Abstract
Object
Investigators conducting the International Study of Unruptured Intracranial Aneurysms, sponsored by the National Institutes of Health, sought to evaluate predictors of future hemorrhage in patients who had unruptured mirror aneurysms. These paired aneurysms in bilateral arterial positions mirror each other; their natural history is unknown.
Methods
Centers in the US, Canada, and Europe enrolled patients for prospective assessment of unruptured intracranial aneurysms. Central radiological review confirmed the presence or absence of mirror aneurysms in patients without a history of prior subarachnoid hemorrhage (SAH) (Group 1). Outcome at 1 and 5 years and aneurysm characteristics are compared.
Results
Of 3120 patients with aneurysms treated in 61 centers, 376 (12%) had mirror aneurysms, which are more common in women than men (82% [n = 308] vs 73% [n = 1992], respectively; p < 0.001) and in patients with a family history of aneurysm or SAH (p < 0.001).
Compared with patients with nonmirror saccular aneurysms, a greater percentage of patients with mirror aneurysms had larger (> 10 mm) aneurysms (mean maximum diameter 11.7 vs 10.4 mm, respectively; p < 0.001). The most common distribution for mirror aneurysms was the middle cerebral artery (34% [126 patients]) followed by noncavernous internal carotid artery (32% [121]), posterior communicating artery (16% [60]), cavernous internal carotid artery (13% [48]), anterior cerebral artery/anterior communicating artery (3% [13]), and vertebrobasilar circulation (2% [8]). When these patients were compared with patients without mirror aneurysms, no statistically significant differences were found in age (mean age 54 years in both groups), blood pressure, smoking history, or cardiac disease. Aneurysm rupture rates were similar (3.0% for patients with mirror aneurysms vs 2.8% for those without).
Conclusions
Overall, patients with mirror aneurysms were more likely to be women, to report a family history of aneurysmal SAH, and to have larger aneurysms. The presence of a mirror aneurysm was not an independent predictor of future SAHs.
Keywords: aneurysm, intracranial aneurysm, mirror aneurysm, ruptured aneurysm, subarachnoid hemorrhage, vascular disorders
Intracranial aneurysms are the most common cause of SAH, which accounts for approximately 10% of strokes and carries a mortality rate of 50%.3,4 Of those patients with unruptured intracranial aneurysms, many (up to 20%–30% in some series) will have multiple such lesions.10,12–14
Unruptured mirror aneurysms are paired aneurysms found within similar distributions on bilateral intracranial arteries. The frequency of mirror aneurysms is unknown. Estimates range from less than 5% of all patients with unruptured intracranial aneurysms to as many as 40% of patients with multiple unruptured intracranial aneurysms.1,9 Mirror aneurysms are not uncommon, but their clinical significance is poorly understood because of the sparse, heterogeneous data from anecdotal reports of these lesions; retrospective databases with variable reporting of aneurysm size, site, and configuration; outcomes extrapolated from patients presenting with SAH; and varying definitions of mirror aneurysms, including, in some cases, a ruptured aneurysm in the mirror pair.5,15
The objective of this study was to determine the frequency and natural history of mirror aneurysms, to define the morbidity and mortality associated with these lesions in patients who had not undergone operations for them, and to describe the risk factors associated with the presence of mirror aneurysms in a prospective cohort.
Methods
The ISUIA was a multicenter epidemiological observational study conducted at 61 treatment centers in the US (27 [44%]), Canada (8 [13%]), and Europe (26 [43%]). The study was funded by the National Institutes of Health and approved by the institutional review board or ethics committee at each site. The overall cohort of 5500 patients was composed of retrospectively identified participants with unruptured intracranial aneurysms diagnosed by means of hard-copy arteriography, and a prospective component of 4000 patients identified between November 1991 and December 1997.
The definition of a mirror aneurysm used in this project was the presence of paired or “twin” unruptured intracranial aneurysms located in similar positions bilaterally on the parent arteries. Central radiological review by a radiologist blinded to the clinical aspects of each case confirmed the presence, location, size, and morphological features of the aneurysms. Participating centers were required to perform high-quality catheter angiography.
Patient eligibility for the study at large required the following: at least 1 aneurysm confirmed by catheter angiography; reasonable neurological functional status (Rankin score of 1 or 2); and no prior patient history of SAH (classified as Group 1).
Patients were classified as Group 2 if they had a history of a ruptured aneurysm at another location that was clipped, trapped, or treated through endovascular obliteration and isolated from circulation.
Exclusion criteria included traumatic, fusiform, or mycotic aneurysms; ruptured aneurysms only; and a patient history of an intracranial hemorrhage with an unrepaired underlying structural lesion or a primary intracranial hemorrhage.
An aneurysm was considered to be a mirror aneurysm if the above entry criteria were met and if the paired aneurysms were more than 2 mm in maximum diameter, as determined by central radiological review.
To focus on the natural history of unruptured intracranial aneurysms, the study group restricted its analysis of outcomes to prospectively enrolled patients in Group 1, which consisted of patients without prior SAH from a separate aneurysm. When available, patient morbidity and mortality were determined by adjudication of clinical records, ancillary imaging studies, death certificates, and autopsy information. A poor outcome was defined as a TICS score of less than 27 or a Rankin score of 3 to 5 or both.
Statistical Analysis
Factors related to the presence or absence of mirror aneurysms were evaluated using univariate analysis (using the t-test and the chi-square test). The associations between mirror aneurysm status and outcomes at 1 and 5 years’ follow-up were tested using the chi-square statistic. The proportional hazards model from Phase 1 of the ISUIA study was augmented by the addition of the mirror aneurysm variable to evaluate whether the presence of mirror aneurysms affected all-cause mortality. All statistical analyses were performed using the SAS version 9.1.3 statistical software package (SAS Institute, Inc.).7
Results
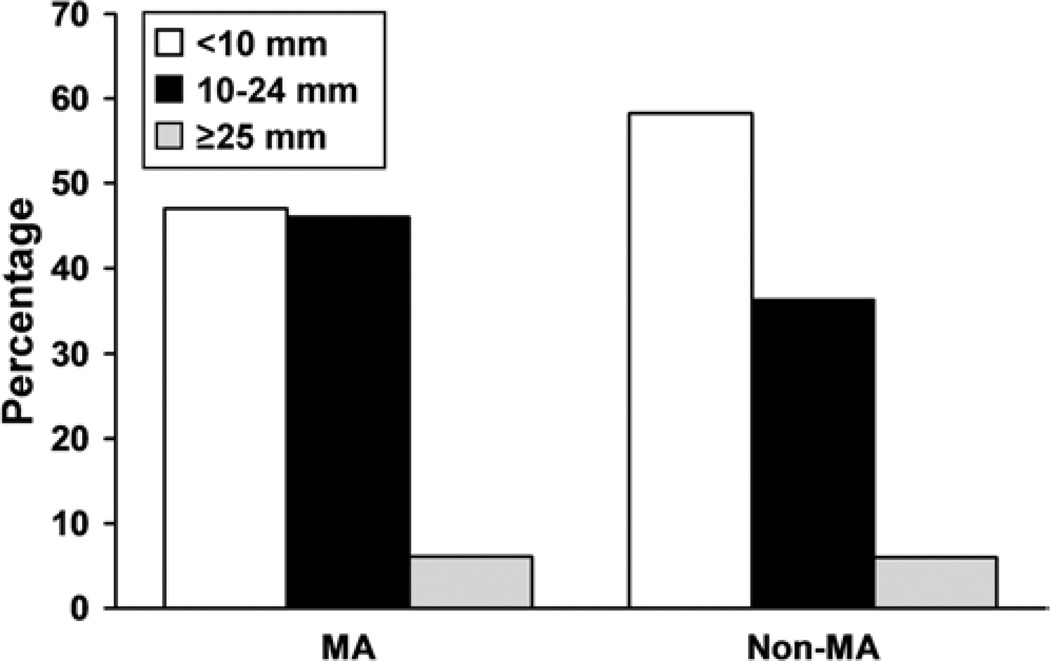
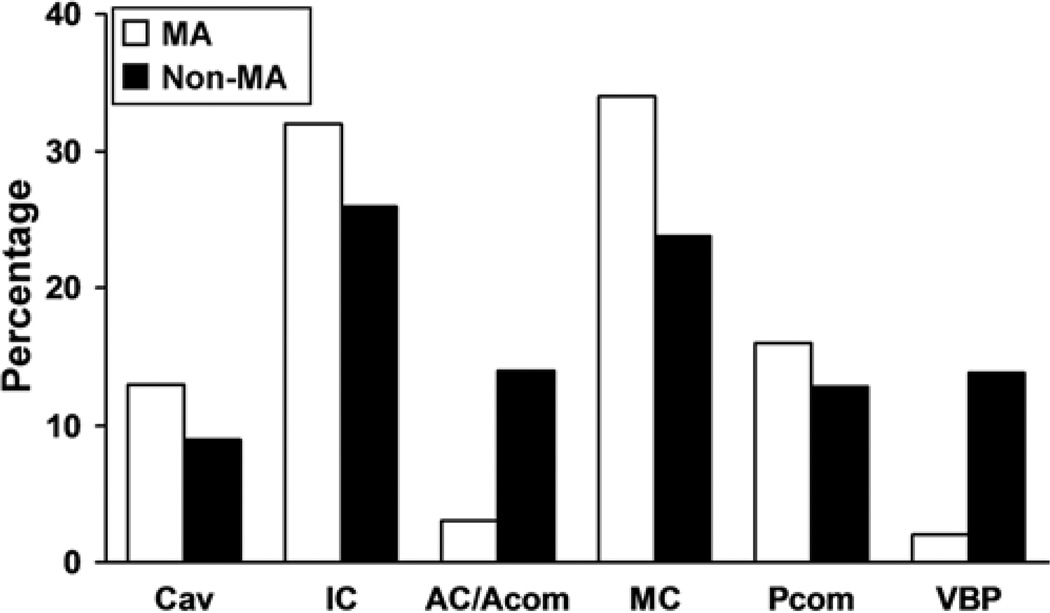
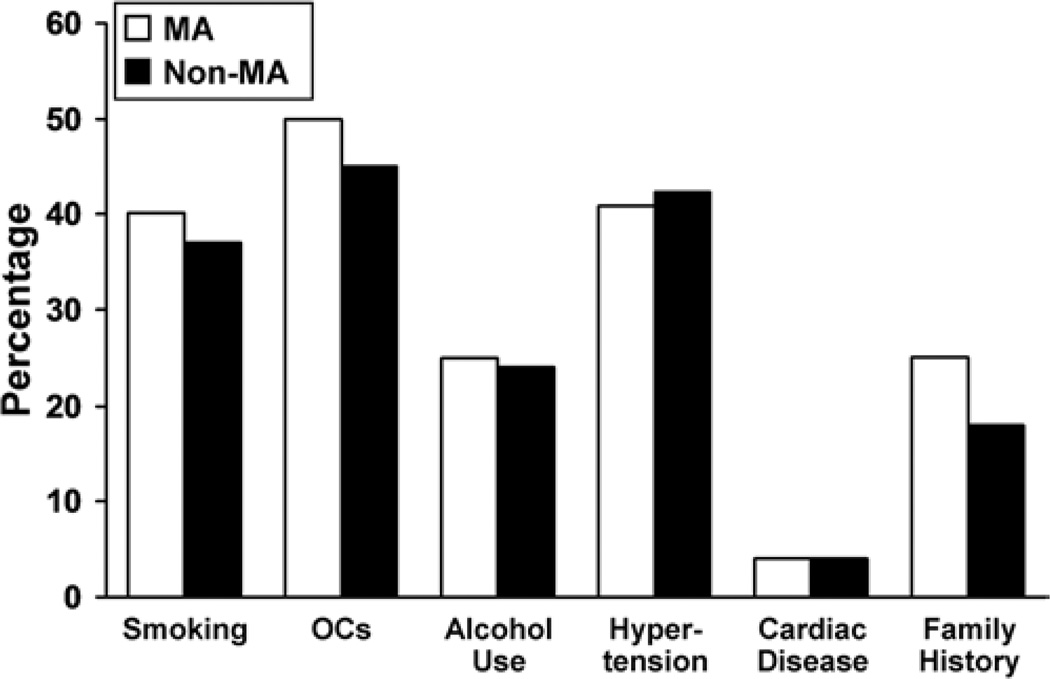
In 61 centers with 3120 prospectively enrolled Group 1 patients, there were 376 patients (12%) with mirror aneurysms. Patients with mirror aneurysms were more likely to be women than were study participants without mirror aneurysms (308 [82%] of 376 vs 1992 [73%] of 2744, respectively; p < 0.001]). Most patients with mirror aneurysms (203 [54%] of 376) had 1 pair of aneurysms. Almost half (149 [40%]) of the patients had additional unpaired aneurysms. Six percent (24) of the 376 patients, had 2 pairs of mirror aneurysms with or without other unpaired aneurysms. Figure 1 illustrates the distribution of maximum aneurysm size among patients in both groups. Patients with mirror aneurysms had larger aneurysms than those without mirror aneurysms (mean maximum diameter 11.7 vs 10.4 mm, respectively; p < 0.001). Patients with mirror aneurysms were also more likely to have at least 1 aneurysm with a maximum diameter greater than 10 mm (53% [198 of 386] vs 42% [1153 of 2744], p = 0.007]). Figure 2 illustrates the location of the type of largest aneurysm in both groups. In the group of patients with mirror aneurysms, the distribution of locations of the largest aneurysms was as follows: middle cerebral artery (34% [126]), noncavernous internal carotid artery (32% [121]), posterior communicating artery (16% [60]), cavernous internal carotid artery (13% [48]), anterior cerebral artery/anterior communicating artery (3% [13]), and vertebrobasilar circulation (2% [8]). Prospectively obtained data on risk factors showed no statistically significant difference in age (mean 54 years for both groups), systolic blood pressure, smoking history, or cardiac disease (Fig. 3). A family history of intracranial aneurysms was reported more frequently in patients with mirror aneurysms than in those without mirror aneurysms (25% vs 18%, respectively; p = 0.023).
Fig. 1.
Comparison of sizes of mirror aneurysms (MA) versus nonmirror aneurysms (non-MA). Frequencies of aneurysms within size categories (maximum diameter) are compared between mirror and nonmirror aneurysms.
Fig. 2.
Comparison of locations of mirror aneurysms versus nonmirror aneurysms. AC = anterior cerebral artery; Acom = anterior communicating artery; Cav = cavernous internal carotid artery; IC = internal carotid artery; Pcom = posterior communicating artery; VBP = vertebrobasilar circulation.
Fig. 3.
Comparison of behavioral risk factors and comorbid conditions in patients with mirror aneurysms versus nonmirror aneurysms. OCs = oral contraceptive medications.
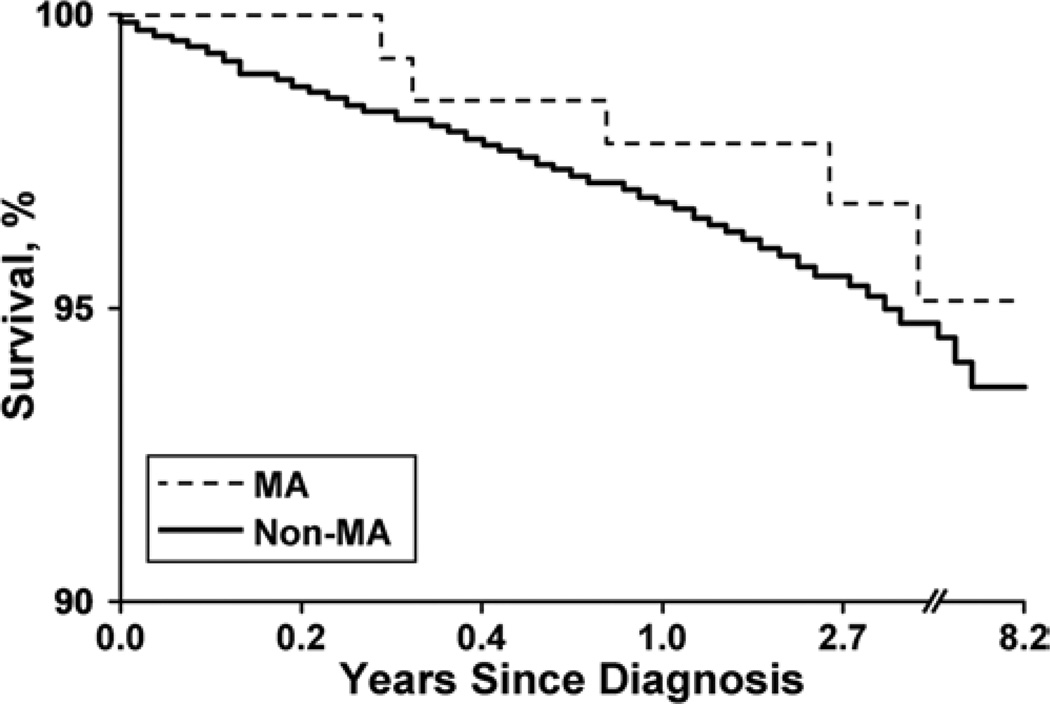
At 1 year and 5 years after diagnosis, outcomes were similar for patients with and without mirror aneurysms. There was no significant difference in the risk of SAH at 1 year (3.0% vs 2.8%) or at 5 years (5.5% vs 4.9%). Table 1 illustrates that there was also no significant difference in the prevalence of deterioration of neurological function as determined by the Rankin scale or the TICS score.2,8 Figure 4 illustrates the survival curves for the mirror aneurysm and nonmirror aneurysm groups, which demonstrated no statistically significant difference in mortality.
TABLE 1.
Outcomes at 1- and 5-year follow-up in 143 patients with and 939 patients without mirror aneurysms*
| FU | Findings | Pts w/ MA | Pts w/o MA | p Value (χ2) |
|---|---|---|---|---|
| 1 yr | poor outcome† | 9/143 (6.3) | 87/930 (9.4) | NS |
| dead | 5/143 (3.5) | 53/930 (5.7) | ||
| 5 yrs | poor outcome† | 5/69 (7.2) | 32/461 (6.9) | NS |
| dead | 14/69 (20.3) | 117/461 (25.4) |
Group 1 (without prior SAH from a separate aneurysm) from the prospective component of the ISUIA study only. Values represent the frequency of the specified outcome in patients with known status at the end of the follow-up period (%).
Abbreviations: FU = follow-up; MA = mirror aneurysm; NS = not significant; Pts = Patients.
Poor outcome was defined as a TICS score of less than 27 or a Rankin score of 3 to 5.
Fig. 4.
Survival curves of patients with mirror aneurysms versus patients with nonmirror aneurysms. Poor outcome is defined as a TICS score of less than 27 or a Rankin score of 3 to 5; the denominator for outcome is based on number of patients with known status at the end of the follow-up period.
Discussion
To date, these ISUIA data represent the largest prospective sample of patients with unruptured mirror aneurysms for whom accurate data on comorbidity and family history of aneurysm rupture are available.
However, much is yet to be learned about mirror aneurysms. Their prevalence in the general population is unclear due to sporadic, incomplete imaging and nonstandardized radiological review. Reports of de novo aneurysms in a mirroring contralateral location suggest a predisposition to their development but also reflect potential underdiagnosis contingent upon the timing and method of reimaging.
One report of a retrospective identification of 33 cases of mirror aneurysms concluded that this type of aneurysm was more likely to be unaccompanied by conventional risk factors (for example, hypertension or smoking) and was more likely to rupture when patients were younger.5
Scientists have postulated that a congenital predisposition in patients with mirror aneurysms causes alterations in blood flow during embryogenesis that account for the reported differences (the higher rate of rupture and the earlier age of diagnosis) in these patients compared with such findings in patients with nonmirror aneurysms.1 Variables associated with the presence of mirror aneurysms (for example, female sex and positive family history) have been reported but relate more to multiplicity in general and not specifically to mirror aneurysms.10,11 The possibility also exists for a congenital or embryologic component. That women are more susceptible to development of aneurysms, including mirror aneurysms, suggests an additional endogenous hormonal effect.6 Patients with mirror aneurysms are more likely to be female, to report a family history of aneurysmal SAH, and to have generally larger aneurysms. Their risk factors and behavioral patterns (for example, smoking or alcohol use) are similar to those of patients without mirror aneurysms. The greater association of mirror aneurysms with female sex was a function of the increased frequency of aneurysm multiplicity in women, rather than a specific characteristic of mirror aneurysms.
Conclusions
The presence of mirror aneurysms does not appear to be a predictor for future SAH, increased mortality, or worsened neurological status at 1- and 5-years’ follow-up. These findings should have important ramifications for the decision-making process with respect to whether and, if so, when to treat patients with mirror aneurysms. Careful attention should be given to recognized risk factors for rupture, including the size and location of aneurysms, the age of the patient, and the presence of previous SAH, irrespective of the presence of mirror aneurysms. Further assessment of complex morphological characteristics using the ISUIA database will clarify if such factors predict a heightened risk of aneurysm rupture in patients with mirror aneurysms.
Acknowledgments
This work was supported in part by grant NS28492 from the National Institutes of Health.
Abbreviations used in this paper
- ISUIA
International Study of Unruptured Intracranial Aneurysms
- SAH
subarachnoid hemorrhage
- TICS
Telephone Interview for Cognitive Status
Footnotes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author contributions to the study and manuscript preparation include the following. Conception and design: Meissner. Acquisition of data: the ISUIA investigators. Analysis and interpretation of data: Meissner. Drafting the article: all authors. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Meissner. Statistical analysis: Meissner, Torner, Rajput. Administrative/technical/material support: Meissner.
References
- 1.Baccin CE, Krings T, Alvarez H, Ozanne A, Lasjaunias P. Multiple mirror-like intracranial aneurysms. Report of a case and review of the literature. Acta Neurochir (Wien) 2006;148:1091–1095. doi: 10.1007/s00701-006-0860-z. [DOI] [PubMed] [Google Scholar]
- 2.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–118. [Google Scholar]
- 3.Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- 4.Brown RD, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Stroke incidence, prevalence, and survival: secular trends in Rochester, Minnesota, through 1989. Stroke. 1996;27:373–380. [PubMed] [Google Scholar]
- 5.Casimiro MV, McEvoy AW, Watkins LD, Kitchen ND. A comparison of risk factors in the etiology of mirror and nonmirror multiple intracranial aneurysms. Surg Neurol. 2004;61:541–545. doi: 10.1016/j.surneu.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 6.Ellamushi HE, Grieve JP, Jäger HR, Kitchen ND. Risk factors for the formation of multiple intracranial aneurysms. J Neurosurg. 2001;94:728–732. doi: 10.3171/jns.2001.94.5.0728. [DOI] [PubMed] [Google Scholar]
- 7.International Study of Unruptured Intracranial Aneurysms Investigators: Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725–1733. doi: 10.1056/NEJM199812103392401. [DOI] [PubMed] [Google Scholar]
- 8.Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2:200–215. doi: 10.1177/003693305700200504. [DOI] [PubMed] [Google Scholar]
- 9.Rinne J, Hernesniemi J, Niskanen M, Vapalahti M. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2–11. doi: 10.1097/00006123-199601000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Rinne J, Hernesniemi J, Puranen M, Saari T. Multiple intracranial aneurysms in a defined population: prospective angiographic and clinical study. Neurosurgery. 1994;35:803–808. doi: 10.1227/00006123-199411000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Torner J, Wiebers D, Brown R, Meissner I, Huston J, Vasquez A. Determinants of the number of intracranial aneurysms. Am J Epidemiol. 2005;161(Suppl):S27. [Google Scholar]
- 12.Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 13.Wiebers DO, Whisnant JP, O’Fallon WM. The natural history of unruptured intracranial aneurysms. N Engl J Med. 1981;304:696–698. doi: 10.1056/NEJM198103193041203. [DOI] [PubMed] [Google Scholar]
- 14.Winn HR, Jane JA, Sr, Taylor J, Kaiser D, Britz GW. Prevalence of asymptomatic incidental aneurysms: review of 4568 arteriograms. J Neurosurg. 2002;96:43–49. doi: 10.3171/jns.2002.96.1.0043. [DOI] [PubMed] [Google Scholar]
- 15.Yamada K, Nakahara T, Kishida K, Yano T, Yamamoto K, Ushio Y. Multiple “mirror” aneurysms involving intracavernous carotid arteries and vertebral arteries: case report. Surg Neurol. 2000;54:361–365. doi: 10.1016/s0090-3019(00)00304-9. [DOI] [PubMed] [Google Scholar]