Abstract
Cilia and flagella are conserved hair-like appendages of eukaryotic cells that function as sensing and motility generating organelles. Motility is driven by thousands of axonemal dyneins that require precise regulation. One essential motility regulator is the central pair complex (CPC) and many CPC defects cause paralysis of cilia/flagella. Several human diseases, such as immotile cilia syndrome, show CPC abnormalities, but little is known about the detailed three-dimensional structure and function of the CPC. The CPC is located in the center of typical [9+2] cilia/flagella and is composed of two singlet microtubules, each with a set of associated projections that extend toward the surrounding nine doublet microtubules. Using cryo-electron tomography coupled with subtomogram averaging, we visualized and compared the three-dimensional structures of the CPC in both the green alga Chlamydomonas and the sea urchin Strongylocentrotus at the highest resolution published to date. Despite the evolutionary distance between these species, their CPCs exhibit remarkable structural conservation. We identified several new projections, including those that form the elusive sheath, and show that the bridge has a more complex architecture than previously thought. Organism-specific differences include the presence of microtubule inner proteins in Chlamydomonas, but not Strongylocentrotus, and different overall outlines of the highly connected projection network, which forms a round-shaped cylinder in algae, but is more oval in sea urchin. These differences could be adaptations to the mechanical requirements of the rotating CPC in Chlamydomonas, compared to the Strongylocentrotus CPC which has a fixed orientation.
Keywords: cryo-electron tomography, axoneme, flagella, Chlamydomonas, Strongylocentrotus
Introduction
Motile cilia and flagella are highly conserved organelles used by eukaryotic cells for generating motility, sensing the environment and signaling. Defects in cilia and flagella have been linked to various human diseases, called ciliopathies [Afzelius, 2004; Fliegauf et al., 2007]. Virtually all motile cilia and flagella share the same microtubule (MT) based core structure, the axoneme, which has a 9+2 arrangement of nine doublet MTs (DMTs) surrounding a pair of singlet MTs known as the central pair complex (CPC). In order to generate the waveforms characteristic of ciliary and flagellar motility, proper assembly and function of 400+ axonemal components is necessary [Pazour et al., 2005] and the mutation of a single protein can lead to paralyzed cilia/flagella, e.g. PF16 [Smith and Lefebvre, 1996]. Structurally, each DMT is built from many copies of a 96 nm-long unit that repeats along the longitudinal DMT axis. Key components within this 96 nm repeat unit are the dynein motors, which are arranged in two rows on the A-tubule of the DMT, the inner and outer dynein arms (IDAs and ODAs). These dyneins are permanently anchored on one DMT and use the energy of ATP to generate the mechanical force necessary for motility by walking along the neighboring DMT, causing the DMTs to slide past one another. This inter-doublet sliding is thought to be restricted, e.g. by the nexin links of the nexin-dynein regulatory complex (N-DRC) which connects neighboring DMTs [Porter and Sale, 2000; Nicastro et al., 2006; Heuser et al., 2009], which transforms linear sliding between DMTs into ciliary bending [Satir, 1968; Summers and Gibbons, 1971]. To generate the beating patterns that are typical of cilia and flagella, only subsets of dyneins must be active at any given time; activation of all dyneins at the same time would render the axoneme rigid and incapable of movement. Coordination of dyneins is achieved via the integration of various signals from axonemal regulatory complexes, such as the radial spokes (RS) [Smith and Sale, 1992; Smith and Yang, 2004], the I1 inner dynein [Porter and Sale, 2000; Wirschell et al., 2007, 2009; Heuser et al., 2012a], the N-DRC [Rupp and Porter, 2003; Heuser et al., 2009], and the CPC [Mitchell, 2009; Smith, 2002; Smith and Yang, 2004].
Cilia and flagella of different organisms have been used to study the CPC, including those from protists (e.g. Chlamydomonas and Paramecium) [reviewed in Smith and Yang, 2004; Omoto and Kung, 1979], echinoderms (e.g. Strongylocentrotus sperm) [Yoshimura and Shingyoji, 1999; Goodenough and Heuser, 1985] and mammals (e.g. murine sperm and epithelia and human sperm flagella) [Sapiro et al., 2000; Lechtreck et al, 2008; Lee et al., 2008; Lesich et al., 2010]. Generally, the CPC appears to have a conserved basic structure, which is summarized in Figure 1: Two singlet MTs, termed C1 and C2, are joined together by structures collectively called the bridge. Attached to each MT are projections that extend out toward the RSs and DMTs. Mutations that adversely affect CPC structure range from a loss of one projection to a complete failure of CPC assembly [Starling and Randall, 1971; Adams et al., 1981; Dutcher et al., 1984; Witman et al., 1978; Smith and Lefebvre, 1996; Mitchell and Sale, 1999; Rupp et al., 2001; Dymek et al., 2004]. Loss of a single projection can cause a significant decrease in the rate of flagellar beating, whereas the absence of one MT or the entire CPC results in the more severe phenotype of flagellar paralysis, suggesting that the CPC is an essential regulator of motility [Ebersold et al., 1962; Warr et al., 1966; Witman et al., 1978; Dutcher et al., 1984; Mitchell and Sale, 1999]. This role of the CPC as a modulator of motility seems to be conserved as mutations in homologous CPC genes are associated with various human ciliopathies characterized by immotile flagella/cilia, such as primary ciliary dyskinesia, infertility, and hydrocephalus [Sturgess et al., 1980; Neugebauer et al., 1990; Stannard et al., 2004; Lechtreck et al., 2008].
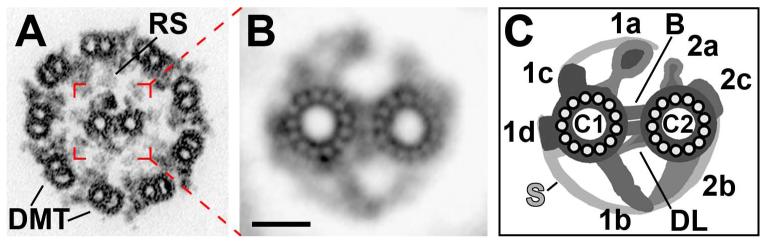
Fig. 1. Organization of the CPC.
(A-C) A cross-section of a Chlamydomonas axoneme (A) shows that the CPC is located in the center of the axoneme surrounded by the nine doublet MTs (DMT). The CPC is composed of two singlet MTs, C1 and C2, and its associated projections which extend toward the radial spokes (RS). Projection designations were determined previously using 2D EM averages (B) and are summarized in a simplified schematic model (C). Scale bar in (B) is 20 nm. All images were adapted from [Mitchell and Sale, 1999] with minor modifications.
The CPC is inherently asymmetric in that the projections of the C1 MT are structurally and biochemically distinct from those of the C2 MT [Hopkins, 1970; Witman et al., 1978; Adams et al., 1981; Dutcher et al., 1984; Mitchell and Sale, 1999; Mitchell, 2003a; Goduti and Smith, 2012]. The localization of known signaling proteins to the CPC projections [Yang et al., 2000; Smith, 2002], along with observations that projections physically interact with the RS heads [Warner, 1970; Warner and Satir, 1974; Goodenough and Heuser, 1985], and genetic studies demonstrating that motility in CPC/RS mutants is rescued by suppressor mutations in dynein activity modulators such as the N-DRC [Huang et al., 1982; Piperno et al., 1992, 1994] and I1 dynein [Dutcher et al., 1988; Porter et al., 1992], has led to a model in which unknown signals pass through the CPC and RSs to ultimately regulate dynein and flagellar activity. Changes in the phosphorylation state of the dynein arms are thought to play a key role in this motility regulation [Habermacher and Sale, 1997; Porter and Sale, 2000; Yang et al., 2000; Yang and Sale, 2000; Elam et al., 2011]. Despite the evidence supporting this CPC-RS interaction model, the precise mechanism of how the CPC regulates dynein activity remains unclear.
Electron microscopy (EM) studies of Chlamydomonas flagella and Tetrahymena cilia demonstrated that the CPC begins distal of the transition zone and extends to the distal tip of the organelle where the MT plus ends anchor to a membrane-associated cap structure [Ringo, 1967; Dentler and Rosenbaum, 1977; Rosenbaum et al., 1969; Dentler, 1984]. In metazoans (e.g. Strongylocentrotus sperm) and the protist Trypanosoma [Ralston et al., 2006; Branche et al., 2006], the CPC has a fixed orientation in the axoneme that remains constant during beating [Gibbons, 1961; Sale, 1986], whereas in most other protists studied (e.g. Chlamydomonas) the CPC is twisted and is thought to rotate during bending [Omoto et al., 1999; Mitchell, 2003b]. The role of this rotation in motility is unknown, although evidence from structural studies of Chlamydomonas suggests that the CPC is an inherently helical structure that rotates passively as a consequence of bend propagation [Mitchell and Nakatsugawa, 2004]. Numerous studies have contributed to our understanding of CPC structure. Currently, the most informative three-dimensional (3D) model of the CPC was deduced using a combination of freeze fracture and thin section EM of Chlamydomonas axonemes [Mitchell, 2003b]. This model proposed that all C2 projections and bridge structures repeat with a 16 nm periodicity, whereas the C1 projections show both 16 nm and 32 nm periodicities.
For a better understanding of the role of the CPC in regulating the motility of cilia/flagella, a detailed 3D in situ structure is needed. Recent structural studies used cryo-electron tomography (cryo-ET) to reveal structural details of the axoneme in its near native state, allowing new insights into structure-function relationships [Nicastro et al., 2006, 2011; Bui et al., 2008, 2009; Heuser et al., 2009, 2012a, 2012b; Pigino et al., 2011, 2012; Barber et al., 2012; Lin et al., 2012]. In this study we used cryo-ET and subtomogram averaging to provide the first detailed 3D structures of the CPC in its near native state in both Chlamydomonas and Strongylocentrotus flagella. Our structural comparison of the CPC between these evolutionary distant organisms reveals remarkable structural conservation of the CPC, yet also novel and organism-specific features.
Materials and Methods
Isolation of Axonemes and Flagella
Axonemes from five Chlamydomonas reinhardtii strains that all have a wildtype CPC (see Table I) were isolated as previously described [Barber et al., 2012; Nicastro, 2009]. In brief, cells were grown in liquid TAP (Tris-acetate-phosphate) medium [Gorman and Levine, 1965] under a 16 hour light/8 hour dark cycle and harvested by centrifugation. Following two washes, cells were resuspended in 10 mM Hepes (pH 7.4, 1 mM SrCl2, 4% sucrose, 1 mM DTT) and exposed to a short pH-shock resulting in flagella detachment from the cell bodies [Witman et al., 1972]. A protease inhibitor mix was added (100 μg/ml of each aprotinin, pepstatin, and leupeptin and 5 mM MgSO4, 1 mM EGTA, 0.1 mM EDTA) and cell bodies were pelleted by centrifugation. The flagella-containing supernatant was further purified in two additional centrifugation steps and finally the flagellar membrane was removed by a 30 minute incubation at 4°C with 1% of the detergent IGEPAL CA-630 (Sigma-Aldrich, St. Louis, MO). We pelleted the axonemes by an 1 hour centrifugation at 35,000 g and 4°C and resuspended the pellet in 10 mM Hepes pH 7.4, 25 mM NaCl, 4 mM MgSO4, 1 mM EGTA and 0.1 mM EDTA. No ATP was added to the buffers during the isolation of axonemes.
Table I.
Strains used for cryo-tomography.
| Name | Strain | Affected structure |
No. of tomograms |
Averaged repeats |
Resolution [nm]a |
|---|---|---|---|---|---|
| Chlamydomonas | 12 | 747 | 3.3 | ||
| pWT | pf2-4::PF2-GFP | Noneb | 2 | 124 | |
| pWT2 | ida6::IDA6-GFP | Nonec | 1 | 57 | |
| bop5-1 | CC-4080 | I1 | 2 | 140 | |
| sup-pf-3 | CC-1399 mt− | N-DRC | 2 | 121 | |
| sup-pf-4 | CC-2366 mt− | N-DRC | 5 | 305 | |
|
| |||||
| Strongylocentrotus | 8 | 451 | 4.0 | ||
All Chlamydomonas strains have a CPC that is indistinguishable from wildtype.
Resolution was estimated using the Fourier Shell Correlation method and the 0.5 correlation score criterion [Harauz and Van Heel, 1986].
The pWT strain (pseudo wild-type) is a mutant rescue with biochemical, structural and phenotypical properties as wildtype [Barber et al., 2012; Heuser et al., 2009; Nicastro et al., 2011; Rupp and Porter, 2003] and was generated by transformation of the N-DRC mutant pf2 with a GFP-tagged PF2 gene from wildtype [Rupp and Porter, 2003]. From Raqual Bower and Mary E. Porter at the University of Minnesota [Bower and Porter, in preparation].
The pWT2 strain was obtained by rescuing the ida6 mutant [Kato et al., 1993] with a GFP-labeled wildtype IDA6 gene. Motility and structure of the pWT2 is identical to wildtype. From Douglas Tritschler and Mary E. Porter at the University of Minnesota [Tritschler et al., in preparation].
Dr. Daniel Chen (Brandeis University) generously provided the sea urchin sperm flagella. Briefly, live Strongylocentrotus purpuratus sea urchins (Monterey Abalone Company, Monterey, CA) were maintained in the laboratory at 4°C in artificial seawater for up to 3 weeks. 1-2 ml of 0.5 M KCl were injected into the perivisceral cavity to induced spawning. Sea urchin sperm were collected and stored on ice without any additives to avoid sperm activation [Gatti and Christen, 1985; Gibbons, 1982]. All samples were processed within 24 hours.
Biochemical Analysis of the Klp1 Knockdown Strain
For the Klp1 knockdown strain [Yokoyama et. al., 2004], cpc1-1 [Mitchell and Sale, 1999] and wildtype (CC-125, mt+ [137c]) cells were grown in MI medium [Sager and Granick, 1953] and deflagellated with 5 mM dibucaine [Witman et. al., 1978]. Axonemes were prepared in HMDEK (30 mM Hepes, 5 mM MgSO4, 1 mM DTT, 0.5 mM EGTA, 25 mM potassium acetate, 1 mM PMSF, pH 7.4) containing 0.2% NP-40. Axonemal samples were run on an 8% SDS-PAGE gel, transferred onto a PVDF membrane and were probed with anti-Klp1 [Bernstein et. al., 1994], anti-Cpc1-A [Zhang and Mitchell, 2004], and anti-Hydin [Lechtreck and Witman, 2007] antibodies. Outer arm dynein detected by the dynein intermediate chain 2 antibody C11.4 [Mitchell and Rosenbaum, 1986] was used as a loading control. Horseradish peroxidase conjugated secondary antibodies were detected using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific, Waltham, MA).
Cryo-ET
After glow discharging for 30 s at −40 mA, Quantifoil holey carbon grids (Quantifoil Micro Tools GmbH, Jena, Germany) were coated with 10 nm colloidal gold (Sigma-Aldrich) and loaded in a self made plunge freezing device. 3 μl of either Chlamydomonas axonemes or Strongylocentrotus sperm flagella were added to the grid and mixed with 1 μl of a tenfold concentrated 10 nm colloidal gold solution. Grids were blotted from the front side for approximately 2 s with a filter paper and rapidly plunge frozen in liquid ethane at approximately −170°C to achieve sample vitrification. Cryo-samples were stored in liquid nitrogen until visualization by EM.
A cryo-holder (Gatan, Pleasanton, CA) was used to transfer vitrified samples to a Tecnai F30 transmission electron microscope (FEI, Eindhoven, NL) equipped with a field emission gun, where they were visualized at 300 keV with −6 to −8 μm defocus, under low dose conditions utilizing the image acquisition software SerialEM [Mastronarde, 2005]. A series of images was recorded by stepwise tilting the sample from approximately −65 to +65° with 1.5 - 2.5° angular increments. After passing an energy filter (Gatan, 20 eV slit width) in zero-loss mode all images were recorded on a 2k × 2k charge-coupled device camera (Gatan) at a magnification of 13,500×, resulting in a pixel size of ~1 nm. The cumulative electron dose was limited to ~100 e/Å2 to minimize radiation damage to the sample. Further details on the preparation of cryo-samples and visualization by cryo-ET were published earlier [Nicastro, 2009].
Image processing
Cryo-electron tomograms were reconstructed from the recorded tilt series using the IMOD software package [Kremer et al., 1996] with fiducial marker alignment and weighted back projection. For further image processing only tomograms of intact, non- or only mildly compressed flagella and axonemes and a well-preserved CPC were selected. A total of twelve axonemes from five different Chlamydomonas strains with wildtype CPC and eight Strongylocentrotus flagella were analyzed in this study (see Table I). Subtomogram averaging of the highly repetitive CPC was employed to increase the inherently low signal to noise ratio of cryo-tomograms and to ultimately improve the resolution. Different periodicities were tested and we found a basic 32 nm long repeat unit of the CPC, confirming previous results [Mitchell, 2003a]. Subtomograms, 100 × 100 × 100 nm sized small tomogram pieces centered on the CPC, were cut out of the cryo-electron tomograms every ~32 nm along the length of the CPC, were aligned to each other and averaged in 3D using the PEET (Particle Estimation for Electron Tomography) software [Nicastro et al., 2006]. By averaging repeats from different positions within one tomogram and from different tomograms, we combined repeat units with many different orientations of the CPC relative to the tilt axis, which allowed us to compensate for the missing wedge, a known limitation in single axis cryo-ET. As a result, our final averages show the 3D CPC structure in high resolution with relatively uniform resolution [Nicastro et al., 2006] (for more details see Table I).
During the subtomogram averaging, we used two different alignment strategies. For a global alignment, the entire CPC (i.e. the C1 and C2 MTs including all associated projections) was used to align repeat units. This method resolved the entire CPC of Strongylocentrotus sperm flagella in excellent and uniform quality and was used to generate all Strongylocentrotus averages shown in this study (Figs. 2-7; Supporting Information Fig. S2). When we utilized the same global alignment approach for Chlamydomonas, we noticed a difference in the average quality within the CPC: while the C1 MT and its projections were averaged in high quality, the C2 MT and associated projections were of lower quality, i.e. they appeared weaker and blurrier (compare C1 and C2 MT in Supporting Information Fig. S1A). To improve the alignment accuracy of the C2 MT, we then employed a local alignment approach, in which only the C2 MT and associated projections were used for alignment of repeat units, while a mask excluded all densities from the C1 MT and its projections. This local alignment approach resolved the C2 MT and projections in high quality, while the C1 MT was averaged poorly (compare C1 and C2 MT in Supporting Information Figs. S1A and S1B). This difference suggests that: a) the relative distance and/or position between C1 and C2 are not constant along the Chlamydomonas CPC, and b) in the global alignment the densities of the C1 MT dominate the alignment. To depict the entire Chlamydomonas CPC in the highest possible quality Figs. 2-7 and Supporting Information Movies S1 and S3 show a composite average consisting of two parts: the global alignment approach was used to average the C1 MT and projections, and the C2 MT including projections was averaged after local alignment. A comparison of both alignment methods is shown in Supporting Information Fig. S1. Only minor differences were observed in the bridge structure between global or local alignment (compare Supporting Information Figs. S1A and S1B). We also generated an average with local alignment focused exclusively on the bridge structure, but the differences between global and local alignment were negligible for the bridge structure (see Supporting Information Figs. S1A and S1B).
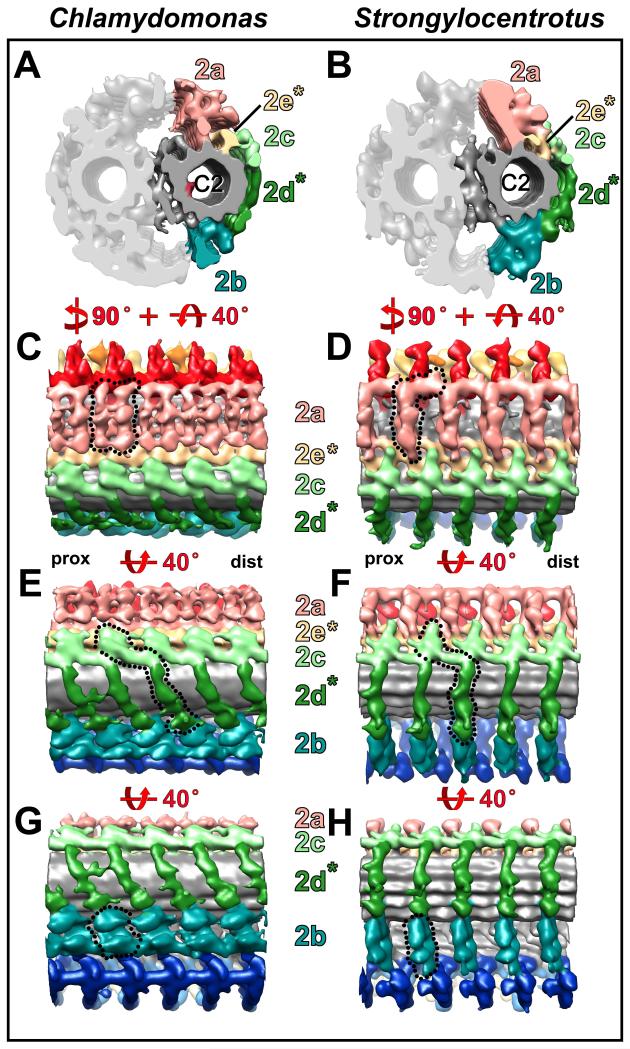
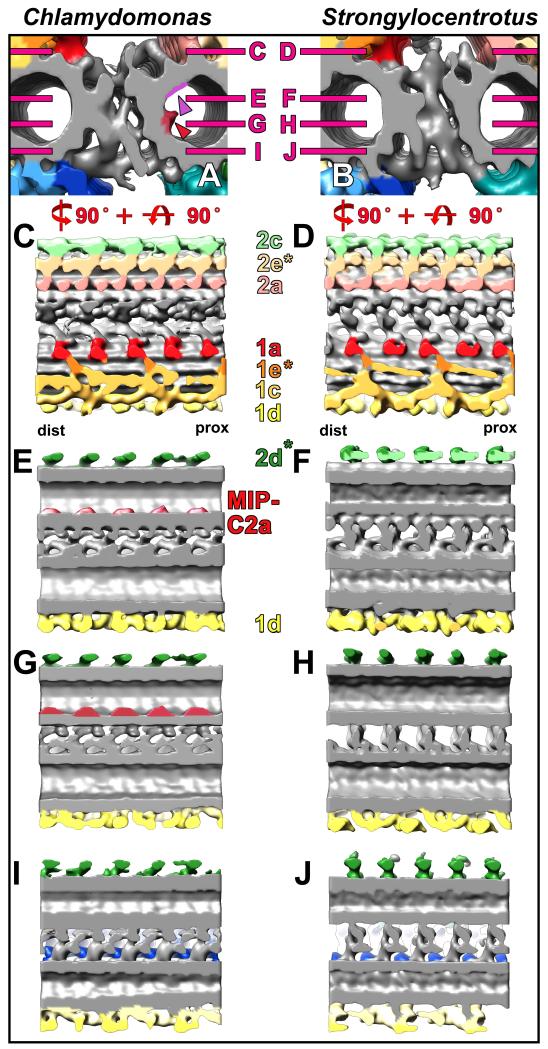
Fig. 2. Comparison of the C1 MT projections between Chlamydomonas and Strongylocentrotus.
(A-H) Isosurface renderings show the C1 MT projections from CPC averages in cross-sectional (A and B) and longitudinal views (C-H), and demonstrate that the C1 projections of Chlamydomonas (A, C, E, and G) bear strong resemblance to those of Strongylocentrotus (B, D, F, and H). Only a few structural differences between Chlamydomonas and Strongylocentrotus C1 projections are present as indicated by dotted outlines in (C, D and G). Note, that the most distal portions of the 1e projection appear to be oriented in opposite directions (compare C and D), and the 1a and 1b projections are longitudinally connected in Chlamydomonas (C and G). The 1a and 1b projections repeat every 16 nm, the remaining 1d, 1c, 1e and 1f projections repeat every 32 nm giving the CPC an overall periodicity of 32 nm. Note the arches (black arrowhead in G) in the 1f projection every 32 nm. An ~80 nm long piece of the CPC (~ 2.5 repeat units) is displayed to highlight the repetitive organization of the CPC. Note that the Chlamydomonas CPC isosurface rendering was obtained from two separate averages of the C1 and C2 MT (see Supporting Information Fig. S1 and Materials and Methods section for details). This averaging approach, color coding of projections and the shown views are preserved in all following Figures. Asterisks highlight projection designations that are described in this study for the first time. For orientation, the proximal (prox) and distal (dist) side of the averages are indicated in (C and D).
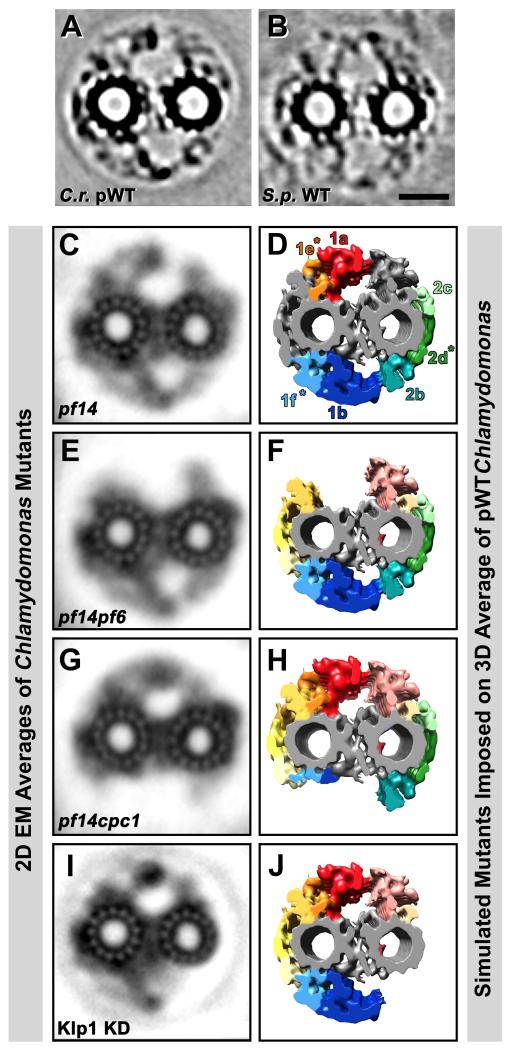
Fig. 7. Classical 2D EM averages of CPC mutants and simulation of corresponding 3D structural defects.
(A-J) Cross-sectional projection images through ~80 nm thick tomographic slices of CPC averages from Chlamydomonas pseudo-wildtype (C.r. pWT, A) and Strongylocentrotus wildtype (S.p. WT, B) are shown for comparison with 2D EM averages of ~80 nm thick plastic sections of the CPC from four Chlamydomonas mutants: pf14 (C) is a mutant lacking the radial spokes, but without CPC defects, i.e. the 2D averages looks like wildtype (compare with Fig. 1B). Note the similarities in densities amongst (A and C), as well as (B). The pf14pf6 (E), pf14cpc1 (G) double mutants and the Klp1 knockdown (I) all show CPC defects. Isosurface renderings of the Chlamydomonas pWT average have been edited by deleting selected projection densities (D, F, H and J) to reflect densities likely to be absent in the depicted Chlamydomonas CPC mutants (C, E, G, and I). The pf14pf6 double mutant in (E) appears to lack projections 1a and 1e. The 1b and 1f projections are almost completely absent in the pf14cpc1 double mutant (G and H). From the Kinesin-like protein 1 knockdown (Klp1 KD) (I) the 2b-d projections appear to be absent (J), which includes Hydin (see Fig. 8). Scale bar (B): 25 nm. Images from (C, E, and G) were adapted from [Mitchell and Sale, 1999] and image (I) was modified from [Yokoyama et al., 2004].
Fig. 3. Comparison of the C2 MT projections between Chlamydomonas and Strongylocentrotus.
(A-H) Cross-sectional (A and B) and longitudinal views (C-H) of isosurface renderings from averaged CPCs show overall a high similarity in the C2 MT projections between Chlamydomonas and Strongylocentrotus, but also reveal differences outlined by black dotted lines in (C-H). In both Chlamydomonas (A, C, E, and G) and Strongylocentrotus (B, D, F, and H) all C2 projections repeat every 16 nm. Projection 2a appears more intricate in Chlamydomonas (C) than in Strongylocentrotus (D), and exhibits a “pseudo-8 nm periodicity” in the alga (C). Note that the 2d projection is angled distally in Chlamydomonas (outlined in E), but runs nearly perpendicular to the MT in Strongylocentrotus (outlined in F). The Chlamydomonas 2b projection (outlined in G) appears broader than in Strongylocentrotus (outlined in H). Projections described in this study for the first time are highlighted by asterisks. The proximal (prox) and distal (dist) side of the averages are indicated in (C and D) for easier orientation.
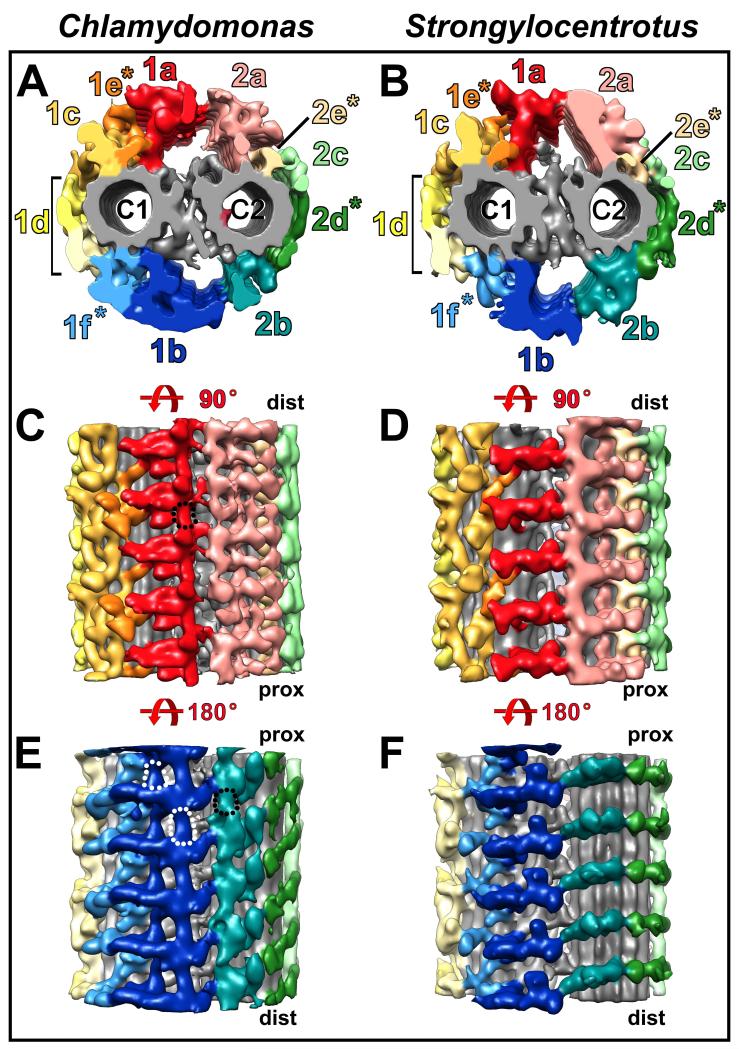
Fig. 4. Connections between C1 and C2 projections.
(A-F) Isosurface renderings from Chlamydomonas and Strongylocentrotus CPC averages show cross-sectional (A and B) and longitudinal views from the top (C and D) and bottom (E and F) of the CPC to highlight connections between C1 and C2 projections. In both organisms the C1 and C2 MTs are linked directly via a complex network of connections, called the bridge (see Fig. 5). Additional connections between the C1 and C2 halves of the CPC are formed by links between the 1a and 2a projections (C and D), as well as between the 1b and 2b projections (E and F). In Chlamydomonas, the 1a projections are also interconnected longitudinally along the MT axis (black outline in C), while they are not connected in Strongylocentrotus (D). Similarly, longitudinal connections between adjacent 1b and 2b projections are present in Chlamydomonas (black and white dotted outlines in E), but not in Strongylocentrotus (F). The proximal (prox) and distal (dist) side of the CPC are indicated for better orientation in (C-F).
Fig. 5. Comparison of the C1-C2 bridge structures between Chlamydomonas and Strongylocentrotus.
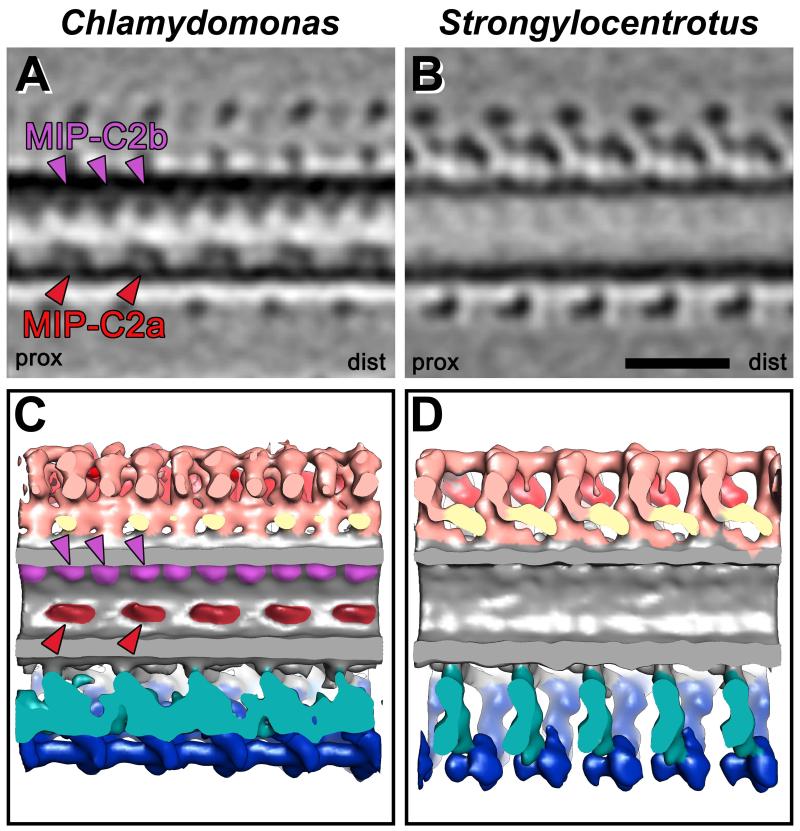
(A-J) Isosurface renderings from CPC averages of Chlamydomonas (A, C, E, G, and I) and Strongylocentrotus (B, D, F, H, and J) display the bridge structure in cross-sectional (A, B) and four different longitudinal views observed from the top (C and D), middle (E-H), and bottom (I and J). A complex network with several distinct connections links the C1 and C2 MTs. It is apparent that some bridge components in Chlamydomonas are arranged diagonally (I) to the MT whereas they are arranged perpendicularly to the MT in Strongylocentrotus (J). All bridge structures appear to have a 16 nm periodicity. Note the positions of the two MT inner proteins MIP-C2a and MIP-C2b (red and purple arrowheads in A) that are only present in Chlamydomonas (A, E and G; see also Fig. 6), but absent from Strongylocentrotus (B, F and H). Magenta lines in (A and B) indicate the orientation of the longitudinal views shown in (C-J). Asterisks highlight newly designated projections and the proximal (prox) and distal (dist) side is indicated to facilitate orientation in (C and D).
Fig. 6. MT Inner Proteins in the C2 MT of the Chlamydomonas CPC.
(A-D) Tomographic slices (A and B) and isosurface renderings (C and D) of the Chlamydomonas (A and C) and Strongylocentrotus CPC averages (B and D) reveal the presence of two MT Inner Proteins, MIP-C2a and MIP-C2b, inside the C2 MT of Chlamydomonas (red and purple arrowheads in A and C), but not in Strongylocentrotus (B and D). The larger MIP-C2a (red arrowheads) shows a 16 nm periodicity, whereas the smaller MIP-C2b (purple arrowheads) repeats every 8 nm. For orientation, proximal (prox) and distal (dist) sides of the averages are indicated in (A and B). Scale bar (B): 25 nm.
We estimated the resolution of our structures with the Fourier Shell correlation method [Harauz and Van Heel, 1986] using the 0.5 correlation score criterion. The over-all resolution of our structures was assessed in 20 × 20 × 20 nm large volumes at the base of the 1b projection (largest projection) and is estimated at 3.3 nm for Chlamydomonas and 4.0 nm for Strongylocentrotus (Table I). We used the same approach to further measure the resolution at the connection of the 2a projection to the C2 MT in order to compare the resolution of the global and local alignment methods in Chlamydomonas. While the global alignment resolves the C1 MT in high resolution, the resolution of the C2 MT was only 4.0 nm (Supporting Information Fig. S1A). The local alignment, however resolves the C2 MT at a better resolution of 3.3 nm (Supporting Information Fig. S1B). The depicted composite of both alignments methods allows us to show the highest resolution structures of both CPC MTs of Chlamydomonas.
We used the UCSF Chimera package [Pettersen et al., 2004] for 3D visualization by isosurface rendering and to measure volume sizes. Small, unconnected densities were considered noise and removed from all isosurface renderings with the “hide dust” function in Chimera. The volume measurements were converted into mass estimates by assuming an average protein density of 1.43 g/cm3 as suggested by Quillin and Matthews [2000]. This means, a volume of 1 A3 is equivalent to a mass of 0.861 Da. As a positive control we used this method to estimate the mass of the CPC MTs. Considering the ambiguity of separating the MT core from associated proteins, it is not surprising that our MT estimate is ~30% higher than that of a theoretical MT consisting of only tubulins, suggesting that the method’s accuracy is within a reasonable range.
Results
Cryo-ET combined with image processing was used to analyze twelve Chlamydomonas axonemes as well as eight Strongylocentratus sperm flagella resulting in the first detailed 3D structures of the CPC in a near native state. Our data confirm previous results, but also reveal several new features, as described below. Previously, seven CPC projections of the C1 and C2 MT, namely 1a-1d and 2a-2c, were assigned based on 2D EM averages of CPC cross sections (Fig. 1) [reviewed in Mitchell, 2009]. For consistency, we have preserved the nomenclature of these projections, but with the improved resolution in our 3D structures, we additionally assigned four new projections, called 1e, 1f, 2d and 2e (described below in more details). These new projections were designated based on their general morphology and direct attachment to either the C1 or C2 MT.
Projections of the C1 MT
Our 3D structures of the Chlamydomonas C1 MT (Figs. 2A, 2C, 2E and 2G; Supporting Information Fig. S1 and Movie S1) and Strongylocentrotus CPC (Figs. 2B, 2D, 2F and 2H; Supporting Information Fig. S2 and Movie S2) revealed new details of all previously-described C1 projections and required the designation of two additional projections, here called 1e and 1f, which are in similar locations to densities previously described as sheath fibers when viewed in the 2D EM average cross-section in Chlamydomonas (Fig. 1). Mass approximations of all CPC projections in both organisms are summarized in Table II. We estimate the entire mass of all projections of the C1 MT to be approximately 14 and 11 MDa per 32 nm repeat unit in Chlamydomonas and Strongylocentrotus, respectively (Table II). Despite small differences in morphology and size, overall, the C1 MT projections are remarkably similar between the algal and sea urchin flagella (Fig. 2; Supporting Information Movie S3).
Table II.
Estimated mass of projections and copy number of projections per 32 nm repeat in the CPC of Chlamydomonas and Strongylocentrotus.
| Projection | Chlamydomonas | Strongylocentrotus | ||
|---|---|---|---|---|
| mass [kDa]/projection | copies/32 nm | mass [kDa]/projection | copies/32 nm | |
| 1a | 1700 | 2 | 1150 | 2 |
| 1b | 1900 | 2 | 1350 | 2 |
| 1c | 1900 | 1 | 2050 | 1 |
| 1d-top | 1200 | 1 | 1150 | 1 |
| 1d-bottom | 1400 | 1 | 1300 | 1 |
| 1e | 550 | 1 | 200 | 1 |
| 1f | 1650 | 1 | 1150 | 1 |
| 2a | 1750 | 2 | 1500 | 2 |
| 2b | 1150 | 2 | 1050 | 2 |
| 2c | 550 | 2 | 400 | 2 |
| 2d | 400 | 2 | 550 | 2 |
| 2e | 400 | 2 | 550 | 2 |
| MIP2a | 50 | 2 | - | - |
| MIP2b | 35 | 4 | - | - |
- : not present; mass estimations assume an average protein density of 1.43 g/cm3 [Quillin and Matthews, 2000]
Among the longest and most massive projections in both organisms are the 1a and 1b projections located on opposite sides of the C1 MT (Fig. 2; Supporting Information Figs. S1 and S2). Viewed in cross-section, the 1a projection has roughly the shape of a hammer, which is slightly more obvious in sea urchin than in the alga (Figs. 2A and 2B; Supporting Information Figs. S1P and S2N). Both the 1a and 1b projections have two identical copies per 32 nm repeat unit, i.e. a 16 nm periodicity, in Chlamydomonas as well as in Strongylocentrotus (Table II). Contrary to sea urchin, the 1a and 1b projection in the alga show additional densities that interconnect individual projection copies along the length of the CPC (compare Figs. 2C with 2D, 2G with 2H, 4C with 4D and 4E with 4F). These additional connecting densities bring the mass of the algal projections 1a and 1b to 1.7 and 1.9 MDa, respectively, compared to their sea urchin counterparts with only 1.2 and 1.4 MDa, respectively (Table II). The 1c projections are very similar between both organisms and repeat only once per 32 nm (Table II). In longitudinal views, part of the 1c density has a wishbone-like shape (Figs. 2C and 2D).
The 1d projection does not protrude far from the C1 MT, but stretches over more than 30 nm along the MT wall when observed in CPC cross-section (Figs. 2A and 2B). In longitudinal views the 1d projections seems to consist of two discrete parts with different motifs. To avoid confusion we have maintained the previously published nomenclature and refer to both parts together as the 1d projection, but to point out differences in both parts more clearly we have colored them differently (bright yellow and light beige; Figs. 2E and 2F) and calculated masses separately for each part (Table II). Although both parts have a 32 nm periodicity, the zigzag-shaped part that resembles a “batman symbol” (bright yellow; Figs. 2E and 2F) has more evenly distributed densities along the length of the CPC. In contrast, the light beige-colored 1d densities form more concentrated complexes with larger density gaps in between (Figs. 2G and 2H).
Some densities have previously been described as “sheath material” because they have a lower contrast than the projections in classical EM cross-sections of the CPC (Fig. 1). However, our averages revealed that these densities have the same characteristics as other projections, such as periodicity and MT-attachment. Thus, based on our 3D structures of the CPC, we assigned two new projections, here called 1e and 1f (Fig. 2). The 1e projection is by far the smallest density attached directly to the C1 MT and has – like most C1 projections – a 32 nm periodicity. While its location, connections to the neighboring 1a and 1c projections and over-all organization show similarities between alga and sea urchin, 1e is arguably the C1 projection that is most different between the studied organisms: in addition to a size difference (~550 kDa in Chlamydomonas and ~200 kDa in Strongylocentrotus), the orientation of the main density of the 1e projection seems to point in opposite directions along the CPC length (compare black dotted outlines in Figs. 2C and 2D). The 1f projection is positioned between 1b and 1d with a 32 nm periodicity that is mainly determined by arches (see arrowhead in Fig. 2G). While the overall shape of 1f is similar between both species, smaller morphological differences exist in the peripheral density and its mass is considerably larger in Chlamydomonas (see Table II; Fig. 2G and 2H).
Projections of the C2 MT
Similar to the C1 MT, multiple projections also extend from the C2 MT. Projections 2a, 2b and 2c were described previously [e.g. Mitchell, 2009] and based on our 3D structure of the CPC we assigned two additional projections, here named 2d and 2e. Table II lists mass estimates for all C2 projections, that together sum up to a total mass of approximately 8.7 MDa per 32 nm repeat unit in Chlamydomonas and 8.1 MDa in Strongylocentrotus, which is significantly smaller than the estimates of the C1 MT projections (14 MDa and 11 MDa, respectively). Overall, the similarities between the C2 projections of the Chlamydomonas and Strongylocentrotus CPC are still high, but less than for the C1 projections due to size and morphological differences (Fig. 3; Supporting Information Movie S3). Also in contrast to the C1 projections that show both 16 and 32 nm periodicities, all C2 projections have a 16 nm periodicity.
The 2a projection is the largest projection of the C2 MT (see Table II). It is located between projections 1a and 2e and repeats twice per 32 nm repeat unit (Fig. 3; Supporting Information Movies S1, S2; Figs. S1 and S2). While in Chlamydomonas each 2a projection consists of two highly similar, elongated parts - with pseudo-8 nm periodicity - that are interconnected at multiple sites, the 2a projection in Strongylocentrotus is built from only one elongated part that connects to its neighboring 2a projections only at one site (compare black dotted outlines in Figs. 3C and 3D).
Positioned opposite to 2a of the C2 MT is the 2b projection that has two copies per 32 nm repeat (Figs. 2E-2H and 4E and 4F). In Chlamydomonas the main densities of the 2b projection are wide and connect to neighboring copies along the MT length, but in Strongylocentrotus these densities are narrower and lack longitudinal connections (see black dotted outlines in Figs. 3G and 3H).
The 2c projections are continuous with the here newly named 2d projections (Figs. 3E and 3F). They repeat with 16 nm periodicity and do not protrude far from the C2 MT, yet together they stretch over ~40 nm along the MT wall when observed in CPC cross-section (Figs. 3A and 3B), similar to the 1c projections. While the overall shape of these two projections is similar between the Chlamydomonas and Strongylocentrotus CPC – with 2c forming a more globular part and 2d a longer and narrower structure, the orientation of 2d is significantly different between the two organisms. In the sea urchin, the 2d projection runs perpendicular to the MT axis to connect to the 2b projection (Figs. 3F and 3H). In contrast, in the alga, the 2d projection is angled towards the distal end of the C2 MT (Figs. 3E and 3G), so that the projection skips the closest neighboring 2b projection and, instead, connects to the 2b projection that is16 nm more distal (compare black dotted outlines in Figs. 3E and 3F).
The new 2e projection, the smallest C2 projection, is sandwiched between 2a and 2c (Figs. 3A and 3B) and has two copies per 32 nm repeat (Figs. 3C and 3D). In Strongylocentrotus the 2e projection has multiple links to the 2a density, including an arch shaped connection (Figs. 3B and 3D), while in Chlamydomonas 2e runs more isolated along the MT wall (Figs. 3A and 3C). Two additional densities on the inside of only the Chlamydomonas C2 MT, two MT inner proteins, are described in a later section.
The C1 and C2 halves of the CPC are connected at three distinct sites
The two CPC MTs, C1 and C2, together with their associated projections have three distinct sites of contact (Figs. 4A and 4B). At the perimeter of the CPC the four largest projections connect at their tips, i.e. 1a links to 2a and 1b to 2b (Fig. 4). On the side with the 1a projections, the linkage between the two CPC halves seems more pronounced in the sea urchin than the alga (compare Figs. 4D and 4C). Another organism-specific difference is that in Chlamydomonas longitudinal connections along the MT axis between neighboring projections are more prominent (see dotted outlines in Figs. 4C and 4E) than in the Strongylocentrotus CPC, where e.g., the side with the 1b projections is completely devoid of such longitudinal linkages between projections (Fig. 4F).
In the CPC center, the C1 and C2 MTs themselves are connected through a massive and complex density network, called the bridge (Fig. 5). This is consistent with early EM studies that described material connecting the C1 and C2 MTs as a bridge [Warner, 1976]. In cross-sectional EM images and 2D averages this bridge appeared to be composed of three separate densities (Fig. 1). However, the higher resolution and 3D nature of our cryo-ET averages revealed that the bridge structures in both Chlamydomonas and Strongylocentrotus are in fact considerably more complex than previously thought (Fig. 5). The bridge consists of multiple densities that are highly interconnected and form a complex, 3D network linking the C1 and C2 MT (Fig. 5). The entire bridge structure seems to follow a 16 nm periodicity in alga and sea urchin, i.e. two copies per 32 nm repeat (Figs. 5C-5J). While the overall arrangement and periodicity is preserved between Chlamydomonas and Strongylocentrotus, details in orientation and morphology vary between species (Fig. 5). Interestingly, the Chlamydomonas bridge seems to contain more diagonal elements connecting neighboring bridge rungs that link C1 and C2 (Figs. 5G and 5I). While the isosurface renderings in Figures 5C and 5D provide close-up views of the dense bridge-network in both organisms, the three isosurface rendered tomographic slices depicted in Figs. 5E/G/I and 5J/H/G show 3D views of the three connecting entities that were previously described for the bridge (compare to Fig. 1), including the diagonal link (Figs. 5I and 5J).
The C2 MT in Chlamydomonas but not in Strongylocentrotus contains microtubule inner proteins
Recently, microtubule inner proteins (MIPs) were discovered in the A- and B-tubules of the outer DMTs of cilia and flagella in several species [Nicastro et al., 2006, 2011; Pigino et al., 2012]. Here we report, to our knowledge, the first MIPs in a MT of the CPC. The higher resolution and the 3D nature of our data enabled us to detect these densities that are much smaller than the previously described CPC structures (see Table II). The C2 MT in Chlamydomonas contains two distinct MIPs that are attached to the inside of the MT wall in the area that faces the bridge and C1 MT (Figs. 5A). The larger CPC MIP, here named MIP-C2a, repeats every 16 nm and has a size of approximately 50 kDa per copy (Table II; Figs. 4A; 5A, 5E, 5G; 6A, 6C; Supporting Information Figs. S1B, S1J and S1R). The second MIP, called MIP-C2b, is smaller with a mass of ~35 kDa and has an 8 nm periodicity (Table II; Figs. 5A; 6A, 6C; Supporting Information Fig. S1B). No MIPs were found in the CPC MTs of Strongylocentrotus (compare Figs. 6B/D with 6A/C), making the MIPs one of the few distinguishing features between the C1 and C2 MTs in Chlamydomonas and an organism-specific difference in an, otherwise, remarkably similar CPC structure.
Discussion
Our subtomogram averages from Chlamydomonas axonemes and Strongylocentrotus flagella provide the first detailed 3D structures of the CPC in a cryo-preserved, near native state and enable a comparison between these two evolutionary distant organisms at unprecedented resolution. The data show that the CPC projections of Chlamydomonas share the same general organization with those of Strongylocentrotus. Despite the overall remarkable similarities, we also observed organism-specific structural differences between the two CPCs, which include additional connections and two MIPs in Chlamydomonas as well as changes in projection orientations. The details of these similarities and differences are discussed below.
The densities previously described as a “sheath” are CPC projections
Most of our knowledge about CPC ultrastructure comes from studies using classical EM and 2D averages of CPC cross-sections [reviewed e.g. in Mitchell, 2009]. These studies identified several CPC projections, namely 1a-1d and 2a-2c, and in addition described a sheath partly surrounding the CPC that was characterized by considerably lower contrast than the projections and no apparent connection to the MTs (Fig. 1C). Our averaged 3D structure of the CPC reveals that the sheath is not an independent density connecting adjacent projections, but rather is the outermost part of projections extending from the C1 and C2 MTs. To enable a better comparison between the traditional 2D EM images of 70-100 nm thick plastic sections and our 3D cryo-ET data, we generated a projection of an 80 nm thick slice through our tomographic averages (Figs. 7A and 7B). The CPC densities seen in these tomographic projection images closely resemble those in traditional 2D cross-sections of the CPC, including the differences in contrast (compare Figs. 1B or 7C to 7A). This allows us to directly relate the densities seen in our 3D structure with previously described features. For example, the sheath between the 1b and 1d projections (Fig. 1C) is built from the outermost densities of the 1f projection and parts of the 1b and 1d projections (Figs. 2A, 2B, 2G, 2H, 7A and 7B). Similarly, the peripheral densities of projections 1a, 1c and 1e make up the sheath between 1a and 1c, and the sheath described between 1b and 2c consists of the outermost parts of the 1b, 2b, 2c and 2d projections (compare Fig. 1C to Figs. 2-4; 7A and 7B). This shows that in both organisms the projections form a highly connected, continuous density network that surrounds the CPC MTs (Fig. 4), which could facilitate communication of regulatory signals throughout the CPC and to neighboring RSs.
The CPC projection network exhibits both structural conservation and adaptation
Previous biochemical and structural studies have shown that the CPC is biochemically and structurally asymmetric [reviewed in Mitchell, 2009] with the projections of the C1 MT being structurally, functionally and biochemically distinct from those of the C2 MT. Our results support this notion by demonstrating that each projection has its own unique shape and size (Figs. 2 and 3). In a comparison of the MTs and their associated projections between Chlamydomonas and Strongylocentrotus, we observed overall astounding similarities in the shape and architecture; some motifs are almost identical, e.g., the 1d projection. This level of structural conservation seems to be higher for C1 projections than C2 projections (Figs. 2 and 3). Although it is established that in vivo the formation of principal and reverse bends is caused by dynein-driven inter-doublet sliding that switches between opposite sides of the axoneme [Satir and Matsuoka, 1989], the relationship between the CPC (fixed and rotating) and the location of dynein activity is not well understood. In vitro, however, sliding disintegration experiments with protease-disrupted Chlamydomonas axonemes show that the C1 MT is predominantly oriented towards the position of active inter-doublet sliding [Wargo and Smith, 2003]. Similar in vitro experiments with protease treated sea urchin axonemes also found inter-doublet sliding in DMTs facing the C1 MT (as well as other locations) [Nakano et al., 2003]. Thus, previous data and our results suggest that the C1 projections could provide unique dynein regulation and that this mechanism of motility regulation through C1 projections is conserved among organisms.
In addition to many similarities, we also observed organism-specific differences, such as the overall shape of the circumference of the projection network. In Chlamydomonas all projections together form a round, cylindrical CPC with a relatively smooth circumference (Fig. 4A). In contrast, the Strongylocentrotus counterpart appears more oval-shaped in cross-section, with the longer axis being perpendicular to a plane connecting both CPC MTs, and the contour is less smooth, interrupted by several protrusions (Fig. 4B). In addition, we noticed a greater number of longitudinal connections between neighboring projections in the algal CPC compared to sea urchin (e.g. projections 1a and 1b in Fig. 4). This high connectivity between projections and smoothly round contour might increase mechanical robustness and allow for free rotation of the CPC in Chlamydomonas flagella, whereas the oval shape of Strongylocentrotus could pose a steric hindrance and resist rotation. Cilia and flagella of all studied metazoans (e.g. Strongylocentrotus) have a fixed CPC orientation in which the plane through the two CPC MTs is perpendicular to the bend plane [reviewed in Mitchell, 2007]. Organisms belonging to Excavata (e.g. Trypanosoma [Branche et al., 2006; Ralston et al., 2006; Gadelha et al., 2006] and Euglena [Melkonian et al., 1982]) also have a fixed or nearly-fixed orientation. In contrast, the CPC has a variable orientation in Chlamydomonas and other organisms belonging to Viridiplantae (e.g. Micromonas, Scourfieldia) and Chromalveolata (e.g. Paramecium, Synura), which is related to an overall helical twist of the CPC structure and a change in the orientation of the CPC during bending, with the plane through the two CPC MTs parallel to the bend plane [Mitchell, 2003b; Mitchell and Nakatsugawa, 2004]. Since the additional connections in the Chlamydomonas CPC occur between projections perpendicular to the plane of curvature of the Chlamydomonas CPC, they could specifically limit flexibility in this perpendicular plane, while their absence could allow flexibility in this plane for the sea urchin CPC.
The distinct structures of CPC projections and previous mutant studies indicate that different projections have unique functions
The 1a projection has the same hammer-like shape in both organisms, but in contrast to the sea urchin CPC, the algal 1a projection is highly connected to neighboring 1a projections (Fig. 4C). Destabilization of 1a in the pf6 Chlamydomonas mutant (Fig. 7E) results in flagella that are immotile, but twitchy [Wargo et al., 2005]. Biochemically, the 1a projection is a stable complex of at least six proteins. PF6 docks with its C-terminus directly to the C1 MT and is thought to recruit the remaining proteins C1a-18, C1a-86, C1a-34, C1a-32, and Calmodulin, that make up the remaining portion of 1a [Rupp et al., 2001; Wargo et al., 2005; Goduti and Smith, 2012]. Based on our 3D structure, we estimate the mass of a single 1a projection in Chlamydomonas to be ~1.7 MDa (Table II). The total estimated mass of the PF6 complex, assuming one copy of all components, is ~400 kDa - considerably smaller than our estimate. Some proteins may be present in higher copy numbers, but it is also likely that 1a contains additional proteins that have failed to co-purify with PF6. When we compared previous 2D EM averages of the pf6 mutant CPC (Fig. 7E; [Mitchell and Sale 1999]) to our 3D structure it appeared that the 2D average was missing not only the 1a projection, but also adjacent material here designated as projection 1e (compare Figs.7E and 7F). Likewise, parts of the PF6 complex may also reach into the 1e projection.
The 1b projection has also been equated with a multi-protein complex, called the Cpc1 complex, that includes enolase, a potential guanylate kinase, a chaperone, an armadillo-repeat protein and Cpc1, with a total mass of ~850 kDa [Mitchell and Sale, 1999; Zhang and Mitchell, 2004; Mitchell et al., 2005]. Mass estimates (Table II) from our data show that each 1b projection is considerably larger at ~1.9 MDa. Similar to projection 1a, this suggests that the Cpc1 complex or individual subunits are present as multiple copies, or additional proteins are so far unidentified. From 2D EM averages (Fig. 7G; [Mitchell and Sale, 1999]) we know that most of projection 1b as well as neighboring densities, here designated as 1f projection, are absent from cpc1 mutants as shown in our simulation of this mutant (Fig. 7H). This near loss of two projections caused by the disruption of a single protein, Cpc1, indicates that Cpc1 is required for stable assembly of both densities and speaks to the highly connected architecture of the CPC.
Aside from differences in mass (Table II), projections 1c and 1d appear remarkably similar between Chlamydomonas and Strongylocentrotus (Figs. 2C-2H). While no biochemical data exist for the 1c projection (no known mutant causing defects in the 1c projection), recent studies found the 1d projection to be a complex of at least five proteins (FAP54, FAP74, FAP46, FAP221, and C1d-87), having a total mass of ~920 kDa [DiPetrillo and Smith, 2010; Brown et al. 2012]. Classical EM of both FAP74ami and fap46-1 strains showed that the entire 1d projections as well as adjacent sheath density between 1d and 1b, here called the 1f projection, are absent [DiPetrillo and Smith, 2010; Brown et al., 2012]. Similar to the previous projections, the total estimated mass of the 1d projections in Chlamydomonas is ~2.6 MDa (Table II), or nearly three-times larger than the sum of all biochemically known subunits.
While the C1 projections appear very similar between Chlamydomonas and Strongylocentrotus, the C2 projections show larger structural differences between organisms. Here we confirm that all C2 projections repeat with a 16 nm periodicity as previously reported [Witman et al., 1978; Mitchell, 2003a], however, the 2a projection is clearly different between Chlamydomonas and Strongylocentrotus (Figs. 3C and 3D). In longitudinal views, each 2a projection consists of two closely-spaced, similar densities in Chlamydomonas (Fig. 3C), which suggests that its interactions with RS heads could differ from those in Strongylocentrotus. A recent 2D EM study reported that the 2a projection appeared larger in human cilia in comparison to the Chlamydomonas CPC [O’Toole et al., 2012]. The CPC’s inherently twisted nature in Chlamydomonas [Mitchell and Nakatsugawa, 2004] makes EM averaging challenging and can lead to an underestimation of projection sizes due to alignment problems. We experienced such complications using a global alignment approach to average the 3D structure of the C2 MT and projections of Chlamydomonas (see Materials and Methods for details). Probably due to the overall smaller size of the C2 projections, our global alignment was biased towards the C1 MT and did not resolve the C2 MT projections sufficiently. We solved these issues by using a local alignment focusing only on the C2 MT and projections, which did resolve these structures in greater detail (compare Supporting Information Figs. S1A and S1B). Our resulting 3D average of the Chlamydomonas C2 MT and associated projections (Fig. 3) looks more similar to the structure of the 2D average of human C2 MT than the 2D Chlamydomonas average [O’Toole et al., 2012], but a comparison of averages obtained by such different methods allows only limited conclusions. Currently, the protein compositions of 2a as well as the adjacent and newly designated 2e projection are unknown.
In contrast, the remaining C2 projections, i.e. 2b-d, are biochemically better characterized in that we know of at least two proteins needed to form these structures. Intriguingly, a member of the kinesin-9 family, Kinesin-like protein 1 (Klp1), was localized to the 2c projection in Chlamydomonas [Bernstein et al., 1994; Yokoyama et al., 2004]. Knockdowns of Klp1 [Yokoyama et al., 2004] resulted in Chlamydomonas with impaired motility and a CPC structure devoid of 2c as well as the sheath material between 2c and 2b, here called the 2d projection. This phenotype was often accompanied by the additional loss of 2b. Although Klp1 was originally thought to be involved in rotation of the CPC, Mitchell and Nakatsugawa [2004] demonstrated that CPC rotation is a passive process. We think that Klp1 is located within the 3D structure of projections 2c and 2d (Figs. 3E and 3F). It should be noted, however, that our estimated mass of the 2c/2d complex is much larger (~950 kDa, Table II) than the mass of Klp1 (~90 kDa) [Bernstein et al., 1994 and Yokoyama et al., 2004] or other kinesins, suggesting that multiple copies and/or additional proteins could be present. The orientation of projection 2d differs between Chlamydomonas and Strongylocentrotus (Fig. 3E and 3F). Contrary to the sea urchin, the algal 2d projection is not perpendicular to the MT axis but diagonal, giving the appearance of a 16 nm “helical shift” that could play a role in the signal transduction from the rotating CPC to the radial spoke heads.
Lechtreck and Witman [2007] identified Hydin as a likely component of the 2b projection. In addition to loss of 2b, knockdown of Hydin was also accompanied by loss of the 2d projection and motility defects. Our biochemical analyses (Fig. 8) show that knockdown of Klp1 also results in the absence of Hydin, suggesting that Klp1 helps anchor 2b to the C2 MT. Overall, the structural defects of Hydin and Klp1 knockdowns appear to be the same [Lechtreck and Witman, 2007 and Yokoyama et al., 2004]. Looking at a 2D EM average of the Chlamydomonas CPC in the Klp1 knockdown (Fig. 7I) [Yokoyama et al., 2004], and comparing it to our wildtype 3D structure, it appears that loss of Klp1 results in the absence of projections 2b-2d due to the highly connected nature of these projections (Fig. 7J). As with projections 1a and 1b, 2b also exhibits lateral connections between neighboring projections in Chlamydomonas, but not in Strongylocentrotus. We estimate the size of projection 2b in Chlamydomonas to be ~1.2 MDa, while Hydin has a mass of only ~540 kDa [Lechtreck and Witman, 2007], suggesting that multiple copies of Hydin could be present in 2b or that 2b is comprised of Hydin as well as other, currently unidentified proteins.
Fig. 8. Biochemical analysis of Chlamydomonas mutations affecting the 1b and 2b projections.
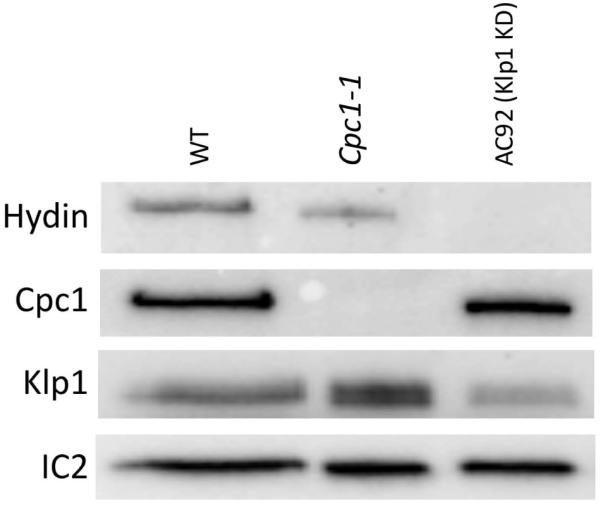
Axonemes from wildtype (WT), cpc1-1 mutant and Klp1-RNAi knockdown (AC92) strains were blotted with different antibodies (left labels) to reveal the level of specific central pair proteins. Hydin is somewhat reduced in axonemes of the cpc1-1 mutant, as previously described [Lechtreck and Witman, 2007], and completely missing from axonemes from Klp1 knockdown strain AC92. The effect of Klp1 knockdown on Hydin has not been reported previously although it is consistent with the loss of the 2b projection in this strain [Yokoyama et. al., 2004]. Klp1 is unaffected in axonemes from the cpc1-1 mutant, and reduced in the knockdown strain. Outer row dynein intermediate chain IC2 was used as a loading control.
The CPC bridge is an intricate set of connections between C1 and C2
The C1 and C2 MTs of the CPC have been described as connected by a set of three structures: a bi-partite bridge and a diagonal link, which, collectively, are known as the bridge with a 16 nm periodicity along the longitudinal axis of the axoneme (Fig. 1C) [Warner, 1976; Linck et al., 1981; Mitchell, 2003a; Nicastro et al., 2005]. Our knowledge of the protein composition of the bridge is limited. Only PF20 has been localized unambiguously to the bridge and is thought to be essential for stable assembly of the CPC, because pf20 mutant axonemes exhibit a 9+0 phenotype, i.e. they lack the entire CPC [Smith and Lefebvre, 1996, 1997]. pf16 axonemeshave a 9+1 phenotype with an unstable C1 MT and PF16 might also localize to the bridge [Zhang et al., 2005]. Our cryo-ET data of the CPC from both Chlamydomonas and Strongylocentrotus show that the bridge is in fact a complex network of connections that link the C1 and C2 MTs (Fig. 5) and that also seem to make several connections to the 1a, 1b, 2a, and 2b projections (Figs.4A and 4B).
The components of the bridge have the same general shape in Chlamydomonas and Strongylocentrotus when viewed in cross-section (Figs. 5A and 5B). In longitudinal slices, however, fewer similarities are obvious (Figs. 5C-5J). Interestingly, in Strongylocentrotus the bridge components seem to be orientated mostly perpendicular to the MTs whereas in Chlamydomonas diagonal components crosslink the bridge rungs (compare Fig. 5I and 5J). It is possible that this interconnected bridge network confers more structural flexibility to the rotating CPC of Chlamydomonas. If so, this could allow for small changes in the relative positions between C1 and C2, which would explain why local alignment focused on individual CPC MTs improved their resolution compared to global alignment (Supporting Information Fig. S1A and S1B). Given the immense complexity of the bridge that we have observed, it is possible that the bridge is not only a fixed structure linking both CPC MTs, but could also have a biochemical function, e.g. relaying signals between the C1 and C2 projections and, ultimately, to the RS heads and dyneins. Learning more about the protein composition of the bridge will help understand its role in axonemal motility.
MIPs could provide stability to the C2 MT in the rotating CPC of Chlamydomonas
MIPs are proteins attached to the inside wall of MTs and were first described in outer DMTs of Chlamydomonas axonemes [Nicastro et al., 2006]. In this study, we have identified two novel MIPs in the C2 MT of the Chlamydomonas CPC. To our knowledge, this is the first description of MIPs in MTs of the CPC. MIP-C2a is ~50 kDa and repeats every 16 nm whereas MIP-C2b is ~35 kDa and repeats every 8 nm (Table II and Fig. 6). The two MIPs are in close proximity to one another and are located on the inner side of the C2 MT which faces the bridge and C1 MT (Figs. 5A, 5B, 6A and 6C). In the outer DMTs, MIPs appear to be highly conserved axonemal structures found in evolutionary distant organisms [Nicastro et al., 2006, 2011; Pigino et al., 2012]. The proteins building MIPs as well as their functions are currently unknown, however, some data suggest that MIPs may contribute to MT stability, because heat-treated DMTs in Chlamydomonas resist depolymerization at protofilaments that have MIPs attached [Nicastro et al., 2006]. Mitchell and Smith [2009] observed a comparable phenomenon in CPC MTs of an uncharacterized Chlamydomonas mutant called unc1; here the only portion of the C2 MT that resisted depolymerization included the protofilaments that are linked to the bridge. Our data show that this is the same part containing MIP-C2a and MIP-C2b, therefore a possible function of the CPC MIPs is to increase MT stability at a site that could experience high mechanical stress.
A previous study by our lab found that MIP1 in the A-tubule of Chlamydomonas DMTs is positioned on the inside of the same tubulin monomer to which the I1 dynein is also attached, but on the MT outside [Heuser et al., 2012a]. This suggests that MIPs may also modulate the tubulin conformation to generate specific attachments sites at the MT outside. Similarly, the locations of the CPC MIPs appear to line up with portions of the bridge, and it is possible that the MIPs play a role in anchoring portions of the bridge to the C2 MT. Aside from linking C1 and C2 together, no other role has been attributed to the bridge and as only one protein has been localized to the bridge unambiguously so far (PF20), a proper analysis of its function is difficult.
We did not observe any MIPs in the CPC of Strongylocentrotus. It should be noted that the resolution of our Strongylocentrotus average was slightly lower than that of Chlamydomonas, probably due to fewer averaged tomograms and the slightly thicker and therefore noisier cryo-tomograms of Strongylocentrotus flagella surrounded by membrane compared to the thinner Chlamydomonas axonemes. Although unlikely, we cannot exclude the possibility that with an increase in resolution, we would also find MIPs present in Strongylocentrotus. Comparing the 3D CPC structures of Chlamydomonas and Strongylocentrotus at the resolution reported in this study, the presence of MIPs in the alga and absence of MIPs in the sea urchin was one of the few striking differences we found in an otherwise highly conserved and remarkably similar CPC architecture in two such evolutionary distant species. The presence of MIPs also in the CPC further stresses their importance and a need to learn more about their composition and the functional roles that they play in assembly and/or motility of cilia and flagella.
The CPC’s striking structural conservation and high connectivity supports its role as a major signaling hub controlling ciliary motility
The majority of structural and functional studies of the CPC have been conducted using Chlamydomonas flagella. From these studies, we have learned that the CPC is a major regulator of motility and most CPC mutations cause flagellar paralysis [Smith and Yang, 2004]. Our cryo-ET data demonstrate remarkable structural similarities between flagella of the single-celled alga Chlamydomonas and sperm flagella of the metazoan Strongylocentrotus, two organisms separated by about 1billion years of evolution (Supporting Information Movie S3). Our structural data revealed that the general architecture of the CPC is highly conserved among species, which is consistent with what one would expect from an important motility regulator and suggests that the detailed regulatory mechanisms may also be conserved. BLAST results of most Chlamydomonas CPC proteins have shown high conservation with proteins from human axonemes [Mitchell, 2009; O’Toole et al., 2012]. Perhaps the most well-known example of this conservation is the protein Hydin, which has been localized to the 2b projection in Chlamydomonas [Lechtreck and Witman, 2007] and mice [Lechtreck et al., 2008]. The absence of Hydin in either organism leads to impaired flagellar/ciliary motility, demonstrating the remarkable conservation of structure and function for this protein from protists to mammals.
In our data, we observed a high degree of connectivity between the CPC projections and between the MTs, which is consistent with the hypothesis of the CPC’s role as a major mechano-chemical signaling hub that controls motility of cilia and flagella. Connections amongst projections occur in both Chlamydomonas and Strongylocentrotus, although longitudinal connections along the MT axis are more prevalent in Chlamydomonas (Fig. 4), which could be related to the higher mechanical stress experienced by the rotating CPC. Visualizing the CPC in 3D at higher resolution provided new, key insights for correlating structure and function of this important regulatory complex. It is also an important step towards the long-term goal of understanding how cilia and flagella work and how they fail in disease.
Supplementary Material
Acknowledgements
The authors are grateful to Raqual Bower, Douglas Tritschler and Mary E. Porter (University of Minnesota) for providing the pf2-4::PF2-GFP (pWT) and ida6::IDA6-GFP (pWT2) rescued strains. We greatly appreciate the exceptional management of the Brandeis EM facility by Chen Xu and thank Daniel T. N. Chen (Brandeis University) for preparing Strongylocentrotus purpuratus sperm. This work was supported by funding from the National Institutes of Health (GM083122 to DN and GM044228 to DRM), from the W. M. Keck Foundation (to DN), the National Science Foundation (DMR-MRSEC-0820492 supporting TH) and by a Pew Biomedical Scholars Award (to DN). BICG was supported in part by the NIH/NIGMS genetics training grant (T32 GM007122).
References
- Adams GMW, Huang B, Piperno G, Luck DJL. Central-pair microtubular complex of Chlamydomonas flagella: Polypeptide composition as revealed by analysis of mutants. J Cell Biol. 1981;91:69–76. doi: 10.1083/jcb.91.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA. Cilia-related diseases. J Path. 2004;204:470–477. doi: 10.1002/path.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber CF, Heuser T, Carbajal-González BI, Botchkarev VV, Jr., Nicastro D. Three-dimensional structure of the radial spokes reveals heterogeneity and interactions with dyneins in Chlamydomonas flagella. Mol Biol Cell. 2012;23:111–120. doi: 10.1091/mbc.E11-08-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein M, Beech PL, Katz SG, Rosenbaum JL. A new kinesin-like protein (Klp1) localized to a single microtubule of the Chlamydomonas flagellum. J Cell Biol. 1994;125(6):1313–1326. doi: 10.1083/jcb.125.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branche C, Kohl L, Toutirais G, Buisson J, Cosson J, Bastin P. Conserved and specific functions of axoneme components in trypanosome motility. J Cell Sci. 2006;119(Pt 16):3443–3455. doi: 10.1242/jcs.03078. [DOI] [PubMed] [Google Scholar]
- Brown JM, DiPetrillo CG, Smith EF, Witman GB. A FAP46 mutant provides new insights into the function and assembly of the C1d complex of the ciliary central apparatus. J Cell Sci. 2012;125(Pt 16):3904–3913. doi: 10.1242/jcs.107151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Molecular architecture of inner dynein arms in situ in Chlamydomonas reinhardtii flagella. J Cell Biol. 2008;183:923–932. doi: 10.1083/jcb.200808050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J Cell Biol. 2009;186:437–446. doi: 10.1083/jcb.200903082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler WL. Attachment of the cap to the central microtubules of Tetrahymena cilia. J Cell Sci. 1984;66:167–173. doi: 10.1242/jcs.66.1.167. [DOI] [PubMed] [Google Scholar]
- Dentler WL, Rosenbaum JL. Flagellar elongation and shortening in Chlamydomonas. III. Structures attached to the tips of flagellar microtubules and their relationship to the directionality of flagellar microtubule assembly. J Cell Biol. 1977;74(3):747–759. doi: 10.1083/jcb.74.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPetrillo CG, Smith EF. Pcdp1 is a central apparatus protein that binds Ca2+-calmodulin and regulates ciliary motlity. J. Cell Biol. 2010;189:601–612. doi: 10.1083/jcb.200912009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Huang B, Luck DJL. Genetic dissection of the central pair microtubules of the flagella of Chlamydomonas reinhardtii. J Cell Biol. 1984;98:229–236. doi: 10.1083/jcb.98.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Gibbons W, Inwood WB. A genetic analysis of suppressors of the PF10 mutation in Chlamydomonas reinhardtii. Genetics. 1988;120:965–976. doi: 10.1093/genetics/120.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymek EE, Lefebvre PA, Smith EF. PF15p is the Chlamydomonas homologue of the Katanin p80 subunit and is required for assembly of flagellar central microtubules. Eukaryot Cell. 2004;3(4):870–879. doi: 10.1128/EC.3.4.870-879.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersold WT, Levine RP, Levine EE, Olmsted MA. Linkage maps in Chlamydomonas reinhardi. Genetics. 1962;47:531–543. doi: 10.1093/genetics/47.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam CA, Wirschell M, Yamamoto R, Fox LA, York K, Kamiya R, Dutcher SK, Sale WS. An axonemal PP2A B-Subunit is required for PP2A localization and flagellar motility. Cytoskeleton. 2011;68:363–372. doi: 10.1002/cm.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–893. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- Gadelha C, Wickstead B, McKean PG, Gull K. Basal body and flagellum mutants reveal a rotational constraint of the central pair microtubules in the axonemes of trypanosomes. J Cell Sci. 2006;119:2405–2413. doi: 10.1242/jcs.02969. [DOI] [PubMed] [Google Scholar]
- Gatti JL, Christen R. Regulation of internal pH of sea urchin sperm. A role for the Na/K pump. J Biol Chem. 1985;260(12):7599–7602. [PubMed] [Google Scholar]
- Gibbons IR. The relationship between the fine structure and direction of beat in gill cilia of a lamellibranch mollusc. J Biophys Biochem Cytol. 1961;11:179–205. doi: 10.1083/jcb.11.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons IR. Sliding and bending in sea urchin sperm flagella. Symp Soc Exp Biol. 1982;35:225–287. [PubMed] [Google Scholar]
- Goduti DJ, Smith EF. Analyses of functional domains within the PF6 protein of the central apparatus reveal a role for PF6 sub-complex members in regulating flagellar beat frequency. Cytoskeleton. 2012;69:179–194. doi: 10.1002/cm.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough UW, Heuser JE. Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol. 1985;100:2008–2018. doi: 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman DS, Levine RP. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 1965;54:1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermacher G, Sale WS. Regulation of flagellar dynein by phosphorylation of a 138-kD inner arm dynein intermediate chain. J Cell Biol. 1997;136:167–176. doi: 10.1083/jcb.136.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harauz G, Van Heel M. Exact filters for general geometry three dimensional reconstruction. Optik (Stuttg) 1986;73:146–156. [Google Scholar]
- Heuser T, Barber CF, Lin J, Krell J, Rebesco M, Porter ME, Nicastro D. Cryoelectron tomography reveals doublet-specific structures and unique interactions in the I1 dynein. Proc Natl Acad Sci USA. 2012a;109(30):E2067–2076. doi: 10.1073/pnas.1120690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser T, Dymek EE, Lin J, Smith EF, Nicastro D. The CSC connects three major axonemal complexes involved in dynein regulation. Mol Biol Cell. 2012b;23(16):3143–3155. doi: 10.1091/mbc.E12-05-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–933. doi: 10.1083/jcb.200908067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins JM. Subsidiary components of the flagella of Chlamydomonas reinhardii. J Cell Sci. 1970;7:823–839. doi: 10.1242/jcs.7.3.823. [DOI] [PubMed] [Google Scholar]
- Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Kato T, Kagami O, Yagi T, Kamiya R. Isolation of two species of Chlamydomonas reinhardtii flagellar mutants, ida5 and ida6, that lack a newly identified heavy chain of the inner dynein arm. Cell Struct Funct. 1993;18(6):371–377. doi: 10.1247/csf.18.371. [DOI] [PubMed] [Google Scholar]
- Kremer JR, Mastronarde DN, McIntosh JR. Computer Visualization of Three-Dimensional Image Data Using IMOD. J Struct Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180(3):633–643. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Whitman GB. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol. 2007;176:473–482. doi: 10.1083/jcb.200611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L, Campagna DR, Pinkus JL, Mulhern H, Wyatt TA, Sisson JH, Pavlik JA, Pinkus GS, Fleming MD. Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol. 2008;28(3):949–957. doi: 10.1128/MCB.00354-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesich KA, Zhang Z, Kelsch CB, Ponichter KL, Strauss JF, 3rd, Lindemann CB. Functional deficiencies and a reduced response to calcium in the flagellum of mouse sperm lacking SPAG16L. Biol Reprod. 2010;82(4):736–744. doi: 10.1095/biolreprod.109.080143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Heuser T, Carbajal-González BI, Song K, Nicastro D. The structural heterogeneity of radial spokes in cilia and flagella is conserved. Cytoskeleton (Hoboken) 2012;69:88–100. doi: 10.1002/cm.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linck RW, Olson GE, Langevin GL. Arrangement of tubulin subunits and microtubule-associated proteins in the central-pair microtubule apparatus of squid (Loligo pealei) sperm flagella. J Cell Biol. 1981;89(2):309–322. doi: 10.1083/jcb.89.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152(1):36–51. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Melkonian M, Robenek H, Rassat J. Flagellar membrane specializations and their relationship to mastigonemes and microtubules in Euglena gracilis. J Cell Sci. 1982;55:115–135. doi: 10.1242/jcs.55.1.115. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. Reconstruction of the projection periodicity and surface architecture of the flagellar central pair complex. Cell Mot Cytoskeleton. 2003a;55:188–199. doi: 10.1002/cm.10121. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil Cytoskeleton. 2003b;56(2):120–129. doi: 10.1002/cm.10142. [DOI] [PubMed] [Google Scholar]
- Mitchell DR. The evolution of eukaryotic cilia and flagella as motile and sensory organelles. In: Jékely G, editor. Eukaryotic membranes and cytoskeleton: origins and evolution. Advances in Experimental Medicine and Biology. Vol. 607. Springer; New York: 2007. pp. 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR. The flagellar central pair apparatus. In: Witman GB, editor. The Chlamydomonas Sourcebook. Vol. 3. Elsevier; New York: 2009. pp. 235–248. Cell Motility and Behavior. [Google Scholar]
- Mitchell DR, Nakatsugawa M. Bend propagation drives central pair rotation in Chlamydomonas reinhardtii flagella. J Cell Biol. 2004;166:709–755. doi: 10.1083/jcb.200406148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell BF, Pedersen LB, Feely M, Rosenbaum JL, Mitchell DR. ATP production in Chlamydomonas reinhardtii flagella by glycolytic enzymes. Mol Biol Cell. 2005;16(10):4509–4018. doi: 10.1091/mbc.E05-04-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Rosenbaum JL. Protein-protein interactions in the 18S ATPase of Chlamydomonas outer dynein arms. Cell Motil Cytoskeleton. 1986;6(5):510–520. doi: 10.1002/cm.970060510. [DOI] [PubMed] [Google Scholar]
- Mitchell DR, Sale WS. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144:293–304. doi: 10.1083/jcb.144.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR, Smith B. Analysis of the central pair microtubule complex in Chlamydomonas reinhardtii. Methods Cell Biol. 2009;92:197–213. doi: 10.1016/S0091-679X(08)92013-6. [DOI] [PubMed] [Google Scholar]
- Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci. 2003;116:1627–1636. doi: 10.1242/jcs.00336. [DOI] [PubMed] [Google Scholar]
- Neugebauer DC, Neuwinger J, Jockenhövel F, Nieschlag E. ‘9 + 0’ Axoneme in spermatozoa and some nasal cilia of a patient with totally immotile spermatozoa associated with thickened sheath and short midpiece. Hum Reprod. 1990;5(8):981–986. doi: 10.1093/oxfordjournals.humrep.a137232. [DOI] [PubMed] [Google Scholar]
- Nicastro D. Cryo-electron microscope tomography to study axonemal organization. Methods Cell Biol. 2009;91:1–39. doi: 10.1016/S0091-679X(08)91001-3. [DOI] [PubMed] [Google Scholar]
- Nicastro D, McIntosh JR, Baumeister W. 3D structure of eukaryotic flagella in a quiescent state revealed by cryo-electron tomography. Proc Natl Acad Sci USA. 2005;102(44):15889–15894. doi: 10.1073/pnas.0508274102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- Nicastro D, Fu X, Heuser T, Tso A, Porter ME, Linck RW. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc Natl Acad Sci USA. 2011;108:E845–E853. doi: 10.1073/pnas.1106178108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell. 1999;10(1):1–4. doi: 10.1091/mbc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omoto CK, Kung C. The pair of central tubules rotates during ciliary beat in Paramecium. Nature. 1979;279(5713):532–534. doi: 10.1038/279532a0. [DOI] [PubMed] [Google Scholar]
- O’Toole ET, Giddings TH, Jr, Porter ME, Ostrowski LE. Computer-assisted image analysis of human cilia and Chlamydomonas flagella reveals both similarities and differences in axoneme structure. Cytoskeleton (Hoboken) 2012;69(8):577–590. doi: 10.1002/cm.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Pigino G, Bui KH, Maheshwari A, Lupetti P, Diener D, Ishikawa T. Cryoelectron tomography of radial spokes in cilia and flagella. J Cell Biol. 2011;195(4):673–687. doi: 10.1083/jcb.201106125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G, Maheshwari A, Bui KH, Shingyoji C, Kamimura S, Ishikawa T. Comparative structural analysis of eukaryotic flagella and cilia from Chlamydomonas, Tetrahymena, and sea urchins. J Struct Biol. 2012;178(2):199–206. doi: 10.1016/j.jsb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Piperno G, Mead K, LeDizet M, Moscatelli A. Mutations in the “dynein regulatory complex” alter the ATP-insensitive binding sites for inner arm dyneins in Chlamydomonas axonemes. J Cell Biol. 1994;125(5):1109–1117. doi: 10.1083/jcb.125.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118(6):1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Power J, Dutcher SK. Extragenic suppressors of paralyzed flagellar mutations in Chlamydomonas reinhardtii identify loci that alter the inner dynein arms. J Cell Biol. 1992;118:1163–1176. doi: 10.1083/jcb.118.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–F42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillin ML, Matthews BW. Accurate calculation of the density of proteins. Acta Crystallogr D Biol Crystallogr. 2000;56(Pt 7):791–794. doi: 10.1107/s090744490000679x. [DOI] [PubMed] [Google Scholar]
- Ralston KS, Lerner AG, Diener DR, Hill KL. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot Cell. 2006;5(4):696–711. doi: 10.1128/EC.5.4.696-711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol. 1967;33(3):543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Moulder JE, Ringo DL. Flagellar elongation and shortening in Chlamydomonas. The use of cycloheximide and colchicine to study the synthesis and assembly of flagellar proteins. J Cell Biol. 1969;41(2):600–619. doi: 10.1083/jcb.41.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp G, O’Toole E, Porter ME. The Chlamydomonas PF6 locus encodes a large alanine/proline-rich polypeptide that is required for assembly of a central pair projection and regulates flagellar motility. Mol Biol Cell. 2001;12:739–751. doi: 10.1091/mbc.12.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp G, Porter ME. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J Cell Biol. 2003;162:47–57. doi: 10.1083/jcb.200303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager R, Granick S. Nutritional studies with Chlamydomonas reinhardtii. Ann NY Acad Sci. 1953;466:18–30. doi: 10.1111/j.1749-6632.1953.tb30261.x. [DOI] [PubMed] [Google Scholar]
- Sale WS. The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol. 1986;102(6):2042–2052. doi: 10.1083/jcb.102.6.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapiro R, Tarantino LM, Velazquez F, Kiriakidou M, Hecht NB, Bucan M, Strauss JF., 3rd Sperm antigen 6 is the murine homologue of the Chlamydomonas reinhardtii central apparatus protein encoded by the PF16 locus. Biol Reprod. 2000;62(3):511–518. doi: 10.1095/biolreprod62.3.511. [DOI] [PubMed] [Google Scholar]
- Satir P. Studies on cilia. 3. Further studies on the cilium tip and a “sliding filament” model of ciliary motility. J. Cell Biol. 1968;39:77–94. doi: 10.1083/jcb.39.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satir P, Matsuoka T. Splitting the ciliary axoneme: Implications for a “switch point” model of dynein arm activity in ciliary motion. Cell Mot. Cyt. 1989;14:345–358. doi: 10.1002/cm.970140305. [DOI] [PubMed] [Google Scholar]
- Smith EF, Sale WS. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science. 1992;257:1557–1559. doi: 10.1126/science.1387971. [DOI] [PubMed] [Google Scholar]
- Smith EF, Lefebvre P. PF16 encodes a protein with armadillo repeats and localizes to a single microtubule of the central apparatus in Chlamydomonas flagella. J Cell Biol. 1996;132(3):359–370. doi: 10.1083/jcb.132.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Lefebvre PA. PF20 gene product contains WD repeats and localizes to the intermicrotubule bridges in Chlamydomonas flagella. Mol Biol Cell. 1997;8(3):455–467. doi: 10.1091/mbc.8.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF. Regulation of flagellar dynein by calcium and a role for an axonemal calmodulin and calmodulin-dependent kinase. Mol Biol Cell. 2002;13:3303–3313. doi: 10.1091/mbc.E02-04-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57:8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stannard W, Rutman A, Wallis C, O’Callaghan C. Central microtubular agenesis causing primary ciliary dskyinesia. Am J Resplr Crlt Care Med. 2004;169:634–637. doi: 10.1164/rccm.200306-782OC. [DOI] [PubMed] [Google Scholar]
- Starling D, Randall J. The flagella of temporary dikaryons of Chlamydomonas reinhardii. Genet Res Camb. 1971;18:107–113. [Google Scholar]
- Sturgess JM, Chao J, Turner JA. Transposition of ciliary microtubules: another cause of impaired ciliary motility. N Engl J Med. 1980;6:318–322. doi: 10.1056/NEJM198008073030606. [DOI] [PubMed] [Google Scholar]
- Summers KE, Gibbons IR. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci USA. 1971;68:3902–3906. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargo MJ, Dymek EE, Smith EF. Calmodulin and PF6 are components of a complex that localizes to the C1 microtubule of the flagellar central apparatus. J Cell Sci. 2005;118:4655–4665. doi: 10.1242/jcs.02585. [DOI] [PubMed] [Google Scholar]
- Wargo MJ, Smith EF. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc Natl Acad Sci USA. 2003;100:137–142. doi: 10.1073/pnas.0135800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FD. New observations on flagellar fine structure. The relationship between matrix structure and the microtubule component of the axoneme. J Cell Biol. 1970;47:159–182. doi: 10.1083/jcb.47.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FD. Ciliary inter-microtubule bridges. J Cell Sci. 1976;20(1):101–114. doi: 10.1242/jcs.20.1.101. [DOI] [PubMed] [Google Scholar]
- Warner JR, Satir P. The structural basis of ciliary bend formation. Radial spoke positional changes accompanying microtubule binding. J Cell Biol. 1974;63:35–63. doi: 10.1083/jcb.63.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr JR, McVittie A, Randall J, Hopkins JM. Genetic control of flagellar structure in Chlamydomonas reinhardii. Genet Res (Cambridge) 1966;7(03):335–351. [Google Scholar]
- Witman GB, Carlson K, Berliner J, Rosenbaum JL. Chlamydomonas flagella. I. Isolation and electrophoretic analysis of microtubules, matrix, membranes, and mastigonemes. J Cell Biol. 1972;54:507–539. doi: 10.1083/jcb.54.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witman GB, Plummer J, Sander G. Chlamydomonas flagellar mutants lacking radial spokes and central tubules. J Cell Biol. 1978;79:729–747. doi: 10.1083/jcb.76.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirschell M, Hendrickson T, Sale WS. Keeping an eye on I1: I1 dynein as a model for flagellar dynein assembly and regulation. Cell Motil Cytoskeleton. 2007;64(8):569–579. doi: 10.1002/cm.20211. [DOI] [PubMed] [Google Scholar]
- Wirschell M, Yang C, Yang P, Fox L, Yanagisawa HA, Kamiya R, Witman GB, Porter ME, Sale WS. IC97 is a novel intermediate chain of I1 dynein that interacts with tubulin and regulates interdoublet sliding. Mol Biol Cell. 2009;20(13):3044–3054. doi: 10.1091/mbc.E09-04-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Sale WS. Casein kinase I is anchored on axonemal doublet microtubules and regulates flagellar dynein phosphorylation and activity. J Biol Chem. 2000;275:18905–18912. doi: 10.1074/jbc.M002134200. [DOI] [PubMed] [Google Scholar]
- Yang P, Fox L, Colbran RJ, Sale WS. Protein phosphatses PP1 and PP2A are located in distinct positions in the Chlamydomonas flagellar axoneme. J Cell Sci. 2000;11:91–102. doi: 10.1242/jcs.113.1.91. [DOI] [PubMed] [Google Scholar]
- Yokoyama R, O’Toole E, Ghosh S, Mitchell DR. Regulation of flagellar dynein activity by a central pair kinesin. Proc Natl Acad Sci USA. 2004;101:17398–17403. doi: 10.1073/pnas.0406817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Shingyoji C. Effects of the central pair apparatus on microtubule sliding velocity in sea urchin sperm flagella. Cell Struct Funct. 1999;24(1):43–54. doi: 10.1247/csf.24.43. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Jones BH, Tang W, Moss SB, Wei Z, Ho C, Pollack M, Horowitz E, Bennett J, Baker ME, Strauss JF., 3rd Dissecting the axoneme interactome: the mammalian orthologue of Chlamydomonas PF6 interacts with sperm-associated antigen 6, the mammalian orthologue of Chlamydomonas PF16. Mol Cell Proteomics. 2005;4(7):914–923. doi: 10.1074/mcp.M400177-MCP200. [DOI] [PubMed] [Google Scholar]
- Zhang H, Mitchell D. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004;117:4179–4188. doi: 10.1242/jcs.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.