Abstract
Caveolae, plasma membrane invaginations with constitutive caveolin proteins, harbour proteins involved in intracellular calcium ([Ca2+]i) regulation. In human airway smooth muscle (ASM), store-operated Ca2+ entry (SOCE) is a key component of [Ca2+]i regulation, and contributes to increased [Ca2+]i in inflammation. SOCE involves proteins Orai1 and stromal interaction molecule (STIM)1. We investigated the link between caveolae, SOCE and inflammation in ASM.
[Ca2+]i was measured in human ASM cells using fura-2. Small interference RNA (siRNA) or overexpression vectors were used to alter expression of caveolin-1 (Cav-1), Orai1 or STIM1. Tumour necrosis factor (TNF)-α was used as a representative pro-inflammatory cytokine.
TNF-α increased SOCE following sarcoplasmic reticulum Ca2+ depletion, and increased whole-cell and caveolar Orai1 (but only intracellular STIM1). Cav-1 siRNA decreased caveolar and whole-cell Orai1 (but not STIM1) expression, and blunted SOCE, even in the presence of TNF-α. STIM1 overexpression substantially enhanced SOCE: an effect only partially reversed by Cav-1 siRNA. In contrast, Orai1 siRNA substantially blunted SOCE even in the presence of TNF-α. Cav-1 overexpression significantly increased Orai1 expression and SOCE, especially in the presence of TNF-α.
These results demonstrate that caveolar expression and regulation of proteins such as Orai1 are important for [Ca2+]i regulation in human ASM cells and its modulation during inflammation.
Keywords: Asthma, caveolae, cytokine, inflammation, Orai1, stromal interaction molecule 1
Caveolae are flask-shaped plasma membrane invaginations expressing the scaffolding caveolin proteins-1 to -3 [1, 2], as well as a variety of proteins important in signal transduction [3]. In other tissue types, caveolae and caveolins have been shown to be critical in the coordination of signalling mechanisms at both the plasma membrane and internal structures, such as the sarco/endoplasmic reticulum [4, 5]. We and others have demonstrated that human airway smooth muscle (ASM) cells express caveolin-1 (Cav-1), and that ASM caveolae contain a number of proteins involved in regulation of intracellular Ca2+ ([Ca2+]i) [6–8]. However, the role of caveolae in communication between plasma membrane and intracellular structures has not been explored in ASM.
In ASM, [Ca2+]i regulation involves both Ca2+ influx and sarcoplasmic reticulum (SR) Ca2+ release and reuptake [6, 9–11]. We and others have demonstrated that following SR Ca2+ depletion (e.g. via agonist stimulation), store-operated Ca2+ entry (SOCE) for SR Ca2+ refilling occurs in ASM [12–14]. Much previous work (including our own) had focused on the role of transient receptor potential (TRP) channels in regulation of SOCE [12, 15–17]. However, more recent evidence highlights the role of two proteins: Orai1 and stromal interaction molecule (STIM)1 [13, 14, 18–20]. Orai1 is thought to be the pore-forming subunit of Ca2+ release-activated Ca2+ channels [21], whereas STIM1 is thought to be localised to the SR (with some plasma membrane expression) and acts as a sensor for SR Ca2+ store status [22]. By sensing [Ca2+]i and intraluminal SR Ca2+ levels, STIM1 interacts with plasma membrane Orai proteins in complex and dynamic ways that are still being explored [22–24]. To date, there are very few studies exploring these novel proteins and their importance for SOCE in ASM [13, 14, 20]. Nonetheless, the plasma membrane localisation of Orai1 versus the SR location of STIM1 raises the question as to whether caveolae facilitate interactions between these molecules and thus contribute to SOCE. This has been suggested by a few studies [6, 25, 26], but has not been specifically examined.
The significance of caveolar regulation of SOCE lies in the role of the latter in enhanced airway contractility with inflammation. We have previously shown that the pro-inflammatory cytokine tumour necrosis factor (TNF)-α increases SOCE in human ASM [15]. Separately, in a recent study, we showed that TNF-α also increases ASM contractility [8]; however, the underlying mechanisms that link caveolae, [Ca2+]i and TNF-α are not known. In the present study, we hypothesised that Cav-1 interacts with Orai1 and STIM1 to regulate SOCE, and in the presence of TNF-α, caveolar regulation of SOCE contributes to increased [Ca2+]i.
MATERIALS AND METHODS
Materials
Chemicals and supplies were obtained from Sigma (St Louis, MO, USA), unless otherwise stated. Tissue culture reagents, including Dulbecco’s modified Eagle’s medium F-12 (DMEM F12) and fetal bovine serum (FBS), were obtained from Invitrogen (Carlsbad, CA, USA). Cav-1-specific caveolin scaffolding domain peptide (CSD) was obtained from Enzo Life Sciences Intl (Plymouth Meeting, PA, USA). Negative CSD was obtained from Calbiochem (La Jolla, CA, USA).
Isolation of human ASM cells
The techniques for isolation of human ASM cells have been previously published [12]. Briefly, third to sixth generation human bronchi were obtained from surgical lung specimens (exempt and not human subjects research acording to the Mayo Clinic Institutional Review Board). ASM layer was dissected and cells enzymatically dissociated (Worthington Biochemical, Lakewood, NJ, USA), resuspended and seeded into tissue culture flasks or Petri dishes. Cells were maintained for a maximum of two subcultures (maintaining ASM phenotype) at 37°C (5% carbon dioxide and 95% air) in DMEM F12 supplemented with 10% FBS. Experiments were performed in cells serum-deprived for 48 h.
Subcellular fractionation
ASM cells at confluence were treated (based on the protocol) and homogenised by sonication (three times, each for 5 s) in sucrose buffer containing 0.25 M sucrose, 20 mM Tris-HCl (pH 7.2) and 10 µg·mL−1 leupeptin. The resulting homogenate was centrifuged at 2,000×g for 10 min to remove cellular debris. The supernatant was centrifuged at 7,000×g for 15 min, then again at 20,000×g for 20 min, and the resultant plasma membrane pellet resuspended in sucrose buffer, while the remaining supernatant was further centrifuged at 100,000×g for 60 min to obtain the microsomal pellet. The final supernatant was saved as the cytoplasmic fraction.
Preparation of ASM caveolar membranes
Caveolin-enriched membrane fractions were prepared from human ASM cells as previously described [6]. Briefly, ASM cells were homogenised in cold buffer A (0.25 M sucrose, 1 mM EDTA and 20 mM Tricine, pH 7.8) and layered on a 30% Percoll gradient. A series of centrifugations, sonications and resuspensions was used to obtain caveolae-enriched fractions (purity confirmed by Western blot analysis for caveolins, with absence of SR or mitochondrial proteins).
Small interfence RNA transfection
Knockdown of proteins including Cav-1 by Small interfence RNA (siRNA) has also been previously described [6, 8]. Transfection was achieved using 20 nM siRNA and Lipofectamine 2000 (Invitrogen) in DMEM F-12 lacking FBS and antibiotics. Fresh growth medium was added after 6 h and cells analysed after 48 h. An 18-basepair Cav-1 siRNA duplex (Dharmacon, Lafayette, CO, USA), as previously described [6], a 21-basepair STIM1 siRNA duplex (GCC UAU AUC CAG AAC CGU Utt) and a 21-basepair Orai1 siRNA duplex (Ambion, Austin, TX, USA) targeting human FLJ14466 mRNA (GCA ACG UGC ACA AUC UCA Att) were used. As a negative control, the Silencer Negative Control #1 (Ambion) was used. Knockdown efficacy and specificity was verified by Western blot analysis.
Cav-1 overexpression
An mRed-tagged Cav-1 construct (generously provided by R. Pagano, Mayo Clinic, Rochester, MN, USA) was transfected into serum-free ASM cells for 24 h. Following transfection, DMEM F-12 media with 10% FBS was added for 24 h, and then withdrawn for 48 h prior to further experimentation. Expression of Cav-1-mRed was verified by mRed fluorescence within cells, specifically at the plasma membrane.
Green fluorescent protein STIM1 transfection
Full-length STIM1 mRNA was generated using RT-PCR and primers designed from published sequences that include endogenous translation start and stop codons. The PCR product was agar purified to eliminate nonspecific PCR product, then cloned into the TA cloning vector pCR4-TOPO (Invitrogen) and transformed into bacteria. Full length mRNA was cloned into the Acc I and Sac I restriction sites of the pAcGFP-N1 expression vector. Correct DNA insertion was confirmed by sequencing. ASM cells were transfected using 0.8 µg·mL−1 green fluorescent protein (GFP)-STIM1 with Lipofectamine.
Western blot analysis
Standard techniques based on SDS-PAGE (Criterion Gel System; Bio-Rad, Hercules, CA, USA; either 15% or 4–15% gradient gels) with transfer to polyvinylidene fluoride membranes (Bio-Rad) were used. Membranes were blocked for 1 h with 5% milk in Tris-buffered saline containing 0.1% Tween (TBST) and then incubated overnight at 4°C with anti-STIM1 (1:1,000; Abcam, Cambridge, MA, USA), Orai1 (1:500; Alomone Labs, Jerusalem, Israel) or Cav-1 (1: 1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and α-actin (1:5,000; Sigma) antibodies. Following three washes with TBST, primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies and signals developed by Supersignal West Pico Chemiluminescent Substrate (Pierce Chemical Co., Rockford, IL, USA) and densitometry quantification using a Kodak ImageStation 4000 mm (Carestream Health, New Haven, CT, USA).
TNF-α exposure
Cells were exposed to 20 ng·mL−1 recombinant human TNF-α (R&D Systems, Minneapolis, MN, USA) for 48 h.
[Ca2+]i imaging
We have published extensively on [Ca2+]i imaging of ASM cells [6, 12]. Briefly, human ASM cells were incubated in 5 µM fura-2/AM (Invitrogen) for 60 min and visualised using an epifluorescence imaging system (MetaFluor; Universal Imaging, Downingtown, PA, USA; Nikon Diaphot inverted microscope (Nikon Instrument Inc., Melville, NY, USA); 40×/1.3 NA oil-immersion lens; 1 Hz acquisition of 512×512 images via a 12-bit Roper Scientific CCD camera (Roper Scientific, Surrey, BC, Canada)). [Ca2+]i responses of 20–30 cells per field were obtained using individual software-defined regions of interest. Fura-2 fluorescence was calibrated for [Ca2+]i using previously described procedures [6].
Store-operated Ca2+ influx
Previously described techniques were utilised for examining SOCE [6, 12, 15]. Briefly, in the absence of extracellular Ca2+ (0 mM Ca2+ Hank’s balanced salt solution containing 1 µM nifedipine and 10 mM KCl), the SR was passively depleted using 10 µM cyclopiazonic acid (CPA) for 5 min, after which 2.5 mM extracellular Ca2+ was rapidly re-introduced (in the continued presence of CPA, nifedipine and KCl), and the observed [Ca2+]i response measured.
Confocal immunofluorescence microscopy
ASM grown on four-chambered Lab-Teks (Nalgene Nunc International, Rochester, NY, USA) were fixed in 2% paraformaldehyde for 15 min. Cells were then washed in 0.1 M tris-buffered saline (TBS), permeabilised with 0.1% Triton-X in TBS for 15 s, washed in TBS and blocked for 60 min in 4% normal donkey serum. Samples were then incubated overnight at 4°C in TBS only (unstained control) or 1 µg·mL−1 of primary anti-Cav-1 (Santa Cruz Biotechnology), anti-STIM1 (Abcam) or anti-Orai1 (Santa Cruz Biotechnology) antibodies. Following washes in TBS, samples were incubated for 1 h in Alexa 488 conjugated donkey anti-mouse (1:200; Invitrogen) or Cy3-conjugated donkey anti-rabbit secondary antibodies (1:200; Millipore, Billerica, MA, USA). Cells incubated with secondary antibodies only served as staining controls. Labelled cells were visualised using a 40X/1.3 or 100X/1.3 oil immersion objective lens on a Nikon Eclipse microscope and a Nikon C2 laser scanning confocal system.
Statistical analysis
Bronchial samples from at least five patients were used to obtain ASM cells, with biochemistry and molecular biology protocols being repeated a minimum of three times. [Ca2+]i experiments were performed in ≥20 cells from each bronchial sample. siRNA/mRed/TNF-α effects on [Ca2+]i responses were compared across sets of cells using two-way ANOVA with Bonferroni correction for repeated comparisons. Statistical significance was established at p<0.05. All values are expressed as mean±se.
RESULTS
Relative subcellular localisation of Cav-1, STIM1 and Orai1 in human ASM
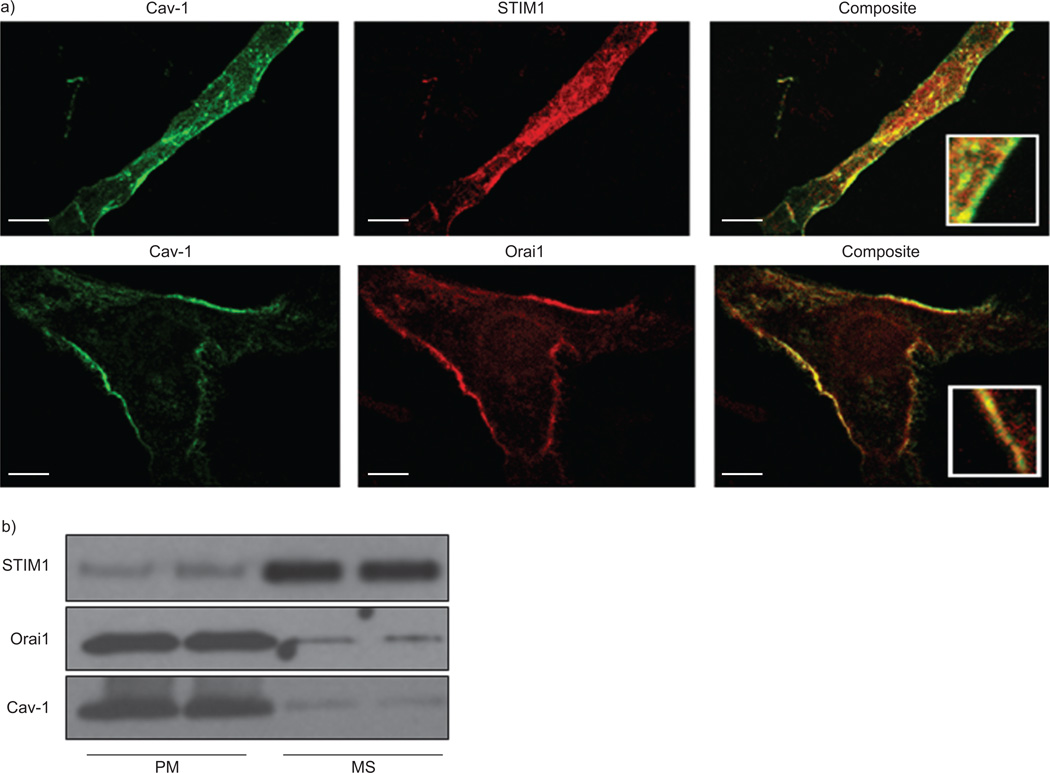
Immunostaining of human ASM cells demonstrated substantial co-localisation of Cav-1 and Orai1, which were primarily expressed within the plasma membrane while STIM1 was expressed within the cytoplasm at the level of the sarcoplasmic reticulum (fig. 1a). There was some level of STIM1 expression also noted near the plasma membrane, albeit to a much smaller extent than intracellular localisation. These results were confirmed by Western blot analysis of cell fractions (fig. 1b). Cav-1 and Orai1 were expressed in plasma membrane fractions, whereas STIM1 was largely expressed in the heavy microsomal fraction.
FIGURE 1.
Localisation of caveolin (Cav)-1, stromal interaction molecule (STIM)1 and Orai1 in human airway smooth muscle (ASM) cells. a) Immunocytochemical staining of human ASM cells and confocal microscopy demonstrated expression of both Cav-1 and Orai1 within the plasma membrane (top row; inset in composite panel magnified 10×). STIM1 was primarily expressed in the intracellular compartments (bottom row; inset in composite panel magnified 10×). Cav-1 secondary stain was Alexa488 (green), while STIM1 or Orai1 were visualised using Alexa568 (red). Composite represents overlap of green and red images from same cell. b) Cav-1, STIM1 and Orai1 expression were also determined in isolated plasma membrane (PM) and microsomal (MS) fractions from ASM cells. Cellular fractions confirmed that Cav-1 and Orai1 were highly expressed in PM fractions, while STIM1 expression was limited to the heavy MS fraction. Scale bar: 10 µm.
Effects of overexpression of Cav-1 and TNF-α
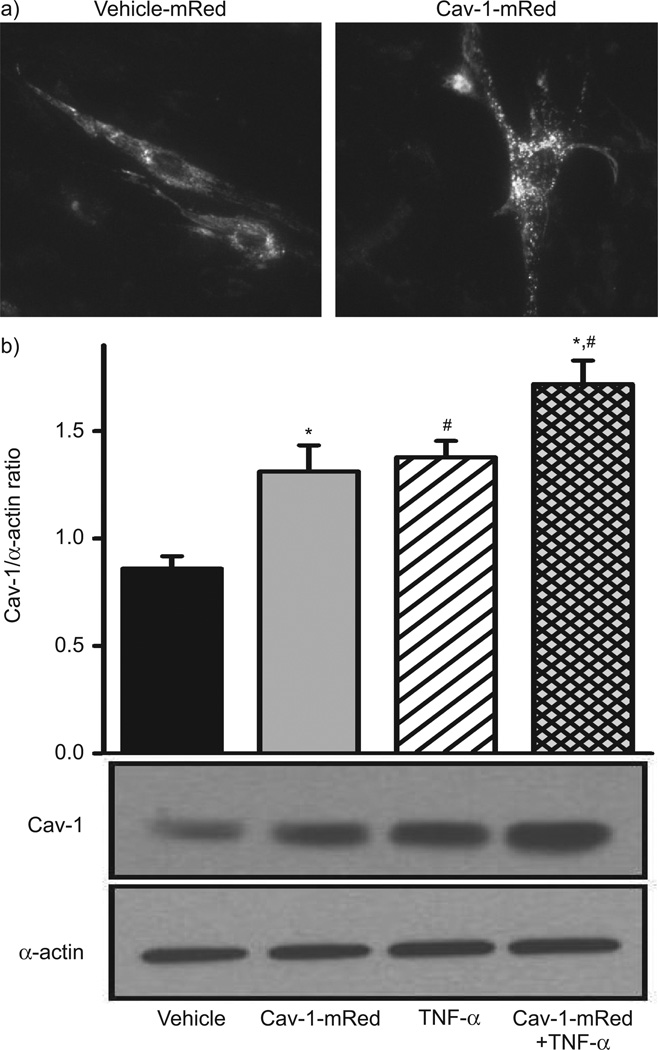
Overexpression of Cav-1 using a Cav-1-mRed construct was confirmed by fluorescence imaging, which showed enhanced fluorescence at the plasma membrane within 24 h (fig. 2a). Exposure to TNF-α not only accelerated the expression of Cav-1-mRed within Golgi but also increased its plasma membrane expression (not shown). Western blot analysis confirmed that transfection with Cav-1-mRed significantly increased Cav-1 expression compared with vehicle (control-mRed transfection). Exposure to TNF-α also significantly increased Cav-1 expression. This effect was even further enhanced when cells overexpressing Cav-1 were exposed to TNF-α (fig. 2b).
FIGURE 2.
Effect of caveolin (Cav)-1 overexpression and tumour necrosis factor (TNF)-α on human airway smooth muscle (ASM) cells. a) Confocal fluorescence imaging demonstrated that overexpression of Cav-1 using Cav-1-mRed significantly increased Cav-1 expression compared with vehicle (control-mRed transfection). b) Western blots analysis showed that exposure to TNF-α increased Cav-1 (as shown before). This effect was further enhanced in the presence of Cav-1-mRed. Data are presented as mean±se. *: significant Cav-1-mRed effect; #: significant TNF-α effect (all p<0.05).
Effect of altered Cav-1 expression on TNF-α-induced enhancement of SOCE
We have previously reported that transfection of human ASM cells with Cav-1 siRNA results in ~75% reduction in Cav-1 levels [6]. We verified a similar extent of reduction in this set of studies. In cells transfected with Cav-1 siRNA, SOCE was significantly decreased when compared with vehicle control or nonspecific siRNA (p<0.05; fig. 3). Exposure to 20 ng·mL−1 of TNF-α for 48 h significantly increased SOCE (p<0.05; fig. 3). This enhancing effect of TNF-α was significantly blunted in cells transfected with Cav-1 siRNA (p<0.05; fig. 3). Cells transfected with Cav-1-mRed showed significantly increased SOCE compared with nontransfected controls (fig. 3). As only ~40–50% of ASM cells showed high levels of Cav-1-mRed, in separate studies, using software-facilitated regions of interest, we compared SOCE in those cells against cells with background fluorescence (which may have overexpressed Cav-1, although not to a large extent). With either method of comparison, cells overexpressing Cav-1 showed significantly higher SOCE. Furthermore, addition of TNF-α to these Cav-1 overexpressing cells showed even further increased SOCE (p<0.05; fig. 3).
FIGURE 3.
Effect of the pro-inflammatory cytokine tumour necrosis factor (TNF)-α, caveolin (Cav)-1 small interference RNA (siRNA) and Cav-1 overexpression (Cav-1-mRed) on store-operated Ca2+ entry (SOCE) in human airway smooth muscle (ASM) cells. a) Representative intracellular Ca2+ ([Ca2+]i) tracings demonstrating SOCE in the different experimental groups. After removal of extracellular Ca2+, sarcoplasmic reticulum Ca2+ stores were depleted with cyclopiazonic acid (CPA). Subsequent rapid introduction of extracellular Ca2+ resulted in activation of SOCE (in the continued presence of CPA). b) Overnight exposure to TNF-α significantly increased SOCE in comparison with vehicle control. In cells transfected with Cav-1 siRNA, SOCE was significantly decreased compared with vehicle control and TNF-α. Overexpression of Cav-1 using Cav-1-mRed significantly increased SOCE compared with vehicle control. Exposure to TNF-α further enhanced SOCE in the presence of Cav-1-mRed. Data are presented as mean±se. *: significant TNF-α effect; #: significant siRNA effect; ¶: significant Cav-1-mRed effect (all p<0.05).
Effect of TNF-α and Cav-1 on STIM1 and Orai1 expression
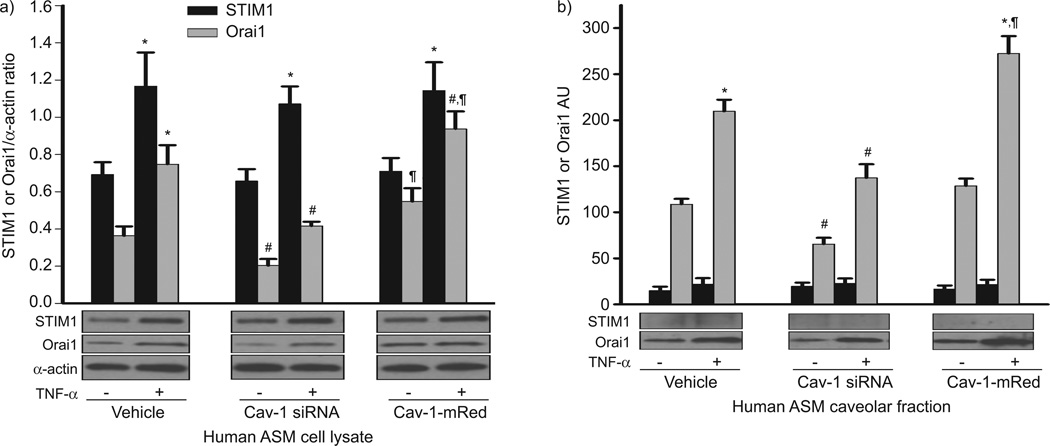
Considering the role of STIM1 and Orai1 in SOCE, we determined whether the mechanism of TNF-α-induced enhancement of SOCE involved increased expression of these proteins. Indeed, exposure to TNF-α significantly increased STIM1 and Orai1 in whole cell lysates of human ASM cells (fig. 4a; p<0.05). With the understanding that Orai1 is plasma membrane bound, we determined whether caveolae played a role. Indeed, suppression of Cav-1 using siRNA significantly reduced Orai1 expression under control conditions as well as in TNF-α-exposed cells (fig. 4a; p<0.05). In separate experiments, we found that nonspecific chelation of plasma membrane cholesterol using 10 mM methyl-β-cyclodextrin (which should reduce caveolar numbers and other lipid rafts) also reduced Orai1 expression (not shown). Overexpression of Cav-1 using Cav-1-mRed significantly increased Orai1 expression, which was further enhanced in the presence of TNF-α (fig. 4a; p<0.05). Interestingly, STIM1 expression with or without the presence of TNF-α was not affected by Cav-1 siRNA or Cav-1-mRed (fig. 4a), suggesting the lack of plasma membrane or caveolar STIM1. Indeed, in a separate set of experiments we analysed caveolar fractions from ASM cells under the same experimental conditions and found that Cav-1 siRNA significantly decreased caveolar Orai1 expression while, conversely, Cav-1 overexpression increased Orai1 expression. Exposure to TNF-α also increased Orai1 expression, an effect further enhanced when Cav-1 was overexpressed (fig. 4b; p<0.05). However, there was negligible expression of STIM1 within caveolar membrane fractions, with no significant changes following TNF-α or Cav-1 siRNA or overexpression (fig. 4b).
FIGURE 4.
Effect of tumour necrosis factor (TNF)-α and caveolin (Cav)-1 on stromal interaction molecule (STIM)1 and Orai1 expression in human airway smooth muscle (ASM) cells. a) Exposure to TNF-α increased both STIM1 and Orai1 expression in ASM cell lysates. In Cav-1 small interference RNA (siRNA)-transfected cells, Orai1 expression significantly decreased in both in cell lysate and caveolar fractions (a and b). In Cav-1-mRed-transfected cells, Orai1 expression significantly increased in both in cell lysate and caveolar fractions in the presence of TNF-α. Interestingly, STIM1 expression was very low within caveolar membrane fractions in comparison to Orai1 and did not show significant changes (b). Data are presented as mean±se. *: significant TNF-α effect; #: significant Cav-1 siRNA effect; ¶: significant Cav-1-mRed effect (all p<0.05).
Relationships between Cav-1, STIM1 and Orai1
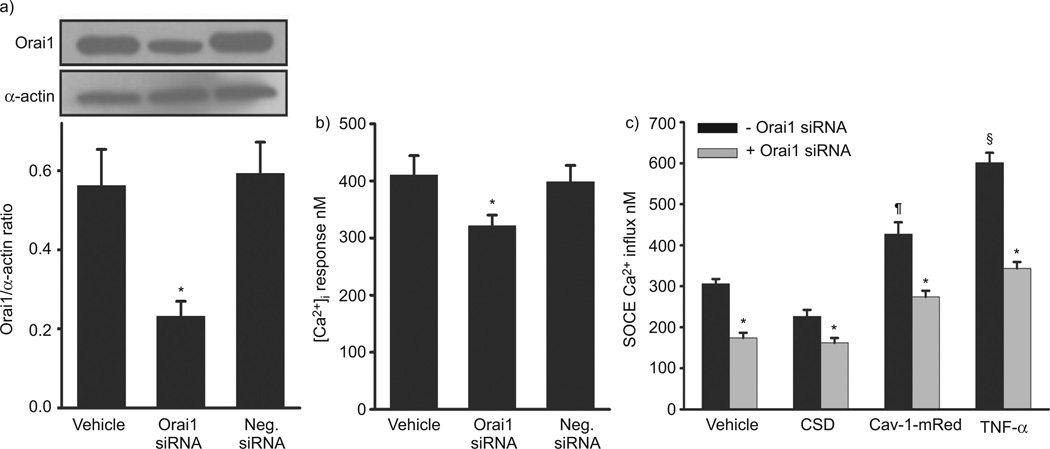
Transfection of human ASM cells with Orai1 siRNA significantly lowered the expression of this protein when compared with both vehicle control and negative siRNA (fig. 5a; p<0.05), and significantly blunted SOCE (fig. 5c; p<0.05). In a separate set of cells, fura-2-loaded human ASM cells exposed to 10 µM histamine demonstrated a typical biphasic [Ca2+]i response with an initial higher peak followed by a lower plateau level (fig. 5b). In cells transfected with Orai1 siRNA, [Ca2+]i responses to histamine were significantly decreased when compared with control and negative control cells (fig. 5b; p<0.05). As Orai1 was substantially expressed within caveolae, additional suppression of Cav-1 via siRNA was considered a futile experiment. Accordingly, to demonstrate the relationship between Cav-1 and Orai1, control and Orai1 siRNA-transfected ASM cells were exposed to 5 µM Cav-1 inhibitor peptide (CSD; 6 h), which inhibits the function of Cav-1 without altering its expression [8, 27]. In normal cells, SOCE was significantly reduced by CSD (fig. 5c), functionally linking Cav-1 to SOCE. Combination of Orai1 siRNA with CSD inhibition of Cav-1 further reduced SOCE (fig. 5c; p<0.05), further linking Orai1, Cav-1 and SOCE. Additional quality control studies performed using a negative CSD found no significant difference from vehicle control (data not shown). Furthermore, we studied SOCE in ASM cells transfected with both Cav-1-mRed and Orai1 siRNA. Overexpression of Cav-1 increased SOCE, while Orai1 siRNA significantly reduced this enhancing effect of Cav-1-mRed on SOCE. To determine the role of caveolar Orai1 in TNF-α-induced enhancement of SOCE, we evaluated SOCE in ASM cells transfected with Orai1 siRNA and then exposed to either vehicle or TNF-α. Orai1 siRNA significantly reduced TNF-α-induced increase in SOCE (fig. 5c; p<0.05).
FIGURE 5.
Effect of Orai1 small interference RNA (siRNA) on intracellular Ca2+ ([Ca2+]i) and store-operated Ca2+ entry (SOCE) in human airway smooth muscle (ASM) cells. a) Transfection efficiency of Orai1 using siRNA was verified by Western blot analysis. Smooth muscle α-actin served as loading control. b) Orai1 siRNA significantly reduced histamine-induced [Ca2+]i responses compared with vehicle and negative control. c) Orai1 siRNA significantly reduced SOCE compared with vehicle control. Exposure to 5 µM Cav-1-specific caveolin scaffolding domain peptide (CSD; 6 h) significantly reduced SOCE (confirming the Cav-1 siRNA effect on SOCE in figure 3). Combination of Orai1 siRNA and CSD also significantly reduced SOCE compared with vehicle control but not in comparison with Orai1 siRNA or CSD alone. Cav-1-mRed significantly increased SOCE, which was decreased by Orai1 siRNA. Orai1 siRNA significantly reduced SOCE in the presence and absence of tumour necrosis factor (TNF)-α, confirming the importance of Orai1 during airway inflammation. Data are presented as mean±se. *: significant Orai1 siRNA effect; #: significant CSD effect; ¶: significant Cav-1-mRed effect; §: significant TNF-α effect (all p<0.05).
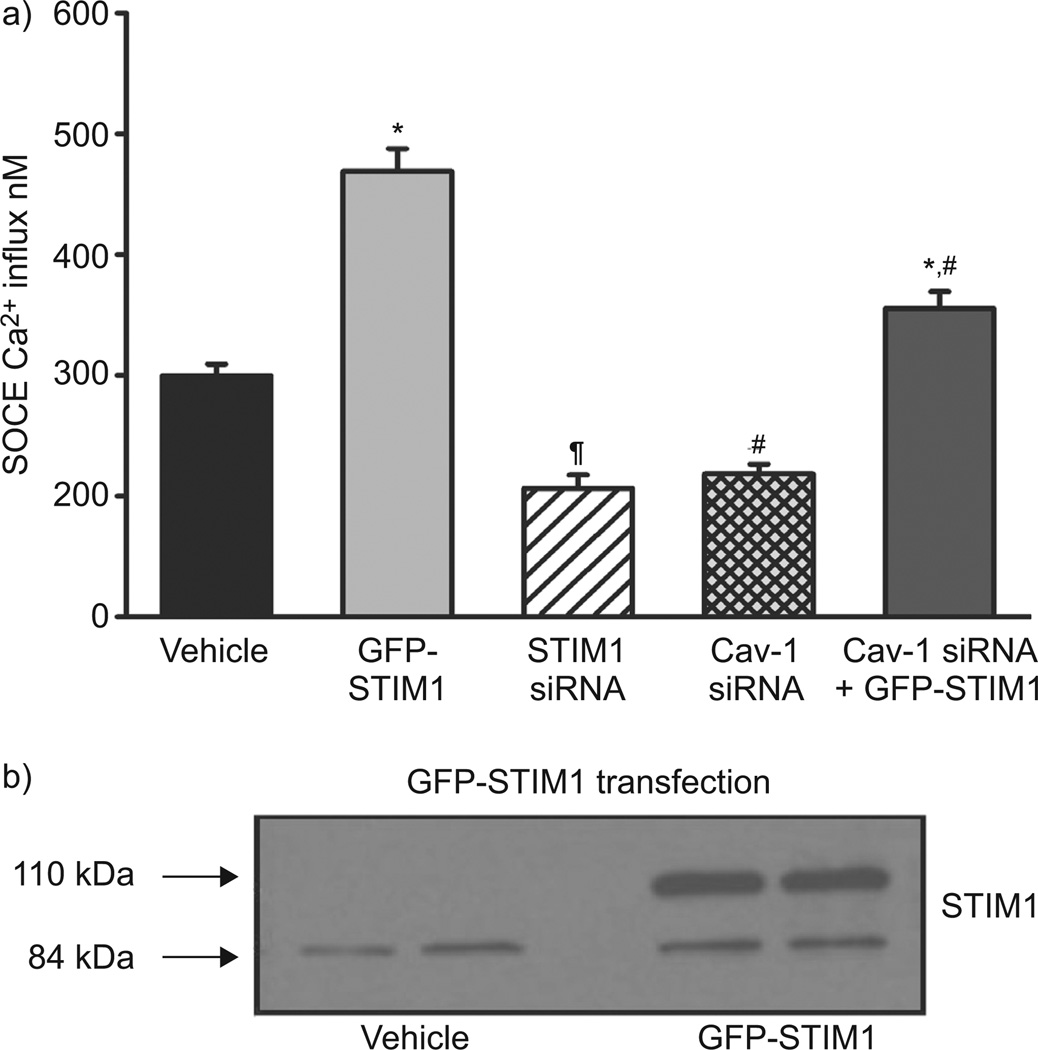
Considering the finding that caveolae express little STIM1, and knowing the individual roles of Cav-1 and STIM1 in SOCE, we investigated the functional relationship between Cav-1 and STIM1. In ASM cells transfected with GFP-STIM1, the expression of STIM1 was substantially increased (as expected and confirmed by Western blot; fig. 6b). STIM1 siRNA substantially reduced SOCE when compared with vehicle control, consistent with its known role in regulating SOCE. Conversely, STIM1 overexpression (GFP-STIM1) substantially enhanced SOCE (p<0.05 compared with control; fig. 6a). In such cells, Cav-1 siRNA significantly blunted GFP-STIM1-enhanced SOCE, albeit not to control levels (fig. 6a; p<0.05).
FIGURE 6.
Effect of stromal interaction molecule (STIM)1 overexpression and caveolin (Cav)-1 small interference RNA (siRNA) on store-operated Ca2+ entry (SOCE) in human airway smooth muscle (ASM) cells. a) Overexpression of STIM1 using green fluorescent protein (GFP)-STIM1 significantly increased SOCE when compared with vehicle control (transfection control). STIM1 siRNA significantly reduced SOCE when compared with vehicle control. Cav-1 siRNA transfection partially reduced the GFP-STIM1-mediated increase in SOCE. b) GFP-STIM1 transfection significantly increased STIM1 expression confirmed by Western blot analysis. Data are presented as mean±se. *: significant GFP-STIM1 effect; #: significant Cav-1 siRNA effect; ¶: significant STIM1 siRNA effect (all p<0.05).
DISCUSSION
There is growing evidence that caveolae contain a variety of proteins that play an important role in [Ca2+]i signalling [1, 2]. By virtue of their shape, caveolae could facilitate interactions between plasma membrane proteins and intracellular organelles. The present study provides evidence that caveolae and specifically Cav-1 facilitate such interactions between the plasma membrane protein Orai1 and the SR protein STIM1 in enhancing Ca2+ influx following depletion of SR Ca2+ stores (i.e. SOCE). Furthermore, we have demonstrated that enhanced [Ca2+]i by pro-inflammatory cytokines such as TNF-α involves SOCE and the proteins Orai1 and STIM1, with a particular role for caveolar Orai1. These findings emphasise the role of caveolae and Cav-1 in regulation of airway contractility and its enhancement in the presence of inflammation. Here, caveolae appear to be important in the communication between intracellular structures, such as the sarcoplasmic reticulum, and STIM1 versus plasma membrane [Ca2+]i regulatory mechanisms, such as SOCE, mediated by Orai1.
Caveolae and SOCE
Caveolae are specialised forms of lipid rafts in the plasma membrane of most cells. They form dynamic assemblies of sphingolipids and cholesterol containing scaffolding domains with different affinities for proteins resulting in a variety of functions [1]. The constitutive Cav-1 protein is distributed ubiquitously, while Cav-2 is usually associated with Cav-1 [3, 28]. Although Cav-3 is thought to be “muscle specific” and is expressed in striated muscles [3, 28, 29], we and others have found that Cav-3 is not expressed within human ASM [6, 30]. Recent studies have established that ASM caveolae contain a number of proteins important to [Ca2+]i regulation (e.g. agonist receptors, Ca2+ influx channels, and force regulatory proteins such as RhoA). In canine ASM, it has been established that caveolar-enriched membrane fractions express Cav-1, L-type Ca2+ channels and plasma membrane Ca2+ ATPase, but not SR proteins such as IP3R or RyR channels [31].
In ASM of different species, in addition to Ca2+ influx through voltage-gated or receptor-operated channels, influx occurs in response to SR Ca2+ depletion. For example, in human ASM, we found that SOCE is triggered by SR Ca2+ release via both IP3R and RyR channels [12, 15]. In this regard, and consistent with previous interests in the role of transient receptor potential channel (TRPC) channels as being key to SOCE, we have also demonstrated a role for TRPC channels in SOCE of ASM [15]. Here, ASM does express several TRPC isoforms, including TRPC1, TRPC3, TRPC4 and TRPC6, with siRNA suppression of TRPC3 and TRPC6 (for example) decreasing SOCE [6, 15]. Furthermore, Cav-1 co-localises with TRPC channels [6] and is important for SOCE regulation in that depletion of lipid rafts with cyclodextrins or suppression of Cav-1 using siRNA decreases SOCE [6, 32].
While this previous work [6, 32] linked Cav-1 to SOCE, the linking mechanism had been thought to be TRPC channels. However, the issue of plasma membrane or caveolar interaction with the SR remained. Here, there has been much recent research on STIM1 and Orai1 as being key to communication between the plasma membrane and SR in triggering SOCE [33]. With regard to the mechanism that senses and triggers SOCE, two models have been proposed: 1) the insertional model, in which STIM1 translocates to the plasma membrane during SR Ca2+ depletion [23]; and 2) the interactional model, where STIM1 is entirely at the level of the SR (although some may be expressed within the plasma membrane) but aggregates and forms clusters with junctional SR following depletion to then activate SOCE channels in the plasma membrane [34, 35]. At this juncture, some studies suggest that Orai1 modulates SOCE (potentially occurring through TRPC channels) [36], while others suggest that Orai1 itself forms the channel that mediates SOCE [23]. Regardless of which mechanism is involved in SOCE, in an interactional role of STIM1, caveolae and Cav-1 would be ideal candidates to serve as a home for the plasma membrane protein mediating/modulating SOCE, and (via caveolar invaginations) for further facilitating such interactions by increasing physical proximity of plasma membrane and SR.
There is now evidence (including data from our current study) that human ASM expresses STIM1 as well as Orai1, and that suppression of either protein decreases SOCE [13, 14]. What was not known is whether caveolae were involved. Our novel finding is that caveolae are important for STIM1–Orai1 interactions in human ASM SOCE, and that caveolar Orai1 is a key factor to SOCE, as evidenced by our siRNA and overexpression studies. This was further confirmed by the lack of additional inhibitory activity by CSD in the presence of Orai1 siRNA. However, what is not known is whether Orai1 is the actual SOCE channel, or whether Orai1 interacts with TRPC channels to modulate SOCE. Based on previous data on reduced SOCE with TRPC siRNA, and on new data showing Orai1 siRNA suppressing SOCE, we suggest that the latter is the case in human ASM. Accordingly, STIM1 aggregation could lead to interactions with caveolae which contain TRPC as well as Orai1, allowing for multiple levels of regulation. These findings are consistent with a report concerning the human submandibular gland [26], which shows that STIM1 clusters with TRPC1 channels and that Cav-1 is required for this interaction.
Caveolae, airway inflammation and SOCE
Inflammation in diseases such as asthma and chronic bronchitis contributes to enhanced bronchoconstriction. Bronchoconstriction results either from an increase in [Ca2+]i or enhanced Ca2+ sensitivity [8, 11]. The fact that overexpression of Cav-1 increases SOCE substantially demonstrates the importance of both in mediating and enhancing bronchoconstriction.
In the current study, we used the pro-inflammatory cytokine TNF-α, which is abundantly produced in the airway and known to be involved in airway inflammation [37]. TNF-α is a potent pro-inflammatory cytokine, which is found in bronchoalveolar lavage fluid and sputum from asthmatic patients and has been implicated as a mediator in the pathophysiology of asthma and COPD. We have previously shown that TNF-α increases SOCE in human ASM [15], an effect involving upregulated TRPC3 expression. Separately, we and others have demonstrated that lipid rafts and Cav-1 are essential for TNF-α-induced activation of signalling pathways associated with ASM contractility and cellular proliferation [8, 38]. Indeed, we recently showed that TNF-α increases Cav-1 expression in ASM [8]. Adding to our previous finding of Cav-1 being important for SOCE [6], these studies link TNF-α, Cav-1 and SOCE. However, in these studies, the assumption was that TRPC3 mediates this linkage. The results of the present study now demonstrate the importance of caveolar Orai1 in TNF-α-induced enhancement of SOCE. Interestingly, although TNF-α increased STIM1 expression in whole cell lysates, it did not affect its caveolar membrane expression, again highlighting the likely SR localisation of this protein in ASM and its role in SOCE regulation. While we previously found some caveolar expression of STIM1 (confirmed in the present study), its role is now less clear. The findings in the present study, which showed that altered STIM1 expression has very little role in caveolar or TNF-α regulation of SOCE, suggest a non-SOCE role for caveolar STIM1.
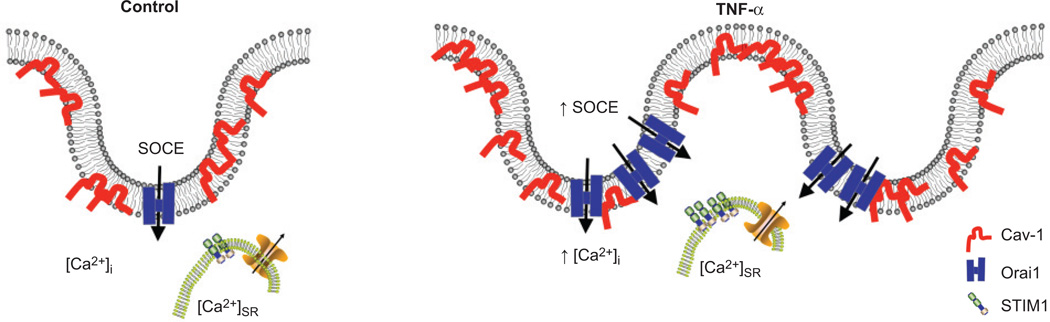
The present data confirm that Cav-1 plays an important role in [Ca2+]i signalling and its enhancement by TNF-α in ASM. The effects of Cav-1 can occur through two scenarios (fig. 7). First, TNF-α can increase the number of caveolae and with it the amount of Cav-1 and other proteins important for [Ca2+]i signalling (e.g. Orai1). With the increased expression of Orai1 and increased SOCE by TNF-α, there is now an environment for enhanced caveolae/Cav-1-mediated plasma membrane–SR interaction (fig. 7). The finding that Cav-1-mRed overexpression increased SOCE supports this model. Secondly, significant increases in the expression of the Ca2+-sensing protein STIM1 during TNF-α exposure, albeit at the level of the SR, could help to sustain refilling of the SR Ca2+. Here, the cytosolic levels of Ca2+ may remain high for a longer period (prolonging refilling) due to decreased sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) expression and activity [11].
FIGURE 7.
Schematic of tumour necrosis factor (TNF)-α effects on caveolae and store-operated Ca2+ entry (SOCE) components (stromal interaction molecule (STIM)1 and Orai1). Caveolae in airway smooth muscle (ASM) cell membranes express caveolin (Cav)-1 as well as major SOCE player Orai1. Sarcoplasmic reticulum (SR) contains STIM1 and other Ca2+ regulatory proteins. Exposure to TNF-α increases the expression of Cav-1 as well as Orai1, with more caveolae being formed or with more regulatory proteins within caveolae. These changes contribute to increased [Ca2+]i in ASM.
In conclusion, the present study demonstrates novel data that caveolae and specifically Cav-1 are important regulators of SOCE in human ASM by influencing plasma membrane SR interactions. While both Orai1 and STIM1 are important in TNF-α-induced enhancement of SOCE, caveolar Orai1 seems to be particularly important.
Acknowledgments
SUPPORT STATEMENT
This study was supported by National Institutes of Health (NIH) grants HL090595 (C.M. Pabelick), HL090595-S2 (C.M. Pabelick), HL088029 (Y.S. Prakash) and HL74309 (G.C. Sieck), and by grants from the Flight Attendant Medical Research Institute (FAMRI; V. Sathish, C.M. Pabelick and Y.S. Prakash).
Footnotes
STATEMENT OF INTEREST
None declared.
REFERENCES
- 1.Chidlow JH, Sessa WC. Caveolae, caveolins, and cavins. complex control of cellular signalling and inflammation. Cardiovasc Res. 2010;86:219–225. doi: 10.1093/cvr/cvq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosens R, Mutawe M, Martin S, et al. Caveolae and caveolins in the respiratory system. Curr Mol Med. 2008;8:741–753. doi: 10.2174/156652408786733720. [DOI] [PubMed] [Google Scholar]
- 3.Cohen AW, Hnasko R, Schubert W, et al. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- 4.Gratton JP, Bernatchez P, Sessa WC. Caveolae and caveolins in the cardiovascular system. Circ Res. 2004;94:1408–1417. doi: 10.1161/01.RES.0000129178.56294.17. [DOI] [PubMed] [Google Scholar]
- 5.Hardin CD, Vallejo J. Caveolins in vascular smooth muscle: form organizing function. Cardiovasc Res. 2006;69:808–815. doi: 10.1016/j.cardiores.2005.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prakash YS, Thompson MA, Vaa B, et al. Caveolins and intracellular calcium regulation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1118–L1126. doi: 10.1152/ajplung.00136.2007. [DOI] [PubMed] [Google Scholar]
- 7.Gosens R, Stelmack GL, Dueck G, et al. Caveolae facilitate muscarinic receptor-mediated intracellular Ca2+ mobilization and contraction in airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1406–L1418. doi: 10.1152/ajplung.00312.2007. [DOI] [PubMed] [Google Scholar]
- 8.Sathish V, Yang B, Meuchel L, et al. Caveolin-1 and force regulation in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2011;300:L920–L929. doi: 10.1152/ajplung.00322.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirota S, Helli P, Janssen LJ. Ionic mechanisms and Ca2+ handling in airway smooth muscle. Eur Respir J. 2007;30:114–133. doi: 10.1183/09031936.00147706. [DOI] [PubMed] [Google Scholar]
- 10.Pabelick CM, Sieck GC, Prakash YS. Significance of spatial and temporal heterogeneity of calcium transients in smooth muscle. J Appl Physiol. 2001;91:488–496. doi: 10.1152/jappl.2001.91.1.488. [DOI] [PubMed] [Google Scholar]
- 11.Sathish V, Thompson MA, Bailey JP, et al. Effect of proinflammatory cytokines on regulation of sarcoplasmic reticulum Ca2+ reuptake in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2009;297:L26–L34. doi: 10.1152/ajplung.00026.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ay B, Prakash YS, Pabelick CM, et al. Store-operated Ca2+ entry in porcine airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2004;286:L909–L917. doi: 10.1152/ajplung.00317.2003. [DOI] [PubMed] [Google Scholar]
- 13.Peel SE, Liu B, Hall IP. A key role for STIM1 in store operated calcium channel activation in airway smooth muscle. Respir Res. 2006;7:119. doi: 10.1186/1465-9921-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peel SE, Liu B, Hall IP. ORAI and store-operated calcium influx in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2008;38:744–749. doi: 10.1165/rcmb.2007-0395OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White TA, Xue A, Chini EN, et al. Role of transient receptor potential C3 in TNF-alpha-enhanced calcium influx in human airway myocytes. Am J Respir Cell Mol Biol. 2006;35:243–251. doi: 10.1165/rcmb.2006-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putney JW, Jr, Trebak M, Vazquez G, et al. Signalling mechanisms for TRPC3 channels. Novartis Found Symp. 2004;258:123–133. [PubMed] [Google Scholar]
- 17.Sweeney M, McDaniel SS, Platoshyn O, et al. Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J Appl Physiol. 2002;92:1594–1602. doi: 10.1152/japplphysiol.00722.2001. [DOI] [PubMed] [Google Scholar]
- 18.Deng X, Wang Y, Zhou Y, et al. STIM and Orai: dynamic inter-membrane coupling to control cellular calcium signals. J Biol Chem. 2009;284:22501–22505. doi: 10.1074/jbc.R109.018655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao Y, Erxleben C, Abramowitz J, et al. Functional interactions among Orai1, TRPCs, and STIM1 suggest a STIM-regulated heteromeric Orai/TRPC model for SOCE/Icrac channels. Proc Natl Acad Sci USA. 2008;105:2895–2900. doi: 10.1073/pnas.0712288105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou JJ, Gao YD, Geng S, et al. Role of STIM1/Orai1-mediated store-operated calcium entry in airway smooth muscle cell proliferation. J Appl Physiol. 2011;110:1256–1263. doi: 10.1152/japplphysiol.01124.2010. [DOI] [PubMed] [Google Scholar]
- 21.Prakriya M, Feske S, Gwack Y, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 22.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hewavitharana T, Deng X, Soboloff J, et al. Role of STIM and Orai proteins in the store-operated calcium signaling pathway. Cell Calcium. 2007;42:173–182. doi: 10.1016/j.ceca.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brazer SC, Singh BB, Liu X, et al. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem. 2003;278:27208–27215. doi: 10.1074/jbc.M301118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pani B, Ong HL, Brazer SC, et al. Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc Natl Acad Sci USA. 2009;106:20087–20092. doi: 10.1073/pnas.0905002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tourkina E, Richard M, Gooz P, et al. Antifibrotic properties of caveolin-1 scaffolding domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol. 2008;294:L843–L861. doi: 10.1152/ajplung.00295.2007. [DOI] [PubMed] [Google Scholar]
- 28.Scherer PE, Lewis RY, Volonte D, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J Biol Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- 29.Song KS, Scherer PE, Tang Z, et al. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J Biol Chem. 1996;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- 30.Gosens R, Stelmack GL, Dueck G, et al. Role of caveolin-1 in p42/p44 MAP kinase activation and proliferation of human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;291:L523–L534. doi: 10.1152/ajplung.00013.2006. [DOI] [PubMed] [Google Scholar]
- 31.Darby PJ, Kwan CY, Daniel EE. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca2+ handling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1226–L1235. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- 32.Sundivakkam PC, Kwiatek AM, Sharma TT, et al. Caveolin-1 scaffold domain interacts with TRPC1 and IP3R3 to regulate Ca2+ store release-induced Ca2+ entry in endothelial cells. Am J Physiol Cell Physiol. 2009;296:C403–C413. doi: 10.1152/ajpcell.00470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor CW. Store-operated Ca2+ entry: a STIMulating stOrai. Trends Biochem Sci. 2006;31:597–601. doi: 10.1016/j.tibs.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luik RM, Wang B, Prakriya M, et al. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454:538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao Y, Plummer NW, George MD, et al. A role for Orai in TRPC-mediated Ca2+ entry suggests that a TRPC:Orai complex may mediate store and receptor operated Ca2+ entry. Proc Natl Acad Sci USA. 2009;106:3202–3206. doi: 10.1073/pnas.0813346106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Erzurum SC. Inhibition of tumor necrosis factor alpha for refractory asthma. N Engl J Med. 2006;354:754–758. doi: 10.1056/NEJMe058266. [DOI] [PubMed] [Google Scholar]
- 38.Hunter I, Nixon GF. Spatial compartmentalization of TNFR1-dependent signaling pathways in human airway smooth muscle cells: lipid rafts are essential for TNF-alpha-mediated activation of RhoA but dispensable for the activation of the NF-kappa B and MAPK pathways. J Biol Chem. 2006;281:34705–34715. doi: 10.1074/jbc.M605738200. [DOI] [PMC free article] [PubMed] [Google Scholar]