Abstract
Background
Efficiently caring for frail, older adults will become an increasingly important part of healthcare reform; telemonitoring within homes may be an answer to improve outcomes. This study sought to determine the difference in hospitalizations and emergency room (ER) visits in older adults using telemonitoring versus usual care.
Methods
This was a randomized trial of adults older than 60 years with high-risk for rehospitalization. Subjects were randomized to telemonitoring with daily input versus patient-driven usual care. Telemonitoring was accomplished by daily biometrics, symptom reporting and videoconference. The primary outcome included a composite end-point of hospitalization and ER visits in the 12 months following enrollment. Secondary end-points included hospital days, hospital admissions, and ER visits. Intention to treat analysis was performed.
Results
Two hundred and five subjects were enrolled with a mean age of 80.3 years. There was no difference in hospitalizations and ER visits between the telemonitoring group (63.7%) and the group receiving usual care (57.3%) (P value 0.345). There was no difference in individual outcomes including hospital days, hospital admissions and ER visits. There also was no significant change between hospitalizations and ER visits in the pre-enrollment and post-enrollment period. Mortality was higher in the telemonitoring group (14.7%), compared to usual care (3.9%) (P value 0.008).
Conclusions
Among elderly patients, telemonitoring did not result in lower hospitalizations or ER visits. There were no differences determined within the secondary outcomes. The cause of the mortality difference is unknown.
Introduction
Increased life expectancy in developed countries 2, 3 has been a remarkable feat. Longer life expectancy challenges the healthcare system to optimally manage those elderly patients with a high-risk of hospitalization.4 Caring for patients in their homes could provide a cost-effective approach with telemonitoring of clinical symptoms and biometric findings. Telemonitoring systems now include monitoring biometric information and videoconferencing asynchronously as well as in real time and allow data to be transmitted easily from patient to provider. Many care organizations will focus on high-risk older adults with many chronic illnesses as a part of medical home initiatives. Home telemonitoring may reduce hospitalizations and ER visits in this population. This is important as hospitalization and ER visits may trigger functional decline in older adults5 or increase mortality.6 Home telemonitoring has been shown to reduce hospital admissions,7 ER visits, and hospital length of stay8 in a variety of different, individual chronic illnesses.9 A systematic review suggests a 20% reduction in hospitalization in patients with cardiovascular disease (primarily heart failure).10 However, the TELE-HF study did not show reductions in hospitalizations.11 In patients with multiple chronic conditions, there remains a lack of evidence supporting the efficacy of telemonitoring to prevent hospitalization or ER visits. To fill this gap, we conducted a randomized control trial comparing daily home telemonitoring (biometrics, videoconference, symptoms) to usual care in older adults at high-risk for hospitalization. We hypothesize that home telemonitoring will reduce hospitalizations and ER visits, as compared to usual care.
Methods
Study design
We conducted a multi-site, randomized controlled trial (RCT) in four sites within Mayo Clinic’s program of Employee and Community Health (ECH). Three of the sites were in Rochester, MN with one in rural Kasson, MN. Patients were randomized to either telemonitoring or usual care. We obtained approval from the Mayo Clinic Institutional Review Board (IRB) on Oct. 30, 2009. All patients provided written informed consent prior to enrollment and randomization.
There were no changes to the protocol or to group allocation after the initiation of the trial. Specifically, there were no changes to the sites of care or to the eligibility criteria.
Participants
Inclusion criteria
Patients were older than 60 years of age, in the ECH primary care panel, and had a high (>15) score on the Elder Risk Assessment Index (ERA). The ERA scored patients electronically for being at risk for hospitalization or ER visits based on administrative data for age, gender, previous hospitalizations, age, gender, and comorbid conditions (heart disease, diabetes, stroke, COPD and dementia). 12 Previous hospitalizations are weighted heavily in a standard fashion but were not required for a high score.12 Patients in the top 10% of the ERA scores in the ECH were identified as eligible for recruitment.
Exclusion criteria
Patients who lived in a nursing home, had a clinical diagnosis of dementia, or had a score of 29 or lower on the Kokmen Short Test of Mental Status were excluded from the study. Subjects who felt they could not use the home telemonitoring system (i.e. visual impairment, inability to use the device) were also excluded from the study.
Settings
Most residents older than 60 years of age in Olmsted County were female (55%) and white (>90%).13 ECH has a combined population of about 21, 000 patients over 60 years of age. Data collection occurred within the subject’s home or the clinical setting, depending on the comfort of the patient. The application of the instruments was applied in a standardized fashion.
Intervention - home telemonitoring
The detailed intervention of the home telemonitoring program was described in our previous protocol publication.1 We utilized the Intel® Health Guide, which is a Food and Drug Administration-approved device, in the patient’s home. The device had real time videoconference capability and peripheral devices (scales, blood pressure cuff, glucometer, pulse oximeter, and peak flow). Patients performed daily 5–10 minute monitoring sessions for symptoms and biometric information. The device worked asynchronously and data was downloaded to a web health site, which was then reviewed by the healthcare team daily including weekends and holidays. One RN oversaw approximately 100 subjects and communicated with the subject via phone or video conference if alerts arose. The nurse provided assessment of symptoms and communicated with the primary provider for treatment options if needed. The decisions for triage were made clinically by the RN with assistance from decision support from the EMR as needed. The participants were advised to call 911 for emergencies because the Intel® Health Guide was not a life-saving device.
Intervention - usual care
Subjects in the usual care intervention had access to primary and specialty office visits. Individuals routinely received post-hospital outpatient visits within a timely fashion and a nurse-generated phone call within 1 business day of hospital dismissal. Subjects also had access to phone nursing, urgent clinic visits, and the ER.
Data collection
Data was collected by the research team and maintained electronically. The investigators also maintained paper records of all information. Investigators and study team members were formally educated on the questionnaire and the examination instruments to ensure uniform application.
Outcomes
The primary endpoints centered upon the occurrence of hospitalization or ER visit within 12 months of enrollment. These outcomes were pre-specified in the original protocol. The ER and hospital utilization data was obtained using administrative billing resources. The secondary endpoints included hospitalization and ER visits as individual outcomes. As a secondary method of evaluation, we also compared total hospital days as an outcome measure. Mortality was also calculated from one year after enrollment and subjects were included in mortality evaluation if they dropped from the study. There were no changes in the determination of the outcomes after initiation of the study.
The baseline factors for all subjects included age, gender, mood, functional status, cognitive status, and quality of life. Each subject was administered a patient health questionnaire PHQ-9 to screen for depression14 and the Kokmen Short Test of Mental Status to assess memory loss.15 The SF-12, which measures quality of life and psychosocial factors,16 was administered to all subjects. Functional status was assessed by measuring grip strength with tonometry,17 the timed up-and-go test,18 and gait speed in meters per second.19 Activities of daily living were measured using the Barthel Index, which utilizes a self-reported questionnaire.20
Sample size
Using an alpha value of 0.05 and a power of 80%, the power calculations were derived from an estimated 76% event rate of hospitalizations and ER visits over 2 years in the high-risk group using ERA.12 Using 100 patients in both groups with a yearly hospitalization/ER rate of 38.2%, we were powered to detect a 36.1% decrease (from 38.2% to 24.4%) in combined outcomes.
Randomization and Blinding
There was block randomization using blocks depending on site. The block size was randomly determined using computer generated allocation as 2–4 individuals in size. Allocated randomization decisions (intervention/ usual care) were placed in sequentially numbered envelopes, depending on site. The randomization was created by statistical services, and study coordinators presented the randomization envelope to the participants after consent.
Given the requirements for the subjects to use the telemonitoring equipment it was not possible to blind the subjects or the study staff to the intervention. The analysis of the final results was performed in a blinded fashion.
Data analysis
All analysis was performed according to the original group using an intention-to-treat method. Wilcoxon rank sum tests, two-sample T tests, or chi-square analysis were used to compare baseline characteristics between the two groups. The primary endpoints of both combined and individual percentages of hospitalizations and ER visits were compared between the two groups using the chi-squared test. Statistical adjustment was planned only if there were statistical differences between the randomized groups on clinical variables. As a secondary method of analysis, the mean number of ER visits and hospitalizations were compared using the Wilcoxon rank sum test. Kaplan-Meier time-to-event analysis was conducted with a combined endpoint for mortality, hospitalization, and ER visits. All tests for significance used a two-sided alpha P value of 0.05. Analyses were done using SAS (SAS version 9.1 for Windows; SAS Institute Inc., Cary, North Carolina).
Results
Five hundred thirteen people were called with 234 visits scheduled for review of the study. Two hundred five people gave consent and were randomized. One hundred and three were assigned to the usual care group, and 102 comprised the telemonitoring group. Recruitment started in November, 2009 and ended in July, 2011. Twenty six (25.5%) of the subjects who received telemonitoring did not complete the trial (15 deaths and 11 withdrawals) compared to 12 subjects (11.73%) in the usual care (4 deaths and 8 withdrawals). The trial was stopped after achieving recruitment and time goals (Figure 1).
Figure 1.
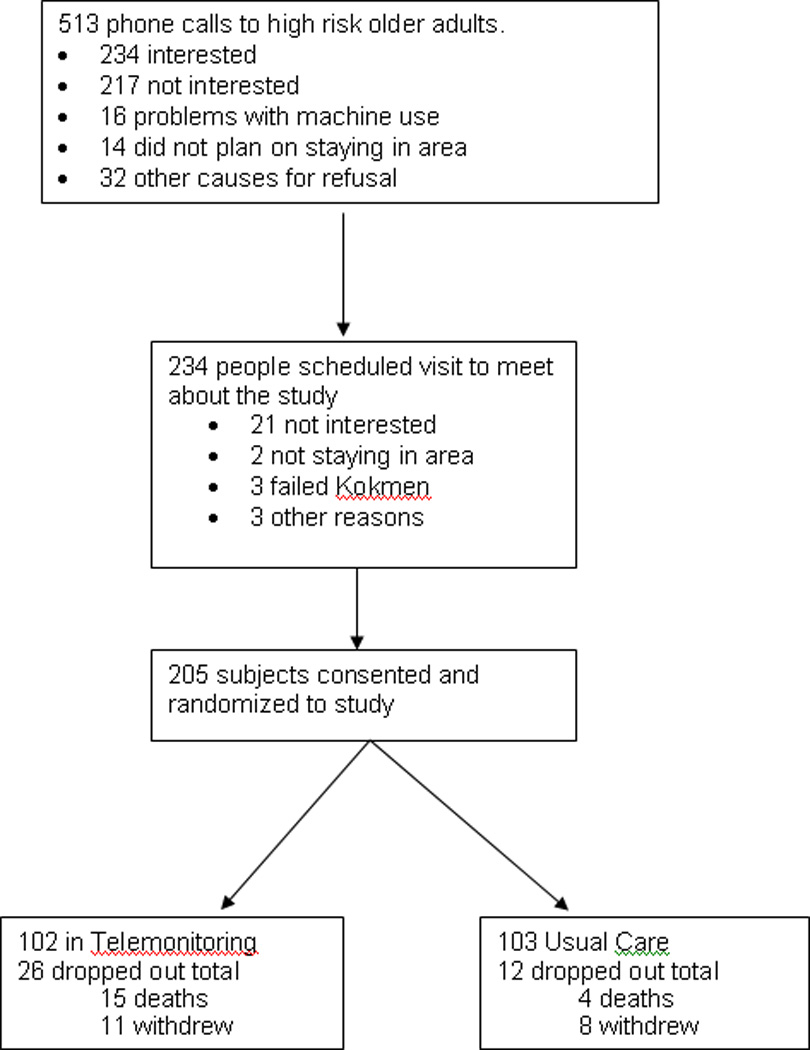
Trial recruitment. Failed Kokmen indicates the individual had a score of 29 or less on the short test of mental status by Kokmen et al.15
The baseline characteristics for both groups were not statistically different except for a slightly lower SF12 mental health composite score in the telemonitoring group (Table 1). The mean age was 80.3 ± 8.9 years in the telemonitoring group, and 80.2 ±7.6 years in the usual care group. The physical quality of life scores were similar, with an SF12 of 35.5 ±10.7 in telemonitoring and 34.7 ± 11.3 in usual care. The SF12 mental status scores of 54.8 ± 8.7 in telemonitoring group and 57.1 ± 7.1 in usual care group did show statistical significance (p=0.0345) with a better score in usual care. Self-reported health was similar in each group. The ERA scores were the same; however, they were lower than expected. With no clinical differences between groups, we made no statistical adjustments.
Table 1.
Baseline Characteristics of Overall Group and by Randomized Group for 205 Patients
| Characteristic | Total (n=205) |
Telemonitoring (n=102) |
Usual Care (n=103) |
P value |
|---|---|---|---|---|
| Age | 80.3 ± 8.2 | 80.3 ± 8.9 | 80.2 ± 7.6 | 0.9427 |
| Male, n(%) | 94 (45.9) | 50 (49.0) | 44 (42.7) | 0.3653 |
| Living Alone, n(%) | 95 (46.8) | 46 (46.0) | 49 (47.6) | 0.8223 |
| Charlson index | 2.9 ± 2.3 | 2.9 ± 2.3 | 3.0 ± 2.3 | 0.6903 |
| Body Mass Index | 29.4 ± 7.2 | 29.2 ± 7.3 | 29.6 ± 7.2 | 0.7207 |
| Blood Pressure (mm Hg) | ||||
| Systolic | 130.2 ± 19.0 | 131.0 ± 19.5 | 129.4 ± 18.5 | 0.5418 |
| Diastolic | 67.6 ± 10.8 | 68.2 ± 11.6 | 67.0 ± 9.9 | 0.4131 |
| Grip Strength | 18.5 ± 9.0 | 18.2 ± 8.6 | 18.8 ± 9.4 | 0.6649 |
| Time up and Go | 14.6 ± 12.0 | 13.3 ± 6.8 | 15.8 ± 15.4 | 0.1521 |
| Gait speed (m/sec) | 0.70 ± 0.36 | 0.70 ± 0.38 | 0.70 ± 0.35 | 0.9238 |
| ERA Score | 17.7 ± 5.8 | 17.8 ± 5.9 | 17.7 ± 5.6 | 0.8970 |
| Comorbidities, n(%) | ||||
| Myocardial infarction | 30 (14.6) | 15 (14.7) | 15 (14.6) | 0.9769 |
| CHF | 75 (36.6) | 40 (39.2) | 35 (34.0) | 0.4365 |
| COPD | 86 (42.0) | 45 (44.1) | 41 (39.8) | 0.5316 |
| Diabetes | 78 (38.1) | 39 (38.2) | 39 (37.9) | 0.9563 |
| Renal disease | 42 (20.5) | 16 (15.7) | 26 (25.2) | 0.0901 |
| Kokmen Mental status score | 34.5 ± 2.3 | 34.5 ± 2.2 | 34.4 ± 2.4 | 0.8550 |
| Barthel ADL | 94.4 ± 9.2 | 94.3 ± 9.7 | 94.6 ± 8.7 | 0.8161 |
| PHQ 9 score for depression | 3.7 ± 3.8 | 4.0 ± 3.8 | 3.4 ± 3.7 | 0.2463 |
| SF 12 physical | 35.1 ± 11.0 | 35.5 ± 10.7 | 34.7 ± 11.3 | 0.5853 |
| SF 12 mental | 55.9 ± 8.0 | 54.8 ± 8.7 | 57.1 ± 7.1 | 0.0345 |
| Chronic Condition Care Satisfaction | 3.6 ± 1.2 | 3.6 ± 1.1 | 3.6 ± 1.2 | 0.9345 |
| Feel on Chronic Condition Care | 2.1 ± 1.1 | 2.2 ± 1.0 | 2.0 ± 1.1 | 0.4518 |
| Very good/excellent health, n(%) | 81 (39.5) | 42 (41.2) | 39 (37.9) | 0.6276 |
Note: continuous variables reported as mean ± standard deviation
The primary outcome of the percentage of patients with either hospitalization or an ER visit was 63.7% in the telemonitoring group, compared to 57.3% in the usual care group resulting in a 6.4% increased risk of the combined outcome (P value=0.345) (Table 2). Considering each outcome separately did not reveal significant differences for hospitalizations, ER visits, number of ER visits, and hospital days. Mortality was different between the groups with 15 deaths (14.7%) in the telemonitoring group and 4 deaths (3.9%) in the usual care group (P value=0.008). The ERA scores at the end of study were not different with a score of 17.3 (SD 6.1) in the telemonitoring group and 16.3 (SD 5.5) in usual care (P value = 0.23). For adherence, of the first 11,212 scheduled visits, 9938 were completed telemonitoring visits (89%). For utilization, 3,942 phone calls were made to participants using the telemonitor. Additionally, there were no direct harms noted nor reported unanticipated problems involving risk to subjects or others (UPIRTSO).21 In a combined endpoint of time-to-event analysis of mortality, hospitalization, and ER visit, there was no difference noted between the telemonitoring group and the usual care group (P value=0.499) (Figure 2).
Table 2.
Hospitalizations and Emergency Room visits in Telemonitoring and Usual Care in 205 Patients
| Telemonitoring (n=102) |
Usual Care (n=103) |
P value | |
|---|---|---|---|
| Primary endpoints: | |||
| Hospitalization or ER visit, n(%) | 65 (63.7) | 59 (57.3) | 0.3454 |
| Hospitalization, n(%) | 53 (52.0) | 45 (43.7) | 0.2359 |
| ER visit, n(%) | 36 (35.3) | 29 (28.2) | 0.2721 |
| Secondary endpoints: | |||
| Death, n(%) | 15 (14.7) | 4 (3.9) | 0.0075 |
| Mean Number of ER visits per person | 0.71 ± 1.3 | 0.45 ± 0.83 | 0.2293 |
| Mean Number of days in hospital per person | 4.1 ± 8.1 | 6.1 ± 20.1 | 0.6055 |
| Mean Number of hospitalizations per person | 1.1 ± 1.7 | 0.83 ± 1.2 | 0.2751 |
| Number of hospitalizations, n(%) | 0.6338 | ||
| 0 | 49 (48.0) | 58 (56.3) | |
| 1 | 28 (27.5) | 23 (22.3) | |
| 2 | 13 (12.8) | 11 (10.7) | |
| 3 | 4 (3.9) | 5 (4.9) | |
| 4 | 4 (3.9) | 5 (4.9) | |
| ≥5 | 4 (3.9) | 1 (1.0) |
Note: continuous variables reported as mean ± standard deviation
Figure 2.
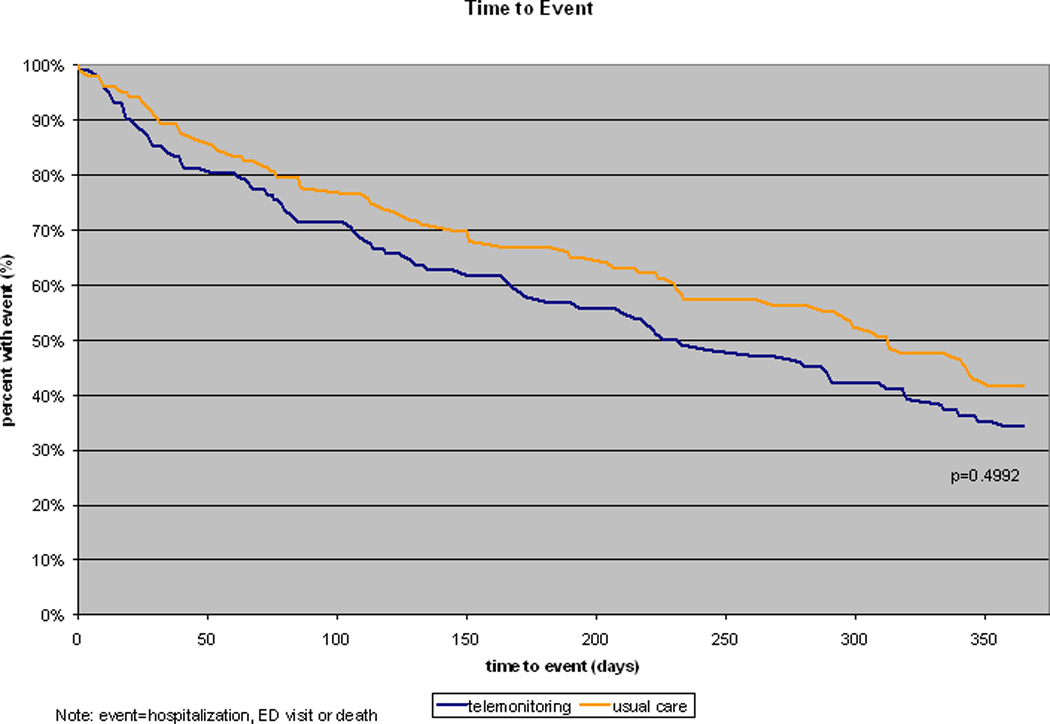
Combined end point for time-to-event analysis of mortality, hospitalizations, and emergency department (ED) visits.
The evaluation of the mean difference of pre-enrollment hospitalizations and ER visits as compared to post-enrollment hospitalizations and ER visits between the two groups was also not significant. The total number of outcomes for both ER visits and hospitalizations are noted in Table 3 for both usual care and telemonitoring. Table 4 reflects the comparison of ER visits and hospitalizations before and during the study.
Table 3.
Emergency Room and Hospitalizations before Study and During Study in 205 Patients
| Total (n=205) |
Telemonitoring (n=102) |
Usual Care (n=103) |
|
|---|---|---|---|
| Number of Emergency Room visits before study | 115 | 74 | 41 |
| Number of Emergency Room visits during study | 118 | 72 | 46 |
| Number of hospitalizations before study | 211 | 102 | 109 |
| Number of hospitalizations during study | 195 | 110 | 85 |
| Number of hospital days before study | 840 | 397 | 443 |
| Number of hospital days during study | 1051 | 420 | 631 |
Table 4.
Comparison of Emergency Room visits and Hospitalizations within a Group for 205 Patients
| Telemonitoring (n=102) | Usual Care (n=103) | |||||
|---|---|---|---|---|---|---|
| Before Study |
After Study |
P value | Before Study |
After Study |
P value | |
| Number (%) with ER visits | 34 (33.3) | 36 (35.3) | 0.2463 | 31 (30.1) | 29 (28.2) | 0.2779 |
| Mean Number of ER visits per person | 0.73 ± 1.5 | 0.71 ± 1.3 | 0.5853 | 0.40 ± 0.73 | 0.45 ± 0.83 | 0.6311 |
| Number (%) with hospitalizations | 46 (45.1) | 53 (52.0) | 0.0345 | 57 (55.3) | 45 (43.7) | 0.2159 |
| Mean Number of hospitalizations per person | 1.0 ± 1.5 | 1.1 ± 1.7 | 0.9345 | 1.1 ± 1.6 | 0.83 ± 1.2 | 0.1810 |
| Mean Hospitalization Days per person | 3.9 ± 7.5 | 4.1 ± 8.1 | 0.4518 | 4.3 ± 7.4 | 6.1 ± 20.1 | 0.3879 |
| Mean length of stay | 3.9 ± 3.4 | 3.8 ± 3.5 | 0.6276 | 4.1 ± 4.0 | 7.4 ±16.8 | 0.3679 |
Note: continuous variables reported as mean ± standard deviation
Comment
Telemonitoring is one potential method for using home case management to reduce ER visits and hospitalization. We did not find a difference between the intervention group (63.7%) and the usual control group (57.3%) in either hospital admissions or ER visits (P value=0.35). Previous studies using telemonitoring in mixed, chronic disease cases were promising, but also showed no improvement in outcomes. In a trial of 53 patients with CHF, COPD, or a chronic wound, there was a trend toward fewer re-hospitalizations in the telemonitoring group (15% versus 42% in usual care, P value of 0.055).22 An RCT of 104 patients with CHF, COPD, and/or diabetes demonstrated a reduction in bed days of care using telemonitoring versus control (1.88 vs. 5.11 beds/ 6 months P 0.0001).23 However, in a telemonitoring trial using the same telemonitor as in our study, there was no difference in hospital admissions or ER visits in heart failure patients using telemonitoring (44.5%), as compared to case management (40.1%).24 In this study, there was a small increase in events with potentially greater costs for support and equipment. This implies that investments in telemedicine may not provide better outcomes in its current delivery of case management.
The secondary aims in our study were also not significant with no difference found in hospitalization between the telemonitoring group (52.0%) and the usual care group (43.7%) (P value 0.24). We also found that the telemonitoring group used the ER 35.3% at least once compared to 28.2% in the usual care group (P value 0.27). In previous studies, there were borderline, non-significant differences in acute visits23 and rehospitalizations as individual outcomes.25 Consequently, the lack of significance in the primary and secondary hypotheses may reflect the lack of clinical infrastructure to process the information. It may be that frequent exposure to the RN resulted in more awareness of symptoms that generated higher ER or hospital use. Protocols can help guide specific illnesses; however, in patients with multiple illnesses, protocols may be more challenging. Further work in care management of these ill, complex adults will be required for success of telemonitoring.
Telemonitoring of individual diseases has shown mixed results in various trials. Although there are limitations in generalizing single disease trials like congestive heart failure (CHF), they do add valuable information to the existing evidence base for telemonitoring. The Home HF study resulted in fewer unexpected admissions for CHF.26 In a meta-analysis of CHF telemonitoring, there was no difference in all cause hospitalizations, but a reduction in CHF hospitalization did occur.27 The Tele-HF trial with 1653 subjects did not reveal differences in hospitalization with non-significant higher rates of admission and readmission in the telemonitoring intervention.11 In the telemonitoring with case management study, there was a lack of difference in ER visits and hospitalizations individually.24 Our study differs and adds to our knowledge as we apply telemonitoring to a group of patients with complex medical problems that will likely be part of the medical home. The findings from our TELE-ERA study provide further evidence relative to the lack of efficacy of telemonitoring impacting hospital stays and ER visits. Given the potential costs of telemonitoring and the lack of efficacy, it may be important for providers and funding organizations to evaluate which patients and which implementation strategy will be most useful.
We found a mortality increase in the telemonitoring group compared to usual care. Using the ERA risk stratification strategy, one expected a 2 year mortality rate of 22% with an extrapolated 13% mortality at one year.28 The 14.7% mortality in the telemonitoring group would be consistent with our previous experience. The mortality in the usual care group was 3.9% which was lower than expected. The difference in mortality between the two groups could be due to the lower than expected mortality in the usual care group or could represent higher mortality in the intervention group due to the increased access to healthcare which occurred with telemonitoring. Unnecessary tests, for example, could have resulted in increased mortality in the intervention group It is possible that the two groups differed by chance, or by another unseen predictor that was not recorded for each group; however, recorded variables were similar at baseline with the exception of the SF-12 mental status measure. The ERA scores were similar at entry into the study. Clinical care and access to medical care remained the same in both groups with the telemonitoring group having similar ER and hospital access.
To date, this novel study represents the largest RCT study of telemonitoring focusing on older adults with multiple illnesses The application of risk stratification reflects real world practice and will likely be used in medical homes. The model used mimics a clinical practice utilizing communication between the telemonitoring team and the primary care provider.1 Additionally, the trial reflects an effort to change clinical practices for primary care providers. However, there were limitations to the study. It was not practical to blind the providers or the patients receiving home equipment and monitoring. This could have led to the Hawthorne effect within the telemonitoring group resulting in bias which should favor an improved effect in the interventional group. The clinical outcomes were derived from Mayo Clinic billing records; thus, subjects receiving care outside of Mayo Clinic may not be recorded. It is also possible that the groups differed by an unmeasured quality such as socioeconomic status, education, transportation, caregiver and/or social support, which might have changed utilization.
The other limitations of the study involved generalizability. The population of Olmsted County is primarily Northern European, which limits the application of these findings. The subjects in usual care had access to a tertiary care hospital and some case management for treatment of heart failure and diabetes. These services would bias the results to show no difference between the groups. We did power the study at the upper end of the clinically reasonable range at 38.2% but saw the results go in a different direction.
Conclusion
In this unique study of 205 patients with multiple comorbid illnesses, there was no difference between combined hospitalization and ER visits for patients receiving telemonitoring as compared to those receiving usual care. The lack of efficacy of telemonitoring may be reflective of the number of patients in the trial, or may illustrate the lack of effective infrastructure needed to fully optimize case management.
Acknowledgments
This project was funded by Mayo Foundation Institutional Funds for clinical support. The Intel® Health Guides and support were provided by CareInnovations (GE/Intel). Other than receipt of this in-kind gift of use of the telemonitors, the authors declare no further funding support and no further competing interests. The project described was further supported by Grant Number 1 UL1 RR024150 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and the NIH Roadmap for Medical Research
References
- 1.Takahashi PY, Hanson GJ, Pecina JL, et al. A randomized controlled trial of telemonitoring in older adults with multiple chronic conditions: the Tele-ERA study. BMC Health Serv Res. 2010;10:255. doi: 10.1186/1472-6963-10-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kulkarni SC, Levin-Rector A, Ezzati M, Murray CJ. Falling behind: life expectancy in US counties from 2000 to 2007 in an international context. Population health metrics. 2011 Jun 15;9(1):16. doi: 10.1186/1478-7954-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonneux LG, Huisman CC, de Beer JA. Mortality in 272 European regions, 2002–2004. An update. European journal of epidemiology. 2010 Feb;25(2):77–85. doi: 10.1007/s10654-009-9415-y. [DOI] [PubMed] [Google Scholar]
- 4.Rula EY, Pope JE, Hoffman JC. Potential Medicare savings through prevention and risk reduction. Population health management. 2011 Feb;14(Suppl 1):S35–S44. doi: 10.1089/pop.2010.0063. [DOI] [PubMed] [Google Scholar]
- 5.Formiga F, Chivite D, Sole A, Manito N, Ramon JM, Pujol R. Functional outcomes of elderly patients after the first hospital admission for decompensated heart failure (HF). A prospective study. Archives of gerontology and geriatrics. 2006 Sep-Oct;43(2):175–185. doi: 10.1016/j.archger.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Sleiman I, Rozzini R, Barbisoni P, et al. Functional trajectories during hospitalization: a prognostic sign for elderly patients. The journals of gerontology. Series A, Biological sciences and medical sciences. 2009 Jun;64(6):659–663. doi: 10.1093/gerona/glp015. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhry SI, Phillips CO, Stewart SS, et al. Telemonitoring for patients with chronic heart failure: a systematic review. J Card Fail. 2007 Feb;13(1):56–62. doi: 10.1016/j.cardfail.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pare G, Jaana M, Sicotte C. Systematic review of home telemonitoring for chronic diseases: the evidence base. J Am Med Inform Assoc. 2007 May-Jun;14(3):269–277. doi: 10.1197/jamia.M2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amarasingham R, Moore BJ, Tabak YP, et al. An Automated Model to Identify Heart Failure Patients at Risk for 30-Day Readmission or Death Using Electronic Medical Record Data. Med Care. 2010 Oct 11; doi: 10.1097/MLR.0b013e3181ef60d9. [DOI] [PubMed] [Google Scholar]
- 10.Axten CW, Foster D. Analysis of airborne and waterborne particles around a taconite ore processing facility. Regul Toxicol Pharmacol. 2008 Oct;52(1 Suppl):S66–S72. doi: 10.1016/j.yrtph.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. The New England journal of medicine. 2010 Dec 9;363(24):2301–2309. doi: 10.1056/NEJMoa1010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crane SJ, Tung EE, Hanson GJ, Cha S, Chaudhry R, Takahashi PY. Use of an electronic administrative database to identify older community dwelling adults at high-risk for hospitalization or emergency department visits: the elders risk assessment index. BMC health services research. 2010;10:338. doi: 10.1186/1472-6963-10-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bureau C. Projected Population of the United States, by Age and Sex, 2000 to 2050. [Accessed September 2009]; [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. 1987 Apr;62(4):281–288. doi: 10.1016/s0025-6196(12)61905-3. [DOI] [PubMed] [Google Scholar]
- 16.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996 Mar;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Judge JO, Schechtman K, Cress E. The relationship between physical performance measures and independence in instrumental activities of daily living. The FICSIT Group. Frailty and Injury: Cooperative Studies of Intervention Trials. J Am Geriatr Soc. 1996 Nov;44(11):1332–1341. doi: 10.1111/j.1532-5415.1996.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 18.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991 Feb;39(2):142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 19.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing. 1997 Jan;26(1):15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 20.Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10(2):64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 21.Services. DoHaH. Guidance on Reviewing and Reporting Unanticipated Problems. Involving Risks to Subjects or Others and Adverse Event. www.hhs.gov/ohrp/policy/advevntguid.pdf.
- 22.Cady R, Finkelstein S, Kelly A. A telehealth nursing intervention reduces hospitalizations in children with complex health conditions. J Telemed Telecare. 2009;15(6):317–320. doi: 10.1258/jtt.2009.090105. [DOI] [PubMed] [Google Scholar]
- 23.Noel HC, Vogel DC, Erdos JJ, Cornwall D, Levin F. Home telehealth reduces healthcare costs. Telemed J E Health. 2004 Summer;10(2):170–183. doi: 10.1089/tmj.2004.10.170. [DOI] [PubMed] [Google Scholar]
- 24.Wade MJ, Desai AS, Spettell CM, et al. Telemonitoring with case management for seniors with heart failure. The American journal of managed care. 2011 Mar;17(3):e71–e79. [PubMed] [Google Scholar]
- 25.Finkelstein SM, Speedie SM, Potthoff S. Home telehealth improves clinical outcomes at lower cost for home healthcare. Telemed J E Health. 2006 Apr;12(2):128–136. doi: 10.1089/tmj.2006.12.128. [DOI] [PubMed] [Google Scholar]
- 26.Dar O, Riley J, Chapman C, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail. 2009 Mar;11(3):319–325. doi: 10.1093/eurjhf/hfn050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarke M, Shah A, Sharma U. Systematic review of studies on telemonitoring of patients with congestive heart failure: a meta-analysis. Journal of telemedicine and telecare. 2011;17(1):7–14. doi: 10.1258/jtt.2010.100113. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi PY, Tung EE, Crane SJ, Chaudhry R, Cha S, Hanson GJ. Use of the elderly risk assessment (ERA) index to predict 2-year mortality and nursing home placement among community dwelling older adults. Archives of gerontology and geriatrics. 2011 Mar 10; doi: 10.1016/j.archger.2011.02.012. [DOI] [PubMed] [Google Scholar]


