations and transmission rates will vary by viral load and stage of disease in the transmitter and by co-infection in each partner, the relative rates of transmission based on coital practice are informative.
A Possible Role for Mucosal Surfaces in HIV-1 Gatekeeping
The overlapping and redundant layers of mucosal protection against HIV transmission are also likely to be responsible for the well-described finding that, while several to many HIV-1 viral variants are present in the bodily secretions of the infected transmitter, the majority of HIV-1 transmission involves only a single CCR5 tropic viral isolate [20]. The gatekeeper function of the mucosal epithelia in selecting the founder/transmitted viral variant was recently reviewed in an informed and comprehensive review by Grivel, Shattock and Margolis [21]. The authors hypothesize that multiple and redundant barriers are part of the mucosal gatekeeping function and that the efficiency of each barrier may vary among potential viral host targets. The preferential transmission of CCR5-tropic variants after direct blood inoculation suggests that post-mucosal selection also occurs.
Vulnerabilities in mucosal barrier function are exploited by HIV-1 to increase transmission efficacy. Among these is the preferential transmission across single cell-layered mucosal barriers (see above) including the endocervix [8–9] and the rectum [10]. Other vulnerabilities include physical breaks in the epithelial barrier, local inflammation and the phenotype and activation status of target cells in subepithelial sites [22]. Each of these vulnerabilities can be introduced or exacerbated by mucosal site co-infections.
A Variety of Interactions between Mucosal Co-infections and HIV-1 transmission and Disease
In this special hot topics issue of Current HIV Researchthe invited contributors were tasked with discussing the effects of specific mucosal co-infections on HIV pathogenesis. An emphasis on HIV transmission was suggested. Contributors were requested to submit primary, original data, topic reviews or a combination of approaches. Each contributing group brought its individual expertise to these submissions. All contributors demonstrate that associations between HIV-1 and other mucosal infections are the result of more than just common risk factors.
Bacterial vaginosis and Trichomonas vaginalis
Mirmonsef, et al., have addressed the interactions between HIV-1 and two common inflammatory conditions of the lower female genital tract. One involves infection with the vaginal and ectocervical pathogen, Trichomonas vaginalis (T. vaginalis); the other involves complex alterations in the normal vaginal flora. The latter has been variably labeled, but is most usually called bacterial vaginosis (BV). Each of these conditions is frequently asymptomatic or minimally symptomatic. For BV, the authors review epidemiologic data linking the presence of BV to HIV-1 acquisition in and transmission by women. They highlight possible mechanisms for this association, including altered vaginal acidity secondary to decreases in hydrogen peroxide (H2O2)-producing lactobacilli, the creation of a pro-inflammatory local microenvironment involving IL-8, IL-1α and -1β, RANTES and IL2, bacteria-associated peptidoglycan stimulation of epithelial TLR2, and direct damage to the vaginal mucosa. The bacteria associated with BV can produce small chain fatty acids, including butyric acid, and sialidases, glycosidases and proteases that can stimulate inflammatory cascades and cell migration or inhibit innate immunity respectively. The authors discuss the intriguing finding that the normal vaginal flora of healthy non-human primates resembles that of women with BV. In their discussion of T. vaginalis as a co-factor in HIV-1 transmission, Mirmonsef, et al., review epidemiologic data linking this relatively asymptomatic infection to increased HIV-1 seroconversion rates in affected women and increased viral shedding in those already infected with HIV-1. They review data mechanistically linking these effects to ectocervical epithelial disruption, to the breakdown of cervical mucus and to pro-inflammatory changes, including recruitment of CD4+ T cell targets and the secretion of IL-1β, IL8 and TNFα into the local microenvironment. Women with T. vaginalis also commonly experience BV, possibly secondary to direct effects of T. vaginalis on H2O2-producing lactobacilli.
Neisseria gonorrhea
Jarvis and Chang have reviewed the very complex data linking infection with Neisseria gonorrhea (N. gonorrhea) with HIV transmission and HIV-specific immune responses. Unlike BV, T. vaginalis, and Chlamydia trachomatis (C. trachomatis), infection with N. gonorrheae is oftentimes quite symptomatic; like C trachomatis, N. gonorrhea is an ascending infection that can spread to the upper female genital tract and cause pelvic inflammatory disease. Most N. gonorrhea infections are highly inflammatory, with adhesion proteins, including pili, opacity-associated (Opa) proteins, lactosyl lipooligosaccharide (LOS) and porin proteins causing cytokine/chemokine secretion via TLR activation and related activities. As seen in T. vaginalis, CD4+ T cell targets are recruited to the endocervices of women infected with N. gonorrheae. The authors review data demonstrating that infection with N. gonorrhea increases viremia in women chronically infected with HIV-1, but advise that population based investigations are conflicting as to the effects of N. gonorrhee on systemic immunity in these women. Further controversies are discussed in terms of the effects of N. gonorrhea on HIV-1 infection of target cells (T cells, dendritic cells and macrophages) in vitro. Here N. gonorrheae exerts cell-type dependent enhancing or inhibiting effects suggesting to the authors that these interactions may be better studied at the tissue level. Gavin and Chang finish their contribution with an exciting review of their own work and that of others on the effects of N. gonorrhea on epithelial cell defensin-mediated enhancement of HIV-1 infectivity and link these finding to the gatekeeping function of mucosal epithelia.
Chlamydia trachomatis
Schust, et. al., have also taken a largely mechanistic approach to their contribution on the effects of C. trachomatis infection on HIV-1 transmission across the female genital tract. Although C. trachomatis is an ascending infection frequently associated with the development of PID, these authors have concentrated their efforts on the cells present at the likely site of atraumatic transmission of both HIV-1 and C. trachomatis—the single-layered endocervical epithelia. Female genital tract epithelial cells have been implicated in HIV-1 transcytosis and CCR5 preferential HIV-1 gatekeeping [23]. The authors have used a newly-described [24–25], primary-like endocervical epithelial cell line to show that infections with non-disseminating genital serovars of C. trachomatis increases the cell surface expression of the alternative HIV-1 receptor, GalCer and of the HIV-1 co-receptors CXCR4 and CCR5 on epithelial cells. They provide primary in vitro data demonstrating that C. trachomatis infection increases the binding of HIV-1 to endocervical epithelial cells and subsequent infection of co-cultured, susceptible MT4-R5 T cells and primary clinical data indicating that CD4+, CXCR4+ and/or CCR5+ T cell targets are recruited to the endocervical mucosae of women with C. trachomatis cervicitis.
Herpes Simplex Virus
Barnabas and Celum have approached their discussion of the effects of co-infection with herpes simplex virus (HSV) on HIV-1 transmission from a largely epidemiologic viewpoint with a specific focus on HSV type 2 (HSV-2). HSV-2 typically enters its host via breaks in the epidermis or across mucosal epithelia. Typical disease manifestations include ulcerative lesions at the site of infection and cycles of latency in the dorsal root ganglia alternating with repeat local lesions and or asymptomatic viral shedding. The authors emphasize the dramatic variations in the worldwide disease prevalence and summarize fairly large data sets showing that HIV-1 transmission and acquisition are increased in HSV-2 infected women and men and in HSV-2 infected men who have sex with men. While dermal disruption with ulcerative lesions is an obvious mechanism by which HSV-2 co-infection may increase HIV-1 transmission, the contributors review other potential mechanisms, including local recruitment of HIV-target cells to sites of HSV-2 infection, HSV-inducted transactivation of HIV-1 long-terminal repeats, increased HIV rna production and the surprising long-term local persistence of HIV-1 target cells at the sites of treated and healed HSV-2 lesions. The contributors then use the co-infection paradigm to discuss lessons learned about HSV-2 pathogenesis from HIV-1 intervention studies. They review several population-based investigations that demonstrate the treatment HSV-2 in HIV-1 infected individuals has little effect on HIV-1 viral load and transmission. These results demonstrate that, despite standard antiviral therapies, HSV-2 infected individuals are in an almost continual phase of disease reactivation and viral shedding. The authors complete their review with a discussion of the promising effects of high dose HSV-2 suppressive therapies on HIV-1 disease progression in co-infected populations.
Mammary and Bronchioalveolar Mucosal Co-infections
This special hot topics issue of Current HIV Research does not contain a contribution on pulmonary co-infections and HIV-1 transmission nor on mammary co-infections and HIV-1 transmission, although each could be the focus of its own special issue. HIV transmission across the pulmonary tract is exceedingly rare, but there is a large body of literature available on the interplay between HIV-1 and pulmonary pathogens, including Mycobacterium tuberculosis (TB) and Pneumocystis carnii (P carnii). One investigator has shown a lung-specific expansion of CD4+ CCR5+ T cells among patients with active TB that is not found in matched peripheral blood nor in patients with non-TB pulmonary disease and suggested that the TB-infected lower respiratory tract and that could serve as a potential site for transmucosal HIV-1 transmission [26]. Others have linked M. tuberculosis infection to HIV-1 reactivation from latency (reviewed in [27]; see below for similar effects by periodontal pathogens). The nearly exclusive place of the mammary gland in HIV-1 transmission is in mother-to-neonate transmission during breastfeeding. Among HIV-positive women that breastfeed, 16–35% will transmit HIV-1 to their infant in the absence of antiretroviral therapy (ART) [28–30]. Breast milk contains both cell-free and cell-associated virus, and higher levels of both have been associated with transmission [31–32]. Generalized breast infection and inflammation (mastitis) are associated with an elevated risk for maternal-to-fetal HIV-1 transmission when compared to HIV-infected mothers without breast complaints; the risk is even higher in the presence of severe and localized breast infections (abscesses) [33]. The discrete point of viral entry in the infant who obtains HIV-1 through breastfeeding is not conclusively known, and infection may occur via the oral mucosa, tonsils, and/or the neonatal gastrointestinal tract. Recent studies using fetal organ tissue models have shown that both cell-free and cell-associated virus can transmigrate across fetal epithelial cells from the oral cavity and the intestines, and thus demonstrate a potential mechanism for transmission of HIV-1 contained in breast milk [34–35]. Studies in primates [36] and mice [37] have shown that cells present in breast milk can survive their transit through the stomach, traverse the intestinal tract and remain functional in the nursing neonate. Other major risk factors for HIV-1 transmission across the oropharyngeal and gastrointestinal tracts include orogenital sexual contact and receptive anal intercourse.
Periodontal pathogens
There is a growing body of literature posing credible mechanisms by which co-infection with the periodontal pathogen Porphyromonas gingivalis (P. gingivalis) might increase transmission of HIV-1 across the oral mucosa. Some of these mechanisms, including P. gingivalis-associated increases in the expression of CCR5 on oral keratinocytes [38], are reminiscent of the effects of C. trachomatis on HIV-1 receptor and co-receptor expression on genital epithelial cells (Schust, et. al., this issue). Also similar are data demonstrating that oral epithelial cells can maintain non-replicative HIV-1 infection [39] and that P. gingivalis infection and its resultant CCR5 induction promotes transmission of CCR5-tropic HIV-1 variants across human oral epithelial cells (gingival and tonsil) to susceptible T cells [40].
The authors of our final contribution in this special Hot Topics issue have also addressed interactions between the periodontal pathogen, P. gingivalis, and HIV-1, but have focused their efforts on their own work on the effects of P. gingivalis on AIDS progression in co-infected individuals. P. gingivalis expresses several virulence factors, including small chain fatty acids (SCFAs), that Imai, et al., have studied in the context of HIV-1 infections [41–42]. The authors review their own published data outlining the specific effects of the SCFA, butyric acid, on the reactivation of integrated HIV-1 from latency [41–42]. They provide evidence supporting a mechanism involving histone deacetylation [42] and primary data demonstrating that these effects are not generalizable to all oral bacteria, including Escherichia coli (E. coli) and Prevotella nigrescens (P. nigrescens), and that local cytokine secretion in the presence of P. gingivalis-induced inflammation may also be involved. Imai, et al., conclude with the intriguing suggestion that HIV-1 reactivation from latency could be similarly induced by anerobic, butyric-acid producing bacteria known to be present at other mucosal sites.
Concluding Remarks
As we continue to increase our knowledge about HIV-1 transmission and pathogenesis, it has become increasingly clear that mucosal co-infections play an important role in both. This will have particular ramifications to strategies aimed at preventing transmission in both resource-poor and more developed settings where many of these co-infections are very common and are inadequately treated. The contributors to this special Hot Topics issue of Current HIV Research have outlined many of the mechanisms involved in mucosal pathogen-associated vulnerabilities to HIV-1 transmission across the otherwise formidable barriers to infection present at most mucosal epithelial sites. These and other experts in infectious disease and mucosal immunology are certain to be at the forefront when advances are made in combating mucosal HIV-1 transmission.
Figure 2. Mechanisms by which co-infections increase HIV-1 transmission and/or pathogenesis at mucosal immune sites.
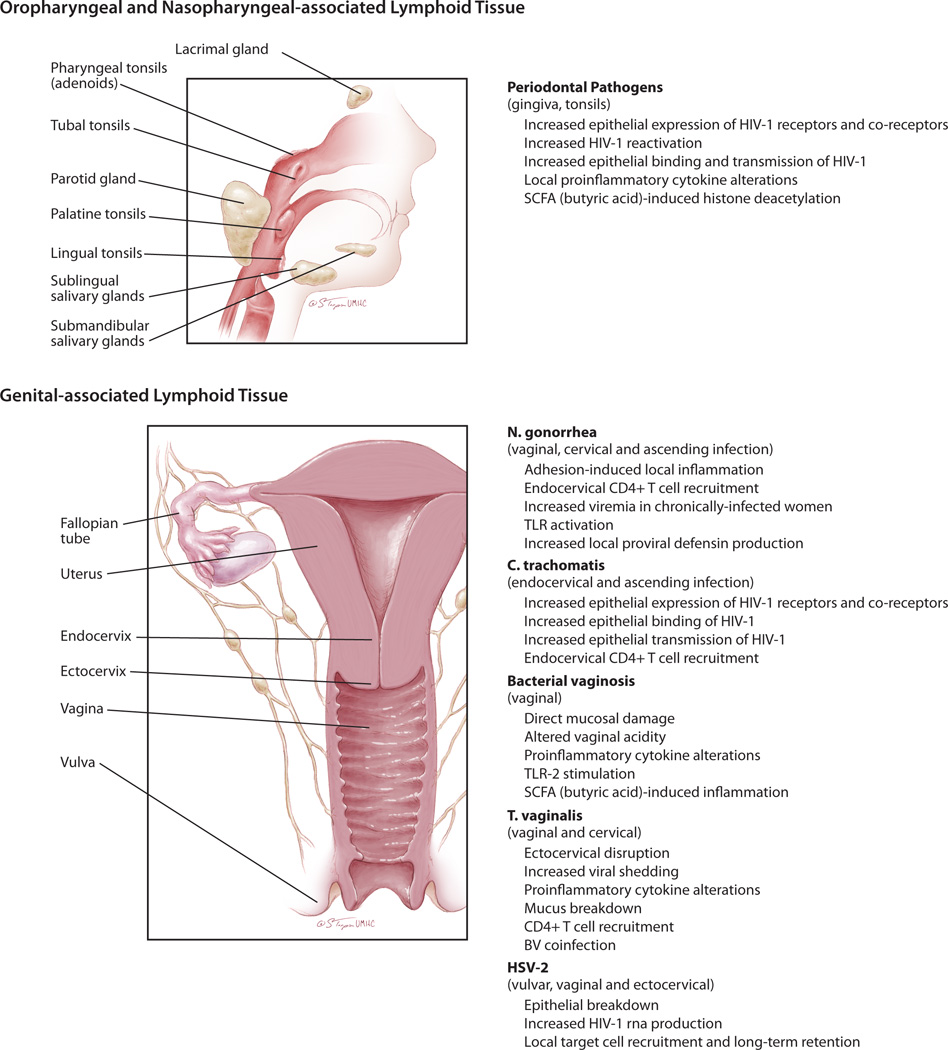
Periodontal pathogens, including P. gingivalis, can infect tissues of the gingival and tonsils. These pathogens have been shown to exert direct effects on the mucosal epithelial cell and indirect effects on the local mucosal immune environment that can aid in HIV-1 transmission and spread after oral-genital exposures. The sexually transmitted infections, C. trachomatis, N. gonorrhea and HSV can also infect oropharyngeal tissues but investigations on their effects on HIV-1 transmission at these sites has not been well-described. Sexually transmitted co-infections with N. gonorrhea, C. trachomatis, T. vaginalis and HSV-2 and the presence of bacterial vaginosis are associated with increased transmission of HIV-1 across the female genital tract and/or increased HIV-1 viremia. The mechanisms causing these increases are variable and pathogen specific, although common mechanisms include epithelial disruption and local recruitment of target immune cells to the subepithelial tissues.
References
- 1.Fischer W, Prassl S, Haas R. Virulence mechanisms and persistence strategies of the human gastric pathogen Helicobacter pylori. Curr Top Microbiol Immunol. 2009;337:129–171. doi: 10.1007/978-3-642-01846-6_5. [DOI] [PubMed] [Google Scholar]
- 2.Kuang Z, Hao Y, Walling BE, et al. Pseudomonas aeruginosa elastase provides an escape from phagocytosis by degrading the pulmonary surfactant protein-A. PLoS One. 2011;6:e27091. doi: 10.1371/journal.pone.0027091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Com E, Bourgeon F, Evrard B, et al. Expression of antimicrobial defensins in the male reproductive tract of rats, mice, and humans. Biol Reprod. 2003;68:95–104. doi: 10.1095/biolreprod.102.005389. [DOI] [PubMed] [Google Scholar]
- 4.Doncel GF, Joseph T, Thurman AR. Role of semen in HIV-1 transmission: inhibitor or facilitator? Am J Reprod Immunol. 2011;65:292–301. doi: 10.1111/j.1600-0897.2010.00931.x. [DOI] [PubMed] [Google Scholar]
- 5.Munch J, Rucker E, Standker L, et al. Semen-derived amyloid fibrils drastically enhance HIV infection. Cell. 2007;131:1059–1071. doi: 10.1016/j.cell.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Olmsted SS, Padgett JL, Yudin AI, et al. Diffusion of macromolecules and virus-like particles in human cervical mucus. Biophys J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pudney J, Quayle AJ, Anderson DJ. Immunological microenvironments in the human vagina and cervix: mediators of cellular immunity are concentrated in the cervical transformation zone. Biol Reprod. 2005;73:1253–1263. doi: 10.1095/biolreprod.105.043133. [DOI] [PubMed] [Google Scholar]
- 8.Li Q, Estes JD, Schlievert PM, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CJ, Li Q, Abel K, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chenine AL, Siddappa NB, Kramer VG, et al. Relative transmissibility of an R5 clade C simian-human immunodeficiency virus across different mucosae in macaques parallels the relative risks of sexual HIV-1 transmission in humans via different routes. J Infect Dis. 2010;201:1155–1163. doi: 10.1086/651274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gray R, Kigozi G, Kong X, et al. The effectiveness of male circumcision for HIV prevention and effects on risk behaviors in a post-trial follow up study in Rakai, Uganda. AIDS. 2012 doi: 10.1097/QAD.0b013e3283504a3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369:657–666. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 13.Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 14.Xiao TS. Subversion of innate immune signaling through molecular mimicry. J Clin Immunol. 2010;30:638–642. doi: 10.1007/s10875-010-9435-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horst D, Verweij MC, Davison AJ, Ressing ME, Wiertz EJ. Viral evasion of T cell immunity: ancient mechanisms offering new applications. Curr Opin Immunol. 2011;23:96–103. doi: 10.1016/j.coi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- 17.Wawer MJ, Gray RH, Sewankambo NK, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–1409. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 18.Baeten JM, Richardson BA, Lavreys L, et al. Female-to-male infectivity of HIV-1 among circumcised and uncircumcised Kenyan men. J Infect Dis. 2005;191:546–553. doi: 10.1086/427656. [DOI] [PubMed] [Google Scholar]
- 19.Jin F, Jansson J, Law M, et al. Per-contact probability of HIV transmission in homosexual men in Sydney in the era of HAART. AIDS. 2010;24:907–913. doi: 10.1097/QAD.0b013e3283372d90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salazar-Gonzalez JF, Salazar MG, Keele BF, et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med. 2009;206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grivel JC, Shattock RJ, Margolis LB. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? J Transl Med. 2011;9(Suppl 1):S6. doi: 10.1186/1479-5876-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keele BF, Estes JD. Barriers to mucosal transmission of immunodeficiency viruses. Blood. 2011;118:839–846. doi: 10.1182/blood-2010-12-325860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saidi H, Magri G, Nasreddine N, Requena M, Belec L. R5- and X4-HIV-1 use differentially the endometrial epithelial cells HEC-1A to ensure their own spread: implication for mechanisms of sexual transmission. Virology. 2007;358:55–68. doi: 10.1016/j.virol.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Buckner LR, Schust DJ, Ding J, et al. Innate immune mediator profiles and their regulation in a novel polarized immortalized epithelial cell model derived from human endocervix. J Reprod Immunol. 2011;92:8–20. doi: 10.1016/j.jri.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herbst-Kralovetz MM, Quayle AJ, Ficarra M, et al. Quantification and comparison of toll-like receptor expression and responsiveness in primary and immortalized human female lower genital tract epithelia. Am J Reprod Immunol. 2008;59:212–224. doi: 10.1111/j.1600-0897.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 26.Santucci MB, Bocchino M, Garg SK, et al. Expansion of CCR5+ CD4+ T-lymphocytes in the course of active pulmonary tuberculosis. Eur Respir J. 2004;24:638–643. doi: 10.1183/09031936.04.000105403. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard A, Montagnier L, Gougeon ML. Influence of microbial infections on the progression of HIV disease. Trends Microbiol. 1997;5:326–331. doi: 10.1016/S0966-842X(97)01089-5. [DOI] [PubMed] [Google Scholar]
- 28.Coutsoudis A, Dabis F, Fawzi W, et al. Late postnatal transmission of HIV-1 in breast-fed children: an individual patient data meta-analysis. J Infect Dis. 2004;189:2154–2166. doi: 10.1086/420834. [DOI] [PubMed] [Google Scholar]
- 29.Liang K, Gui X, Zhang YZ, et al. A case series of 104 women infected with HIV-1 via blood transfusion postnatally: high rate of HIV-1 transmission to infants through breast-feeding. J Infect Dis. 2009;200:682–686. doi: 10.1086/605123. [DOI] [PubMed] [Google Scholar]
- 30.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 31.Rousseau CM, Nduati RW, Richardson BA, et al. Association of levels of HIV-1-infected breast milk cells and risk of mother-to-child transmission. J Infect Dis. 2004;190:1880–1888. doi: 10.1086/425076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 33.John GC, Nduati RW, Mbori-Ngacha DA, et al. Correlates of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission: association with maternal plasma HIV-1 RNA load, genital HIV-1 DNA shedding, and breast infections. J Infect Dis. 2001;183:206–212. doi: 10.1086/317918. [DOI] [PubMed] [Google Scholar]
- 34.Tugizov SM, Herrera R, Veluppillai P, et al. Differential transmission of HIV traversing fetal oral/intestinal epithelia and adult oral epithelia. J Virol. 2011 doi: 10.1128/JVI.06578-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tugizov SM, Herrera R, Veluppillai P, et al. HIV is inactivated after transepithelial migration via adult oral epithelial cells but not fetal epithelial cells. Virology. 2011;409:211–222. doi: 10.1016/j.virol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain L, Vidyasagar D, Xanthou M, et al. In vivo distribution of human milk leucocytes after ingestion by newborn baboons. Arch Dis Child. 1989;64:930–933. doi: 10.1136/adc.64.7_spec_no.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiler IJ, Hickler W, Sprenger R. Demonstration that milk cells invade the suckling neonatal mouse. Am J Reprod Immunol. 1983;4:95–98. doi: 10.1111/j.1600-0897.1983.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 38.Giacaman RA, Nobbs AH, Ross KF, Herzberg MC. Porphyromonas gingivalis selectively up-regulates the HIV-1 coreceptor CCR5 in oral keratinocytes. J Immunol. 2007;179:2542–2550. doi: 10.4049/jimmunol.179.4.2542. [DOI] [PubMed] [Google Scholar]
- 39.Vacharaksa A, Asrani AC, Gebhard KH, et al. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology. 2008;5:66. doi: 10.1186/1742-4690-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giacaman RA, Asrani AC, Gebhard KH, et al. Porphyromonas gingivalis induces CCR5-dependent transfer of infectious HIV-1 from oral keratinocytes to permissive cells. Retrovirology. 2008;5:29. doi: 10.1186/1742-4690-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imai K, Ochiai K. Role of histone modification on transcriptional regulation and HIV-1 gene expression: possible mechanisms of periodontal diseases in AIDS progression. J Oral Sci. 2011;53:1–13. doi: 10.2334/josnusd.53.1. [DOI] [PubMed] [Google Scholar]
- 42.Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182:3688–3695. doi: 10.4049/jimmunol.0802906. [DOI] [PubMed] [Google Scholar]


