Abstract
The formation of disulfide bonds between cysteine residues is crucial for the stabilization of native protein structures and, thus, determination of disulfide linkages is an important facet of protein structural characterization. Nonetheless, the identification of disulfide bond linkages remains a significant analytical challenge, particularly in large proteins with complex disulfide patterns. Herein, we have developed a new liquid chromatography mass spectrometric (LC/MS) strategy for rapid screening of disulfides in an intact protein mixture after a straightforward reduction step with tris(2-carboxyethyl)phosphine. LC/MS analysis of reduced and non-reduced protein mixtures quickly revealed disulfide-containing proteins owing to a 2 Da mass increase per disulfide reduction and, subsequently, the total number of disulfide bonds in the intact proteins could be determined. We have demonstrated the effectiveness of this method in a protein mixture composed of both disulfide-containing and disulfide-free proteins. Our method is simple (no need for proteolytic digestion, alkylation, or the removal of reducing agents prior to MS analysis), high throughput (fast on-line LC/MS analysis) and reliable (no S-S scrambling), underscoring its potential as a rapid disulfide screening method for proteomics applications.
Keywords: Disulfide, Intact Proteins, Proteomics, Liquid Chromatography, TCEP, Mass Spectrometry
The formation of disulfide bonds between cysteine residues is crucial for the stabilization of native protein structures and, thus, determination of disulfide linkages is an important facet of protein structural characterization [1–2]. The potential for utilizing mass spectrometry (MS) to determine the disulfide linkages in proteins was realized more than a decade ago and, consequently, MS has become the preferred tool for disulfide bond analysis [1].
MS methods for the determination of disulfide bonds generally require proteolytic or chemical digestion of proteins, followed by chromatographic separation of the disulfide-linked peptides, prior to MS analysis [1, 3–5]. In most cases, the disulfide-containing proteins/peptides need to be subjected to full or partial reduction due to the difficulty of fragmenting the disulfide-linked peptides. Furthermore, alkylation of the digested peptides is often required to prevent disulfide scrambling [1, 3, 5–6]. Although these methods have proven to be useful for the determination of disulfide bonds, the major drawbacks are the requirement of multiple sample preparation procedures, which can be time-consuming, as well as incomplete information regarding the overall number of disulfide linkages due to partial sequence coverage of peptides recovered from digestion.
A top-down MS methodology greatly simplifies the sample preparation as no proteolytic digestion is required, which also reduces the mixture complexity [7–9]. Another great advantage of the top-down approach is its ability to provide a complete view of protein post-translational modifications [10–11]. We and others have demonstrated that top-down MS provides comprehensive characterization of purified disulfide-containing proteins [12–14]. Nonetheless, to analyze complex protein mixtures in proteomics applications, a chromatographic separation of intact proteins is needed prior to MS analysis. Herein, we have developed a simple liquid chromatography (LC) MS-based method for rapid determination of disulfide bonds in intact proteins.
First, we developed a one-step sample preparation method to completely and rapidly reduce the disulfide bonds in intact proteins by incubation of protein mixtures with an excess of tris(2-carboxyethyl)phosphine (TCEP) in 0.1 M citric buffer (pH 3) (total protein:TCEP 1:5000) at 65 °C for 30 min (Detailed experimental procedures in Supporting Information). We chose TCEP as a reducing agent since it has several advantages over other traditional reducing agents such as DTT and β-mercaptoethanol for disulfide bond reduction [5]. The most attractive advantages are: 1) TCEP reduces disulfide bonds under highly acidic conditions (pH 3), which significantly suppresses disulfide scrambling (which typically occurs under neutral and alkaline pH) thus obviating the need for alkylation of free thiols; 2) removal of excess TCEP is usually not required because, unlike DTT, TCEP does not contain a sulfhydryl group; 3) TCEP has no pungent odor, but can provide complete (or selective) quantitative reduction and does not react with other functional groups on proteins. Therefore, the sample preparation step is greatly simplified and we can directly inject the reduced intact proteins in a LC/MS system without an additional sample clean-up procedure, which significantly enhanced the throughput of each analysis.
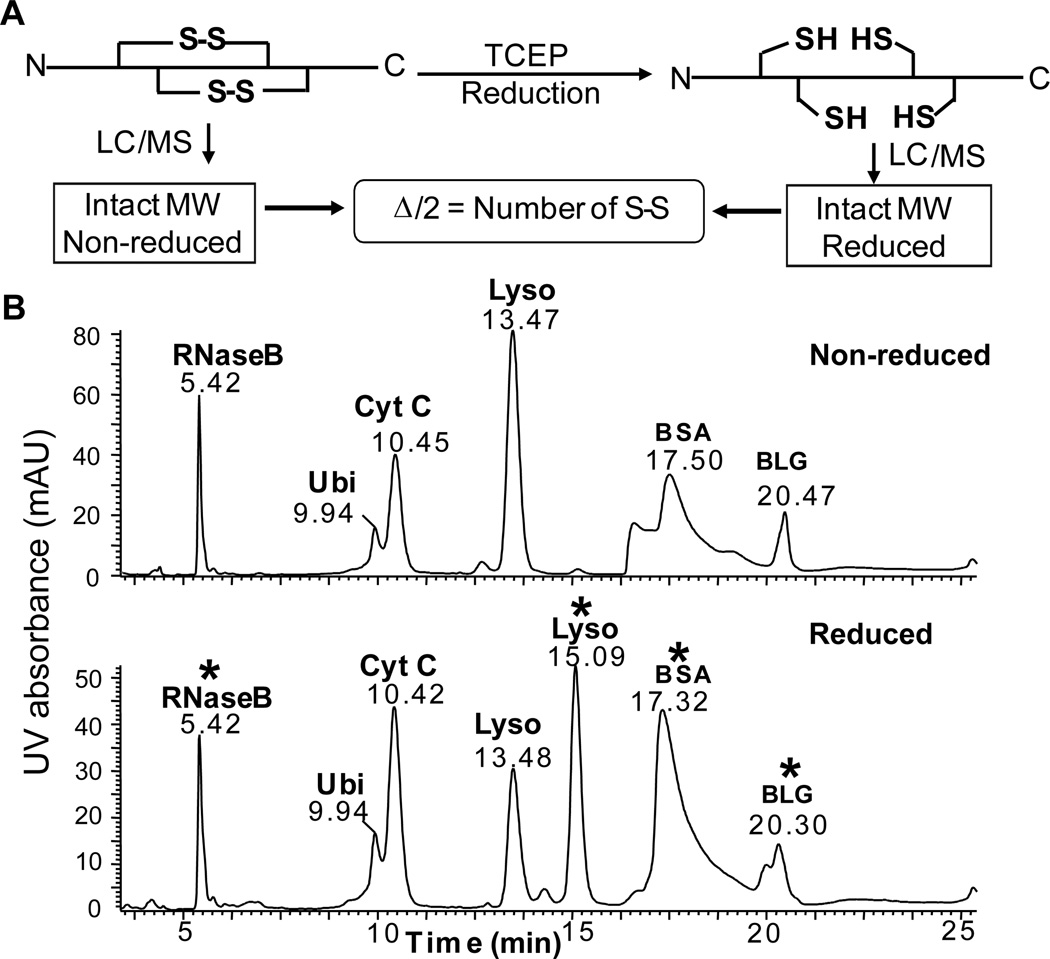
Next, we analyzed the fully reduced and non-reduced protein mixtures by LC/MS side-by-side to determine the number of disulfide bonds. Owing to a mass increase of 2 Da per disulfide reduction [12–13, 15–16], the number of disulfide bonds can be directly determined by a comparison of the molecular weights (MWs) of the reduced vs. non-reduced intact proteins (Figure 1A). Here we employed a LC/MS system using a Waters 2695 HPLC connected on-line to a Waters quadrupole time-of-flight (Q-TOF) mass spectrometer (Waters, Milford, MA, USA). The use of lower resolution mass spectrometers such as Q-TOF instruments greatly expanded the capability of top-down MS, which has traditionally been demonstrated on high resolution Fourier transform MS [17]. Both reduced and non-reduced protein mixtures containing bovine serum albumin (BSA), ubiquitin (Ubi), cytochrome C (Cyt C), β-lactoglobulin (BLG), lysozyme (Lyso), and ribonuclease B (RNase B) were separated on an ACE C18 reversed-phase column (250×4.6 mm, 90 Å, ACE, Aberdeen, Scotland). The separation was carried out with a linear gradient from 73% solvent A (0.025% TFA and 0.1% formic acid (FA) in H2O) and 27% solvent B (0.1% FA in acetonitrile (ACN)) to 50% solvent B in 40 minutes with a flow rate of 0.6 mL/min. As shown in Figure 1B, all the intact proteins were eluted and separated in less than 25 min. The order of retention time of the proteins was RNase B < Ubi < Cyt C <Lyso < BSA < BLG. As expected, for proteins that do not contain disulfides, the retention time remained the same before and after the reduction. Interestingly, a significant shift in retention time was observed for proteins such as Lyso and BSA, but not for other disulfide-containing proteins, following disulfide bond reduction. Since reverse-phase chromatography separates proteins based on hydrophobicity, the shift in retention time may be the result of either sequestration or exposure of hydrophobic residues following the disruption of protein structure via the reduction of disulfide bonds. However, the degree of such alteration in protein structure and hydrophobicity may vary from protein to protein. As observed here, some disulfide-containing proteins, i.e., Lyso and BSA, showed drastically altered retention times after reduction while others, such as BLG and RNase B, showed little or no change in retention time, respectively. Therefore, alteration in retention time alone cannot be used as the criteria for determining whether a protein contains disulfide bonds, which makes MS analysis a necessity.
Figure 1. A. Schematic representation of the LC/MS workflow for deducing the number of disulfide bonds. B. On-line LC separation of (top) non-reduced and (bottom) reduced protein mixture.
UV detection at 214 nm. The separation was carried out with a linear gradient from 73% solvent A (0.025% TFA and 0.1% FA in Water) and 27% solvent B (0.1% FA in ACN) to 50% solvent B in 40 minutes. Flow rate was 0.6 mL/min. RNase B, ribonuclease B; Ubi, ubiquitin, Cyt C, cytochrome C; Lyso, lysozyme; BLG, β-lactoglobulin; BSA, bovine serum albumin. *, disulfide-containing proteins.
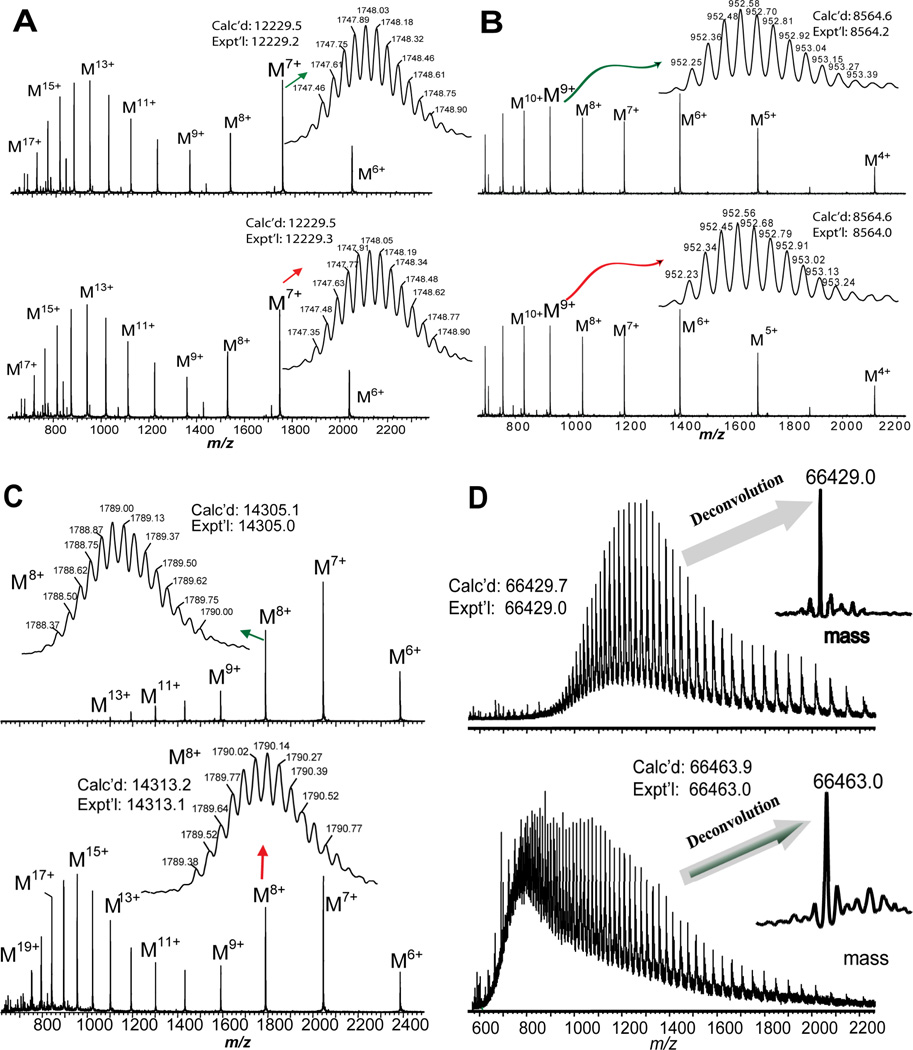
Subsequently, both reduced and non-reduced intact proteins were then directly analyzed by electrospray ionization (ESI)/Q-TOF/MS after HPLC separation. The MWs of the intact proteins of both completely reduced and non-reduced proteins were acquired with high accuracy and this information was used to determine the total number of disulfide bonds in the proteins (Figure 2, Supplemental Figures 1–2). For RNase B, ESI/Q-TOF/MS analysis revealed a shift in the MW following reduction with TCEP, suggesting that this protein contains disulfide linkages. High resolution Q-TOF/W mode/MS spectra (Supplemental Figure 1, right panel) clearly shows an 8 Da mass shift between reduced and non-reduced RNase B, corresponding to the reduction of four disulfide bonds within RNase B by TCEP. This is consistent with previous studies showing that RNase B contains 124 amino acids that are linked by four disulfide bonds: Cys 26-Cys 84, Cys 40-Cys 95, Cys 58-Cys 110, and Cys 65-Cys 72 [18]. Additionally, RNase B is known to be heavily glycosylated. Indeed, we observed multiple glycosylated forms for both reduced and non-reduced RNase B and the MWs of these species are summarized in Supplemental Table 1. The experimental MWs matched well with the calculated ones for RNase B with complex glycosylations varying from 5 to 9 mannoses in high mannose structure. Meanwhile, all of these glycosylated forms also showed 8 Da mass shifts between their reduced and non-reduced forms, which further confirmed the presence of 4 disulfide bonds in non-reduced RNase B.
Figure 2. A. ESI/MS analysis of (top) non-reduced and (bottom) reduced bovine cytochrome C.
Main panel, low resolution V mode detection of multiply charged molecular ions; Inset, high resolution W mode detection of molecular ions (M7+). B. ESI/MS analysis of (top) non-reduced and (bottom) reduced bovine ubiqutin. Main panel: low resolution V mode detection of multiply charged molecular ions; Inset, high resolution W mode detection of molecular ions (M9+). C. ESI/MS analysis of (top) non-reduced and (bottom) reduced chicken egg lysozyme. Main panel, low resolution V mode detection of multiply charged molecular ions; Inset, high resolution W mode detection of molecular ions (M8+). D. ESI/MS analysis of (top) non-reduced and (bottom) reduced BSA. Main panel, low resolution V mode detection of BSA molecular ions; Inset, deconvoluted mass of BSA.
For Ubi and Cyt C, no mass differences were observed between the reduced and non-reduced forms (Figure 2A, B), suggesting that these proteins do not contain disulfide bonds. This is consistent with the fact that Ubi and Cyt C are well-known as disulfide-free proteins. As expected, the charge state distributions of the multiply-charged molecular ions before and after reduction remained the same. In contrast, for Lyso and BSA, we observed drastic changes in the profiles of charge state distributions of the multiply-charged molecular ions after the reduction and significant MWs shifts were observed for both proteins following treatment with TCEP (Figure 2C, D). For Lyso, 4 disulfide bonds were deduced based on the mass difference of 8 Da between the non-reduced form (Expt’l Mr: 14305.0; Calc’d Mr: 14305.1) and completely reduced form (Expt’l Mr: 14313.1, Calc’d Mr: 14313.2), which was consistent with results reported previously [15]. For BSA (66.4 kDa), the isotopic distribution of the molecular ions could not be resolved due to the limited resolution of Q-TOF; thus, we used the deconvoluted MWs generated by MaxEnt (Waters, Milford, MA, USA) (66463.0 and 66429.0 for completely reduced and non-reduced BSA, respectively) for our analysis instead. The 34 Da mass shift confirms a total of 17 disulfide bonds in native BSA. BSA contains 607 amino acids with 35 cysteine residues linked by 17 disulfide bonds [SwissProt database, http://www.expasy.ch/sprot, P02769], which makes it challenging to determine the total disulfide bonds by traditional methods. Here we have completely reduced BSA under a quick TCEP reduction and maintained the reduced state of BSA during LC/MS analysis to yield the total number of disulfide bonds without the need for additional alkylation.
MS analysis of BLG revealed the presence of two natural variants, A and B, which can be easily distinguished in each charge state of both reduced and non-reduced forms of BLG (Supplemental Figure 2). The 4 Da mass shift between the deconvoluted MWs of BLG in reduced and non-reduced forms (Non-reduced forms: variant A, Expt’l Mr: 18363.0, Calc’d Mr: 18363.2; variant B, Expt’l Mr: 18277.3, Calc’d Mr: 18277.2. Reduced forms: variant A, Expt’l Mr: 18366.8, Calc’d Mr: 18367.3; variant B, Expt’l: 18281.0, Calc’d: 18281.2), suggests the presence of 2 disulfide bonds in both BLG variants. This is in agreement with a previous report that BLG contains 5 cysteine residues that are linked by 2 disulfide bonds [15]. It is important to note that, although HPLC is able to separate the intact protein from the protein mixture, it is unnecessary to have a baseline separation for all protein components. Even in the case of poorly resolved chromatographic peaks representing two protein variants, interpretation of Q-TOF data is still possible for determination of mass shift as described above.
In summary, we have presented here a simple LC/MS-based method with a one-step reduction for the rapid screening of disulfide-containing proteins. After reduction with TCEP, disulfide-containing proteins show distinct LC/MS patterns from those lacking a disulfide bond, making this method ideal for the rapid determination of disulfide linkages. Furthermore, our method is very effective for the analysis of large disulfide-containing proteins with complex disulfide bonding patterns such as BSA (66 kDa with 17 disulfide bonds). In addition, this method is also suited to analyze proteins with complex glycosylation patterns as shown here for RNase B. The major advantages of our method are 1) simplicity (no need for proteolytic digestions, alkylation, or removal of the reducing agent prior to MS analysis); 2) high throughput (on-line HPLC separation of reduced and non-reduced protein mixtures); and 3) reliability (no S-S scrambling). The use of high resolution MS can alleviate the need for base-line separation of intact proteins, which makes it feasible for detecting disulfide containing proteins in complex mixtures in proteomics applications. The future work includes the application of this method to a more complex biological system such as cell lysate or tissue lysate using multi-dimensional chromatography separation and identification of proteins by MS/MS.
Supplementary Material
Acknowledgement
We would like to acknowledge the financial support by NIH R01HL096971 and R01HL109810 (to YG). ZG would like to thank the NIH training grant T32GM008688.
Abbreviations
- LC
liquid chromatography
- MS
mass spectrometry
- MW
molecular weight
- BSA
bovine serum albumin
- Cyt C
cytochrome C
- BLG
β-lactoglobulin
- Lyso
chicken egg lysozyme
- RNase B
ribonuclease B
- Ubi
ubiquitin
- TCEP
tris(2-carboxyethyl)phosphine
- TFA
trifluoroacetic acid
- DTT
dithiothreitol
- FA
formic acid
- Q-TOF
quadrupole time-of-flight
- ACN
acetonitrile
- ESI
electrospray ionization
Footnotes
Conflict of Interests Disclosure
None.
References
- 1.Gorman JJ, Wallis TP, Pitt JJ. Protein disulfide bond determination by mass spectrometry. Mass Spectrom Rev. 2002;21:183–216. doi: 10.1002/mas.10025. [DOI] [PubMed] [Google Scholar]
- 2.Wedemeyer WJ, Welker E, Narayan M, Scheraga HA. Disulfide bonds and protein folding. Biochemistry. 2000;39:4207–4216. doi: 10.1021/bi992922o. [DOI] [PubMed] [Google Scholar]
- 3.Yen TY, Joshi RK, Yan H, Seto NOL, et al. Characterization of cysteine residues and disulfide bonds in proteins by liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2000;35:990–1002. doi: 10.1002/1096-9888(200008)35:8<990::AID-JMS27>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Chait BT. Identification and characterization of posttranslational modifications of proteins by MALDI ion trap mass spectrometry. Anal Chem. 1997;69:4002–4009. doi: 10.1021/ac970489n. [DOI] [PubMed] [Google Scholar]
- 5.Wu J, Watson JT. A novel methodology for assignment of disulfide bond pairings in proteins. Protein Sci. 1997;6:391–398. doi: 10.1002/pro.5560060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu SL, Jardine I, Hancock WS, Karger BL. A new and sensitive on-line liquid chromatography/mass spectrometric approach for top-down protein analysis: the comprehensive analysis of human growth hormone in an E coli lysate using a hybrid linear ion trap/Fourier transform ion cyclotron resonance mass spectrometer. Rapid Commun Mass Spectrom. 2004;18:2201–2207. doi: 10.1002/rcm.1609. [DOI] [PubMed] [Google Scholar]
- 7.Kelleher NL, Lin HY, Valaskovic GA, Aaserud DJ, et al. Top down versus bottom up protein characterization by tandem high-resolution mass spectrometry. J Am Chem Soc. 1999;121:806–812. [Google Scholar]
- 8.Ge Y, Lawhorn BG, ElNaggar M, Strauss E, et al. Top down characterization of larger proteins (45 kDa) by electron capture dissociation mass spectrometry. J Am Chem Soc. 2002;124:672–678. doi: 10.1021/ja011335z. [DOI] [PubMed] [Google Scholar]
- 9.Ge Y, Rybakova IN, Xu Q, Moss RL. Top-down high-resolution mass spectrometry of cardiac myosin binding protein C revealed that truncation alters protein phosphorylation state. Proc Natl Acad Sci USA. 2009;106:12658–12663. doi: 10.1073/pnas.0813369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Ge Y. Comprehensive analysis of protein modifications by top-down mass spectrometry. Circ Cardiovasc Genet. 2011;4:711. doi: 10.1161/CIRCGENETICS.110.957829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J, Dong X, Hacker TA, Ge Y. Deciphering modifications in swine cardiac troponin I by top-down high-resolution tandem mass spectrometry. J Am Soc Mass Spectrom. 2010;21:940–948. doi: 10.1016/j.jasms.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng Y, Chen X, Sato T, Rankin SA, et al. Purification and high-resolution top-down mass spectrometric characterization of human salivary alpha-amylase. Anal Chem. 2012;84:3339–3346. doi: 10.1021/ac300083y. [DOI] [PubMed] [Google Scholar]
- 13.Thevis M, Loo RRO, Loo JA. Mass spectrometric characterization of transferrins and their fragments derived by reduction of disulfide bonds. J Am Soc Mass Spectrom. 2003;14:635–647. doi: 10.1016/S1044-0305(03)00199-5. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Cui W, Zhang H, Dewald HD, Chen H. Electrochemistry-assisted top-down characterization of disulfide-containing proteins. Anal Chem. 2012;84:3838–3842. doi: 10.1021/ac300106y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loo JA, Edmonds CG, Udseth HR, Smith RD. Effect of reducing disulfide-containing proteins on electrospray ionization mass-spectra. Anal Chem. 1990;62:693–698. doi: 10.1021/ac00206a009. [DOI] [PubMed] [Google Scholar]
- 16.Scigelova M, Green PS, Giannakopulos AE, Rodger A, et al. A practical protocol for the reduction of disulfide bonds in proteins prior to analysis by mass spectrometry. Eur J Mass Spectrom. 2001;7:29–34. [Google Scholar]
- 17.Kellie JF, Tran JC, Lee JE, Ahlf DR, et al. The emerging process of Top Down mass spectrometry for protein analysis: biomarkers, protein-therapeutics, and achieving high throughput. Mol Biosys. 2010;6:1532–1539. doi: 10.1039/c000896f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu GQ, Zhai HL, Narayan M, McLafferty FW, Scheraga HA. Simultaneous characterization of the reductive unfolding pathways of RNase B isoforms by top-down mass spectrometry. Chem Biol. 2004;11:517–524. doi: 10.1016/j.chembiol.2004.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.