Abstract
Similar to other protein-based hydrogels, extracellular matrix (ECM) based hydrogels, derived from decellularized tissues, have a narrow range of mechanical properties and are rapidly degraded. These hydrogels contain natural cellular adhesion sites, form nanofibrous networks similar to native ECM, and are biodegradable. In this study, we expand the properties of these types of materials by incorporating poly(ethylene glycol) (PEG) into the ECM network. We use decellularized myocardial matrix as an example of a tissue specific ECM derived hydrogel. Myocardial matrix-PEG hybrids were synthesized by two different methods, cross-linking the proteins with an amine-reactive PEG-star and photo-induced radical polymerization of two different multi-armed PEG-acrylates. We show that both methods allow for conjugation of PEG to the myocardial matrix by gel electrophoresis and infrared spectroscopy. Scanning electron microscopy demonstrated that the hybrid materials still contain a nanofibrous network similar to unmodified myocardial matrix and that the fiber diameter is changed by the method of PEG incorporation and PEG molecular weight. PEG conjugation also decreased the rate of enzymatic degradation in vitro, and increased material stiffness. Hybrids synthesized with amine-reactive PEG had gelation rates of thirty minutes, similar to the unmodified myocardial matrix, and incorporation of PEG did not prevent cell adhesion and migration through the hydrogels, thus offering the possibility to have an injectable ECM hydrogel that degrades more slowly in vivo. The photo-polymerized radical systems gelled in four minutes upon irradiation allowing for 3D encapsulation and culture of cells, unlike the soft unmodified myocardial matrix. This work demonstrates PEG incorporation into ECM-based hydrogels can expand material properties, thereby opening up new possibilities for in vitro and in vivo applications.
1. Introduction
Extracellular matrix (ECM) based hydrogels, formed from decellularized tissues, have recently emerged as tissue specific scaffolds for both in vivo as well as in vitro tissue engineering [4, 6, 10, 25, 27, 32]. These materials are capable of assembling into a nanofibrous network, reminiscent of the native ECM, at physiological conditions [16]. Unlike synthetic or single component protein hydrogels, these materials retain a complex mixture of tissue specific biochemical cues, including both proteins and polysaccharides [5]. These hydrogels have been used in in vivo applications to promote tissue repair and regeneration, and have also been utilized as model systems for in vitro culture. One example is an ECM hydrogel derived from decellularized porcine myocardial ECM, which has been developed for cardiac tissue engineering applications [27]. The benefits of the tissue specificity of this material have been demonstrated in 2D in vitro culture where it enhanced the maturation and differentiation of human embryonic stem cell derived cardiomyocytes and cardiac progenitor cells versus gelatin, collagen, and a non-tissue specific adipose matrix [5, 9]. In vivo it has demonstrated the potential to increase cardiac muscle, reduce fibrosis, prevent negative left ventricular remodeling, and improve cardiac function after injection into the infarct region in porcine and rat MI models [24, 28].
While the myocardial matrix hydrogel has shown promise as a therapeutic and cell culture material, it has a limited range of properties typical of protein-based hydrogels [16, 26]. It forms a soft hydrogel upon self-assembly with a storage modulus of 5–10 Pa at 1 Hz, which is much softer than native heart tissue. This property in particular does not allow for in vitro 3D culture since cells rapidly migrate through the soft hydrogel. Increasing the stiffness closer to that of native myocardium may have improved outcomes in vivo and could also permit 3D cell culture, providing a tissue specific in vitro platform for cardiac tissue engineering. Increasing the weight percent of the myocardial matrix can double the stiffness to 9.5 Pa, however, this is still far below native tissue [16]. Cross-linking the myocardial matrix with 0.1 % glutaraldehyde increased the stiffness to 136 Pa [26]; however, increasing the amount of glutaraldehyde could lead to toxicity [17]. Mixing the myocardial matrix with collagen increased the mechanical strength to 59.3 Pa, which allowed for encapsulation of human embryonic stem cells, and increased their cardiac differentiation compared to collagen alone [8]; however, adding in other proteins such as collagen disrupts the appropriate tissue specific ratio of cardiac ECM components. The myocardial matrix is also enzymatically degraded in 2–3 weeks in vivo [24]. This degradation time frame has shown positive in vivo results including the recruitment of neovasculature and c-kit+ stem cells [24, 28]; however, adjusting the degradation time may allow for a more prolonged recruitment of progenitor cells. While glutaraldehyde has been shown to adjust degradation time [26], questions regarding safety of this cross-linker remain.
One promising approach to altering the properties of protein-based hydrogels is to dope in synthetic polymers to create a protein-synthetic hybrid hydrogel [15, 31]. For example, the properties of collagen hydrogels were altered by cross-linking the protein-based hydrogel with multi-armed poly(ethylene glycol) (PEG) stars containing activated esters on the termini that reacted with amine residues on the protein [23]. PEG is an ideal candidate to add to protein hydrogels because it is biocompatible and inert [2, 33], and therefore the biochemical cues in the hydrogel remain the same. Herein, we demonstrate a new approach to tissue specific ECM-based hydrogels for tissue engineering by synthesizing myocardial matrix-PEG hybrid hydrogels by two different methods. These approaches allow for expanded material properties, potentially opening up additional applications for the in vivo and in vitro use of ECM hydrogels.
2. Materials and Methods
2.1. Synthesis of decellularized porcine myocardial matrix
Porcine myocardial tissue was harvested, decellularized, and enzymatically digested as previously described [27]. Briefly, porcine left ventricular tissue was sliced into small pieces and placed into 1% (wt/vol) sodium dodecyl sulfate (SDS) solution in 1x PBS pH 7.4 with 0.5% penicillin/streptomycin. The solution was changed every 24 hours. After the fourth day the tissue was rinsed with sterile deionized (DI) water for an additional 24 hours. The ECM was then frozen, lyophilized, and milled into powder using a Wiley Mini Mill. The myocardial matrix (10 mg/mL) was then enzymatically digested at room temperature with pepsin (1 mg/mL) in 0.1 M hydrochloric acid (sterile filtered) for 48 hours. The pH of the digest was then adjusted to give myocardial matrix (6 mg/mL) in 1x PBS pH 7.4. The digest was then frozen, lyophilized, and stored at −20 °C until needed.
2.2. Synthesis of myocardial matrix/star PEG-NHS hybrid hydrogels
Myocardial matrix was resuspended in sterile DI water. Four-arm polyethylene glycol N-succinimidyl glutarate (star PEG-NHS, pentaerythritol core, Mn = 20,000 g/mole, JenKem Technology) was dissolved with sterile DI water at 100 mg/mL. Myocardial matrix and PEG-NHS were mixed to give two different formulation of myocardial matrix/PEG-NHS: N12 (myocardial matrix at 6 mg/mL and PEG-NHS at 12 mg/mL) and N24 (myocardial matrix 6 mg/mL and PEG-NHS at 24 mg/mL). Gelation was induced by incubation at 37 °C for 30 minutes and gel formation was determined as the inability of the solution to flow.
2.3. Synthesis of myocardial matrix/star PEG-acrylate hybrid hydrogels
Myocardial matrix was resuspended in sterile DI water that had been degassed with argon and contained N-hydroxy succinimde acrylate (NHS-acrylate, Aldrich) at 0.67 mg/mL. Four-arm polyethylene glycol acrylate (star PEG-acrylate, pentaerythritol core, Mn = 20,000 g/mole, JenKem Technology) was dissolved with sterile DI water at 100 mg/mL. Irgacure 2959 (50 mg/mL, Aldrich) was dissolved in degassed ethanol. Myocardial matrix, PEG-acrylate, and Irgacure 2959 were mixed to give different formulations of myocardial matrix/PEG-acrylate: P12 (myocardial matrix at 6 mg/mL and PEG-acrylate at 12 mg/mL) and P24 (myocardial matrix at 6 mg/mL and PEG-acrylate at 24 mg/mL). The final concentrations of Irgacure and NHS-acrylate were both 0.5 mg/mL for P12 and P24. Radical polymerization was induced with 305 nm light (4 mW/cm2) for four minutes. Gel formation was determined as the inability of the solution to flow.
2.4. Synthesis of myocardial matrix/PEG-diacrylate hybrid hydrogels
Myocardial matrix was resuspended in sterile DI water that had been degassed with argon and contained N-hydroxy succinimde acrylate (NHS-acrylate) (0.67 mg/mL). PEG-diacrylate (PEGDA, Mn = 545) (100 mg/mL) was dissolved with degassed, sterile DI water, and Irgacure 529 (50 mg/mL) was dissolved in degassed ethanol. Myocardial matrix, PEG-diacrylate, and Irgacure 2959 (1 in 1000 dilution) were mixed to give different formulations of myocardial matrix/PEG-diacrylate: D12 (myocardial matrix at 6 mg/mL and PEG-diacrylate at 12 mg/mL) and D24 (myocardial matrix at 6 mg/mL and PEG-diacrylate at 24 mg/mL). The final concentrations of Irgacure and NHS-acrylate were both 0.5 mg/mL for P12 and P24. Radical polymerization was induced with 305 nm light (4 mW/cm2) for four minutes. Gel formation was determined as the inability of the solution to flow.
2.5. Enzymatic degradation
Enzymatic degradation of hybrid hydrogels with bacterial collagenase was performed as previously described with slight modifications [26]. Myocardial matrix/PEG hybrids formulations N24, N12, P24, P12, D24, and D12 were made as described above. Myocardial matrix (MM) without PEG at 6 mg/mL was used as the control for this study. Myocardial matrix formulations (20 μL) were pipetted in 1.5 mL Eppendorf tubes. Gels were incubated at 37 °C for 24 hours, then collagenase (300 units/mL, Worthington Biomedical Corporation, 20 μL) in 0.1 M tris(hydroxyethyl)aminomethane buffer pH 7.4 with 0.25 M CaCl2 was added on top of each gel and Epperndorf tubes were returned to the 37 °C incubator. Three time-points, 5 hours, 24 hours, and 48 hours, were investigated for each group (MM, N24, N12, P24, P12, D24, and D12, n=8/group). At each time-point, samples were spun down at 15000 rpm for 5 minutes and 5 μL was removed and added to a 0.5 mL Eppendorf tube containing 5 μL of 2% Ninhydrin reagent solution (Sigma). Samples were boiled for 10 minutes on heating block at 110 °C, and then 190 μL of DI water was added to each tube and thoroughly mixed. A 100 μL aliquot was added to a 96 well plate and the corrected absorbance at 570 nm was measured on a BioTek Synergy 4 (Biotek Instruments). All hydrogel samples were blanked against collagenase in an equal volume of PBS pH 7.4.
2.6. Parallel plate rheometry
Parallel plate rheometry was performed on the hydrogels as previously described with slight modification [26]. Gel formulations (N24, N12, P24, P12, D24, and D12) were mixed as described above and 300 μL aliquots were pipetted between two dichlorodimethylsilane coated glass slides with 1 mm spacers. Gels were incubated at 37 °C for 24 hours in a humidity chamber. A TA instruments AR-G2 rheometer was used to test the matrix rheological properties after cross-linking. Gels were tested using a 20 mm geometry with 0.75 mm gap height at 37 °C. Samples were run in triplicate for each group.
2.7. Scanning electron microscopy
Samples (n=3) were prepared and analyzed for scanning electron microscopy as previously described [16]. Briefly, fixed and dehydrated hydrogels were loaded into Teflon sample holders and processed in an automated critical point drier (Leica EM CPD300, Leica, Vienna) with 40 exchange cycles of CO2 at medium speed and 40% stirring. The fill and heating steps were performed at slow speed, while the venting step was performed at medium speed. After drying, the samples were removed and adhered to double-sided carbon tabs on aluminum stubs. The mounted samples were then sputter coated (Leica SCD500, Leica, Vienna) with approximately 7 nm of platinum while being rotated. The samples were then imaged on a FE-SEM (Sigma VP, Zeiss Ltd., Cambridge, UK) at 0.6 kV using the in-lens SE1 detector. SEM images were taken of three different samples per formulation. A total of five images per formulation were analyzed. Four lines were then drawn the same on every image and fibers were counted until ten fibers were obtained per each image totaling 50 fibers per formulation.
2.8. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)
Matrix formulations for all groups (MM, N24, N12, P24, P12, D24, D12) were prepared as described above and 20 μL was added to an Eppendorf tube and incubated at 37 °C for 24 hours. The gels were then frozen and lyophilized. The gels were rehydrated with a sodium dodecyl sulfate (5 weight percent (wt%) in sterile DI water, 15 μL) solution, followed by addition of 2x sample loading buffer (15 μL, Invitrogen), 10x reducing agent (3 μL, Invitrogen), and heated at 80 °C for 20 minutes. The boiled gel solutions (20 μL) were loading into a 12% Bis-Tris gel (Invitrogen). Protein bands were visualized with Imperial Protein Stain (Thermo Scientific).
2.9. Infrared spectroscopy
Matrix formulations for all groups (MM, N24, N12, P24, P12, D24, D12) were prepared as described above and 20 μL was added to an Eppendorf tube and incubated at 37 °C for 24 hours. The gels were then incubated with sterile DI water (1 mL) for 24 hours at 37 °C, followed by ethanol (1 mL) for 24 hours at 25 °C, and dried for 48 hours under vacuum. Infrared Spectra (1256 scans, n=2 for each formulation) were recorded on a Thermo Scientific Nicolet 6700 FTIR with the diamond ATR accessory.
2.10.1. Cell Culture
Murine 3T3 fibroblasts (ATCC, Manassas, VA) were cultured per previously established methods in growth media (GM) made up of Dulbecco’s Modified Eagle Medium (Gibco, Grand Island, NY) supplemented with 10% fetal bovine serum (Hyclone) and 0.5 % penicillin/streptomycin (Gibco, Grand Island, NY)[22]. All cultures were maintained in a humidified 37 °C incubator and 5 % CO2. Media was replaced every two days and cells were split prior to reaching confluence.
2.10.2. Cell Labeling
Murine 3T3 fibroblasts were rinsed with Dulbecco PBS, trypsinized and spun down into a cell pellet (10 million cells per pellet). The cells were then resuspended in PBS, centrifuged at 1,200 rpm for 5 minutes at 25 °C, and the PBS removed from the pellet. The cells were then labeled with PKH26 (red cell membrane dye) following manufacture’s protocol with slight modification. Cells were resuspended with Diluent C (1 mL) followed by quick addition of Diluent C (1 mL)-PKH26 dye (4 μL) solution, gently mixed, and incubated at 37 °C. After 5 minutes a sterile 1wt% bovine serum albumin in PBS (2 mL) was added to the cell suspension and incubated at 37 °C for 1 minute. The cells were then centrifuged at 1,200 rpm for 10 minutes at 25 °C. The cell pellet was resuspended in complete media (10 mL), transferred to a new tube, and centrifuged at 1,200 rpm for 5 minutes at 25 °C. The cell pellet was then resuspended in complete media (10 mL) and centrifuged at 1,200 rpm for 5 minutes two more times. The red-membrane labeled cells were then labeled with calcein AM following a previous procedure [3]. Briefly, the cells were resuspended (1 million cells/mL) with PBS. For every 1 million cells 2.5 μg of calcein AM in dimethylsulfoxide (4 mM) was added and the cell suspension was incubated at 37 °C for 30 minutes. The cells were then centrifuged at 1,200 rpm for 5 minutes at 25 °C and resuspended with PBS (10 mL) three times.
2.10.3. Cell Seeding
Labeled cells in complete media (2.5 million cells/mL, 100 μL) were seeded on myocardial matrix and myocardial matrix/star PEG-NHS hybrid hydrogels (N12 and N24) (n=4) that had been incubated at 37 °C for 1 hour. Cells seeded on top of hydrogels and fluorescent images were taken at 1 hour and 24 hours using a Nikon Eclipse TE2000-U microscope with motorized, programmable stage using a CoolSnap HQ camera controlled by Metamorph 7.6 software. Images (10x) were processed with Fiji software.
2.10.4. Cell encapsulation
Labeled cells (1 million) were pelleted. The cell pellets were then resuspended with pre-hydrogel formulations (400 μL) of either myocardial matrix (6 mg/mL)/star PEG-acrylate (12 mg/mL) (P12), myocardial matrix (6 mg/mL)/star PEG-acrylate (24 mg/mL) (P24), myocardial matrix (6 mg/mL)/PEG-diacrylate (12 mg/mL) (D12), or myocardial matrix (6 mg/mL)/PEG-diacrylate (24 mg/mL) (D24). Cells suspended in hydrogel formulation were then pipetted in wells (100 μL x 4) of a glass bottomed 96 well plate and placed directly on top of a 350 nm UV light with a surface intensity of 0.85 mW/cm2 for 4 minutes. After UV irradiation, complete media (100 μL) was added and the encapsulated cells were incubated at 37 °C. Fluorescent images were taken at 1 hour and 24 hours using a Nikon Eclipse TE2000-U microscope with motorized, programmable stage using a CoolSnap HQ camera controlled by Metamorph 7.6 software. Images (10x) were processed with Fiji (Image J) software.
2.10.5. Alamar Blue assay
Cells were encapsulated in hydrogels (100 μL) as described above. After 24 hours media was removed (100 μL) and fresh media was added (100 μL) with Alamar Blue reagent (10 μL). Fluorescent measurements (excitation 550 nm and emission 585 nm) were taken at various time-points and depicted as fold change of fluorescence relative to the first measurement performed at 2 hours (n=4/group).
2.11. Statistical analysis
All groups were analyzed by one-way ANOVA with a two-tailed distribution followed by a Tukey post-hoc t-test. Significance was accepted at p < 0.05.
3. Results
3.1. Synthesis of myocardial matrix-PEG hybrid hydrogels
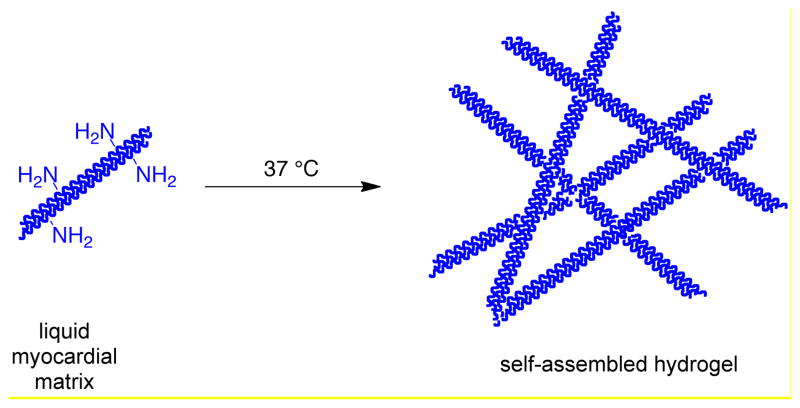
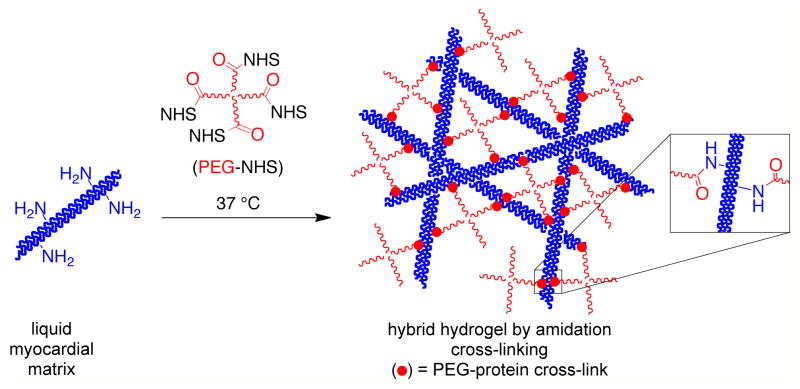
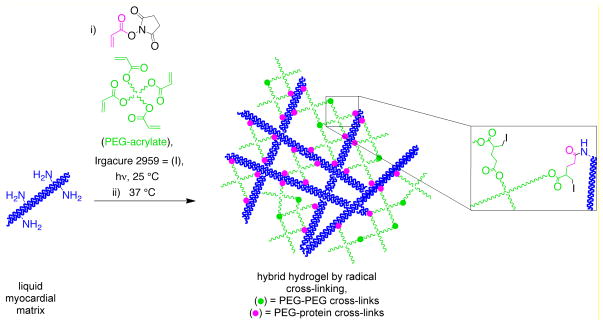
Without modification, the myocardial matrix self-assembles into a nanofibrous hydrogel (Scheme 1). We investigated two different approaches to conjugate PEG into the myocardial matrix protein network to alter the properties of the material: amidation with an amine-reactive PEG (Scheme 2) and radical polymerization of vinyl functionalized myocardial matrix with multi-armed PEG acrylates (Scheme 3). For all hydrogels investigated the myocardial matrix final concentration was 6 mg/mL (Table 1) [27]. For the amidation cross-linked system, two different concentrations of a four-armed PEG star in which the arms were terminated with N-hydroxy succinimides (star PEG-NHS) with molecular weight of ~20,000 g/mole were investigated, 24 mg/mL (N24) and 12 mg/mL (N12). Two different types of PEG-acrylates were investigated for the radical polymerization system (Table 1). A four-armed PEG star in which the arms were terminated with acrylates (star PEG-acrylate) with molecular weight of ~20,000 g/mole at 24 mg/mL (P24) and 12 mg/mL (P12) and a PEG-diacrylate (PEGDA) with a molecular weight of 575 g/mole at 24 mg/mL (D24) and 12 mg/mL (D12) were photopolymerized with acrylamide-functionalized myocardial matrix. Both of the amidation systems (N24 and N12) and the myocardial matrix alone gelled in 30 minutes at 37 °C. All four radical systems (P24, P12, D24, and D12) formed gels after four minutes of irradiation with ultraviolet light. Gelation was determined as the inability of the solution to flow in a vial (data not shown).
Scheme 1.
Self-assembly of the myocardial matrix into a hydrogel at physiological temperature.
Scheme 2.
Synthesis of myocardial matrix-PEG hybrid hydrogel by amidation.
Scheme 3.
Synthesis of myocardial matrix-PEG hybrid hydrogel by radical crosslinking.
Table 1.
Myocardial matrix-PEG hybrid hydrogels.
| Group | [ECM] mg/mL | PEG incorporation | [star PEG-NHS] mg/mL | [star PEG-acrylate] mg/mL | [PEG-diacrylate] mg/mL |
|---|---|---|---|---|---|
| MM | 6 | - | - | - | - |
| N24 | 6 | amidation | 24 | - | - |
| N12 | 6 | amidation | 12 | - | - |
| P24 | 6 | radical | - | 24 | - |
| P12 | 6 | radical | - | 12 | - |
| D24 | 6 | radical | - | - | 24 |
| D12 | 6 | radical | - | - | 12 |
3.2. PEG incorporation into the protein-hydrogel network
3.2.1 SDS-PAGE of myocardial matrix-PEG hybrid hydrogels
PEG incorporation into the protein network of the MM-PEG hybrids was assessed by SDS-PAGE. The myocardial matrix was the only hydrogel sample that was completely solubilized, while all of the hybrid hydrogel samples contained unsolubilized material after heating at 80 °C. Equal volume aliquots were then removed from each sample and analyzed by SDS-PAGE. Strong protein and peptide bands are seen in the myocardial matrix only lane (Figure 1, lane 2) while these same bands are either weak or not present for the hybrids systems (Figure 1, lanes 3–8). Faint bands at 100 kDa, 65 kDa, and 39 kDa are observed for both N12 and N24 with N24 having a darker band near the stacking gel. The bands for N12 are more pronounced then the bands for N24 indicative of less cross-linked protein for N12. Both P24 and P12 have faint bands at 90–100 kDa with the intensity of high molecular weight species (signal >100 kDa) increasing with increasing PEG concentration. D24 and D12 have no bands below 200 kDa with darker smears near the top of gel. D12 has less solubilized protein in the gel than D24. This is likely the result of limited PEGylation of acrylate functionalized-MM in D12 and the result of the proteins radically cross-linking together. The distinct differences with this system were also as verified by IR and SEM below. Color intensity is a relative indication of protein/peptide content and all six hybrid systems have less solubilized protein than the myocardial matrix only gel, suggesting cross-linking of PEG to the protein network.
Figure 1.
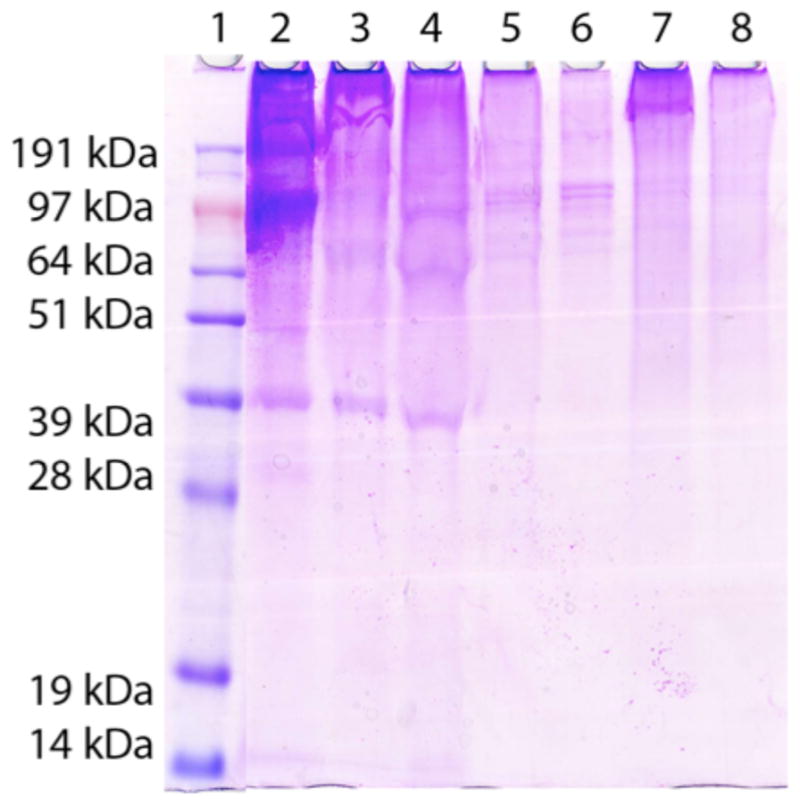
SDS-PAGE of myocardial matrix hybrids. Lane 1) protein ladder, 2) myocardial matrix, 3) star PEG-NHS hybrid 24 mg/mL, 4) star PEG-NHS hybrid 12 mg/mL, 5) star PEG-acrylate hybrid 24 mg/mL, 6) star PEG-acrylate hybrid 12 mg/mL, 7) PEGDA hybrid 24 mg/mL, 8) PEGDA hybrid 12 mg/mL.
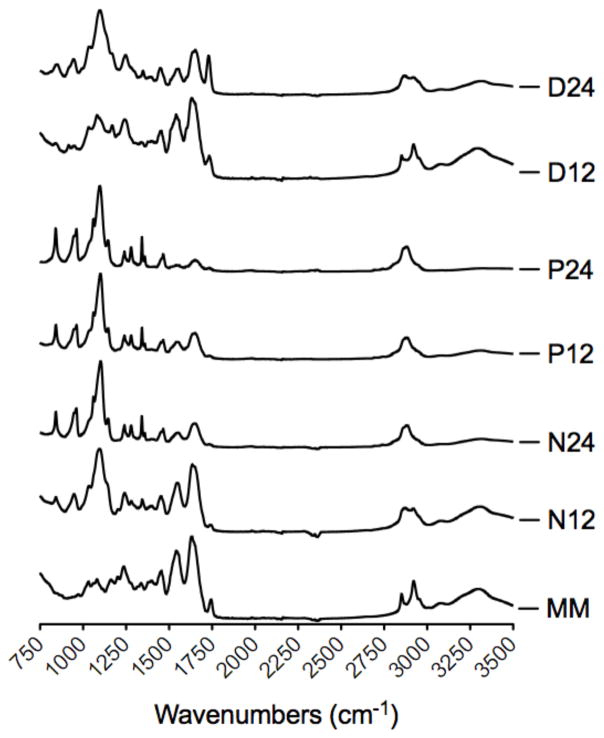
3.2.2. Infrared spectroscopy of myocardial matrix-PEG hybrid hydrogels
Infrared spectroscopy was also used to confirm the presence of PEG in the hydrogels (Figure 2). The myocardial matrix has no strong peak at 1150 cm−1, while all of the myocardial matrix-PEG hybrids have a strong peak at 1150 cm−1 indicative of a C-O stretch present from PEG. Additionally, the peaks from 1250–1750 cm−1 in all hybrid spectra decrease in intensity relative to the C-O peak with increasing PEG concentration. The PEGDA groups have relatively weaker CO peaks than the star PEG-NHS and star PEG-acrylate systems. These spectra indicate that PEG is covalently attached to the protein network of the myocardial matrix.
Figure 2.
Infrared spectrum of myocardial matrix and myocardial matrix hybrids.
3.4. Rheometry on myocardial matrix-PEG hybrid hydrogels
Parallel plate rheometry was performed on myocardial matrix-PEG hybrids (Figure 3) to quantify if the method and amount of PEG used to synthesize the MM-PEG hybrids resulted in an increase in stiffness. The storage modulus was larger than the loss modulus for all six of the hybrid systems indicative of a gel like state. The star PEG-NHS and PEGDA systems had storage moduli ranging from 5–30 Pa similar to the myocardial matrix alone [16] (~5 Pa) and previous glutaraldehyde cross-linked gels [26]. The star PEG-acrylate system had a storage modulus of 719 Pa and 127 Pa for the P24 and P12 hybrids, respectively.
Figure 3.
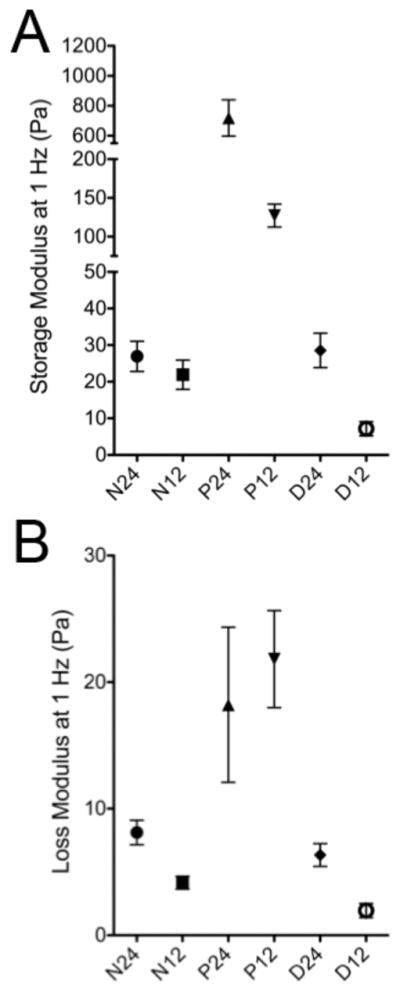
Parallel plate rheometry of myocardial matrix-PEG hybrid hydrogels. A) Storage modulus at 1 Hz. B) Loss modulus at 1 Hz.
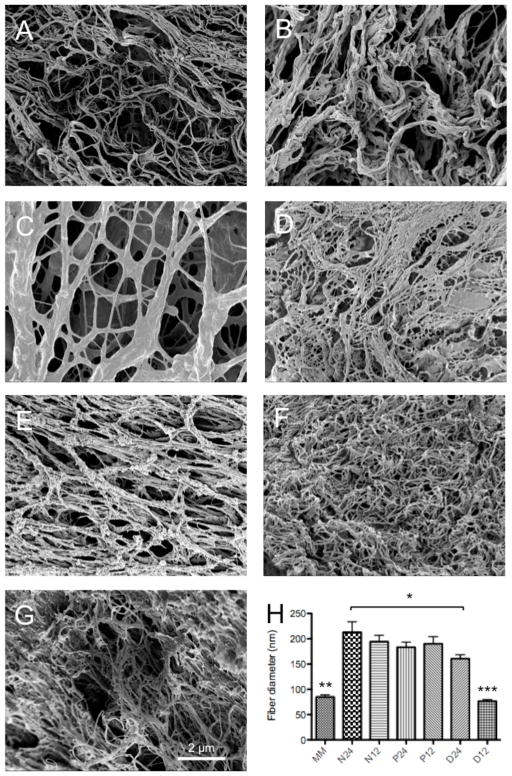
3.5. Scanning electron microscopy analysis of myocardial matrix-PEG hybrid hydrogels
Scanning electron microscopy (SEM) was used to determine if the hybrid hydrogels still retained the nanofibrous structure of the myocardial matrix, and if PEG incorporation changed the fiber diameter of the ECM network. All of the hydrogels exhibited a fibrous like structure (Figure 4a–g). Myocardial matrix (MM) (85 nm +/− 28 nm) and D12 (76 nm +/− 22 nm) had similar diameter while fibers in the other hybrids systems were statistically larger than MM and D12 (Figure 4h). N12 and N24 had fiber diameters of 190 nm +/− 90 nm and 210 nm +/− 150 nm, respectively. P12 and P24 had fiber diameters of 190 nm +/− 90 nm and 180 nm +/− 73 nm, respectively. D24 had a fiber diameter of 160 nm +/− 54 nm, which was statically smaller than the fibers found in the N24 group.
Figure 4.
Scanning electron microscope images of myocardial matrix and myocardial matrix-PEG hybrid hydrogels). A) myocardial matrix alone, B) N12, C) N24, D) P12, E) P24, F) D12, G) D24 and H) fiber diameter of myocardial matrix and myocardial matrix-PEG hybrids. *p<0.05; **p<0.001 compared to N24, N12, P24, P12, D24; *** p<0.001 compared to all MM-PEG hybrids. All images are at 5,000x magnification.
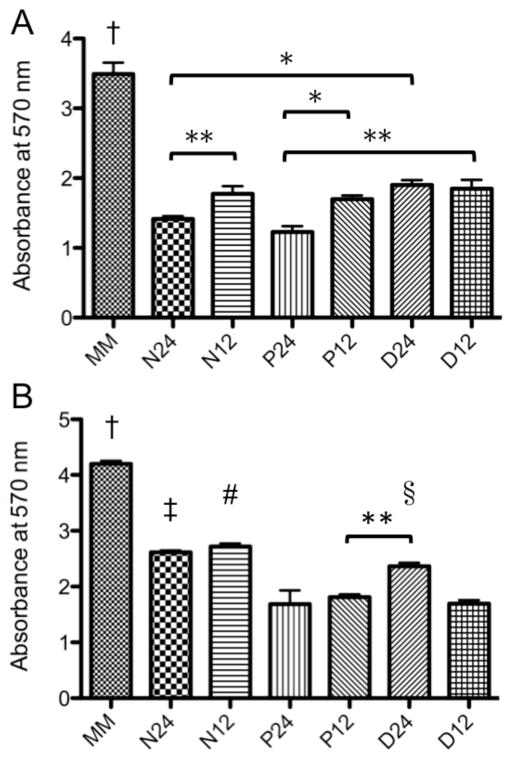
3.7. Enzymatic degradation of myocardial matrix and myocardial matrix-PEG hybrids hydrogels
The ability to tune the rate of enzymatic degradation of hydrogels is important for in vivo and in vitro application [7]. Enzymatic degradation of the hybrid hydrogels was quantified with the Ninhydrin assay. A higher absorbance value relates to more soluble amines in solution, which is indicative of faster enzymatic degradation. After five hours, the myocardial matrix alone was degraded approximately two to three times faster than all of the hybrids (Figure 5a). Slowest degradation rates were seen with N24 and P24. After 48 hours the myocardial matrix still showed significantly more degradation than all of the hybrid gels (Figure 5b). P24, P12, and D12 had significantly less degradation than N24 and N12. P12 was also less degraded than D24.
Figure 5.
Enzymatic degradation of myocardial matrix-PEG hybrid hydrogels. Soluble amines quantified by ninhydrin after A) 5 hours and B) 48 hours. *p<0.05; **p<0.01; †p<0.001 compared to all MM-PEG hybrids; ‡p<0.001 compared to P24, P12, and D12); #p<0.001 compared to P24, P12, and D12); § p<0.001 compared to P24 and D12.
3.6. Adhesion and migration through myocardial matrix-PEG-NHS hybrid hydrogels
Unlike the photo-polymerized systems, the PEG-NHS hybrids only requires mixing of two components, and therefore could allow for in vivo injection using a two-barrel injection system. Since these hybrids slow enzymatic degradation of the myocardial matrix (Figure 5), they could be used to adjust degradation of the material in vivo. It was however important to confirm that PEG incorporation did not prevent cell adhesion and infiltration into the material, which would be critical in vivo. We therefore formed myocardial matrix, N12, and N24 gels in a 96 well plate, and fibroblasts, labeled with a membrane dye as well as calcein AM (green) to indicate live cells, were seeded on top. Images taken 1 hr (Figure 6a–c) and 24 hr (Figure 6d–f) after seeding show green labeled cells within the hydrogel, demonstrating that cells were still capable of attaching and migrating through the PEG-NHS hybrid hydrogels. Green staining overlapped with the red membrane dye (data not shown).
Figure 6.
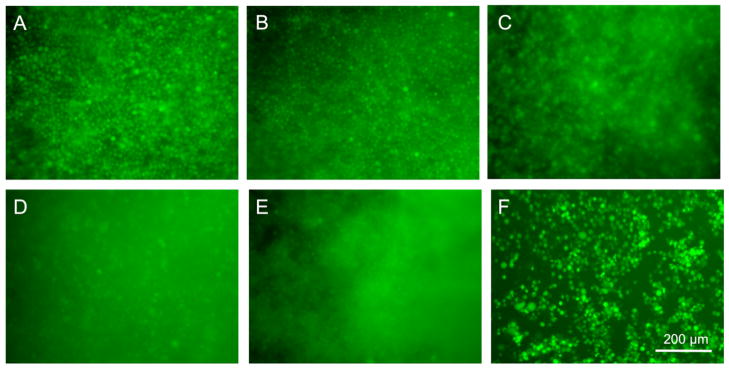
Calcein AM labeled fibroblasts cells within myocardial matrix and hybrid hydrogels. Green stained fibroblsts are observed within the myocardial matrix (A, D), N12 (B, E), and N24 (C, F) after 1 (A–C) and 24 hours (D–F).
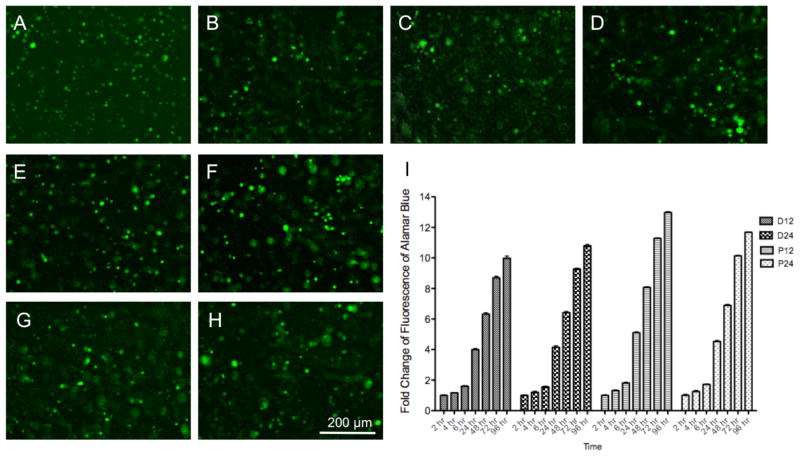
3.7. Cells encapsulation in radical cross-linked myocardial matrix-PEG hybrid hydrogels
The standard myocardial matrix hydrogel is too weak on its own to support 3D encapsulation since cells rapidly migrate to the bottom of the well. Radical cross-linking is however frequently used with PEG-based hydrogels to encapsulate cells for in vitro applications. We were therefore interested to determine if the radically cross-linked PEG-MM hybrids were able to efficiently encapsulate cells, which would enable 3D culture with the appropriate biochemical cues of the myocardial matrix. Fibroblasts were labeled as previously described, and encapsulated in myocardial matrix-PEG hydrogels (P12, P24, D12, and D24) by photo-initiated radical polymerization. Cells were imaged 1 hr (Figure 7a–d) and 24 hrs (Figure 7e–h) after encapsulation and green cells are seen distributed throughout all hydrogels at both time points. We also investigated the metabolic activity of the encapsulated cells with the Alamar blue assay. The media was removed after 24 hours and replaced with media containing Alamar Blue to monitor metabolic activity of the encapsulated cells over 4 days. Initial fluorescent readings were similar for all groups. Metabolic activity is expressed as fold change of fluorescence over time normalized to the reading after 2 hours. All four groups showed an increase in fluorescence over time indicative of metabolically active cells with greater than 10-fold fluorescence achieved for all groups after 4 days (Figure 7i).
Figure 7.
Fibroblast encapsulation in radical cross-linked myocardial matrix-PEG hybrids. A) P12, B) P24, C) D12, and D) D24 after 1 hour. E) P12, F) P24, G) D12, and H) D24 after 24 hours. I) Fold change of fluorescence over time of Alamar Blue of encapsulated cells.
4. Discussion
Tissue-specific ECM-based hydrogels have gained attention for a variety of in vitro and in vivo applications [4, 6, 10, 24, 25, 27, 32]. In particular an ECM based, nanofibrous hydrogel derived from decellularized myocardium, termed myocardial matrix, has shown promise for both in vitro and in vivo cardiac tissue engineering [5, 9, 24, 28]. However, the versatility of this material, like all predominantly collagen-based hydrogels, is restricted because of its limited range of properties, mainly degradation rate and stiffness [16, 26]. The ability to access a broader range of mechanical properties and a tunable rate of enzymatic degradation would expand the scope of this and other ECM based hydrogels. Cross-linking the myocardial matrix with glutaraldehyde has been shown to increase the stiffness of the hydrogel as well as decrease the rate of degradation [26]; however, application of glutaraldehyde in a clinical setting could be problematic due toxicity issues. Blending collagen-based materials with other polymers and proteins is an attractive approach to improve the properties of a material while maintaining many of the positive biochemical cues [15, 18, 31]. In this study, we developed myocardial matrix-PEG hybrid hydrogels in order to tune the properties of this ECM based hydrogel.
PEG is an attractive material to incorporate into ECM hydrogels. PEG-based materials are nontoxic, nonimmunogenic, and allow nutrient and oxygen transport [1, 12, 21]. PEG materials can also be fabricated under conditions compatible with cell culture and encapsulation as well as accessing a broad range of mechanical properties [21, 30]. Cells are unable to bind directly to PEG since it resists protein adsorption. This makes it an attractive additive to ECM hydrogels to preserve their bioactivity [14]. PEG can be added directly by reacting amine residues on the proteins with activated carboxylic acid functionalized PEGs [29]. With this approach PEG can only react with amine residues on the protein thus functioning as a bioinert macromolecule cross-linker [23, 29]. This approach has been used to synthesize collagen-PEG hybrid hydrogels with improved properties [23]. PEG can also be incorporated into the network through radical polymerization of multi-armed PEG acrylates in the presence of acryl-functionalized proteins [14]. In this case, PEG can react with acryl groups on the proteins and with acrylates on other PEGs.
We were interested in investigating both amidation and radical polymerization to synthesize myocardial matrix-PEG hybrid hydrogels to vary the mechanical and degradation properties of the material. For the amidation system we used a 20,000 g/mole four-arm star-PEG terminated with N-hydroxysuccinimde groups (PEG-NHS) which had been previously used to synthetize PEG-collagen hybrid hydrogels [23, 29]. The PEG-collagen system investigated concentration ranges of 12.5–200 mg/mL and 2.5–30 mg/mL of PEG-NHS and collagen; respectively [23]. We were interested in using a lower concentration of ECM for our hybrids, specifically 6 mg/mL since this concentration of myocardial matrix had already been demonstrated to be an effective treatment to improve cardiac function post-MI in rats and pigs [24, 28], while maintaining a PEG-NHS concentration between 2.5–30 mg/mL. For the radical system we investigated two different multi-armed PEG acrylates: a four-arm star-PEG terminated with acrylates (molecular weight (MW) = 20,000 g/mole) (PEG-acrylate) and a PEG-diacrylate (MW = 454 g/mole) (PEGDA). For PEG-NHS, PEG-acrylate, and PEGDA we investigated two different concentrations of PEG, 12 mg/mL and 24 mg/mL, while maintaining a constant myocardial matrix concentration of 6 mg/mL. PEG incorporation into protein networks by radical polymerization has been shown to not alter the biological properties of the proteins, but can have an effect on cell morphology [11]. As a result, the lowest PEG concentrations were chosen based upon the minimal concentration of PEGDA required to form a hydrogel with acryl-functionalized myocardial matrix; this amount was also doubled to investigate how the PEG content altered the properties of the hybrid hydrogels. Both approaches, amidation and radical cross-linking, allowed for PEG incorporation into the nanofibrous network of the myocardial matrix. SDS-PAGE demonstrated that the majority of the protein content did not enter the gel indicative of high molecular weight species. These high molecular species were the result of PEG cross-linking to the ECM. PEGylation of the myocardial matrix was also verified by IR in which all of the gel formulation contained peaks consistent with the ether group in PEG.
Addition of PEG to the myocardial matrix did increase the stiffness of the hydrogels, although this was greater with the radical polymerization with the four-armed PEG. An increase in stiffness with PEG is consistent with the results obtained when synthesizing PEG-hybrids hydrogels by radical polymerization with fibrin [11] and gelatin [14]. PEG-NHS concentration was shown previously to have minimal effect on mechanical properties of collagen-PEG hybrids when the collagen concentration was below 10 mg/mL [23], and therefore a lower stiffness compared to the radical system is not surprising. This result highlights key design factors when synthesizing polymer-protein hybrid hydrogels. The four-armed PEG acrylate can react with itself as well as with acryl-functionalized proteins allowing it to form a stiffer network than the four-arm PEG-NHS. The four-armed PEG-acrylate has four acrylate groups, while the PEGDA only has two acrylate groups, allowing PEG-acrylate to form a stiffer network. Additionally, protein concentration is an important factor to consider when using macromolecular protein cross-linkers to alter material properties.
Interestingly, all of the hybrid hydrogels maintained a nanofibrous nature indicative of the myocardial matrix material. All of the groups, except D12 (PEGDA 12 mg/mL, myocardial matrix 6 mg/mL), had fiber diameters that were 2–2.5 times larger than the myocardial matrix. The similarity of fiber size of D12 to the myocardial matrix is likely the result of minimal cross-linking of PEGDA and minimal incorporation of the PEGDA into the protein network as verified by IR. Functionalization of the myocardial matrix with PEG-NHS still allowed for protein self-assembly. This is consistent with results found when PEGylating collagen mimetic peptides. Previously, it has been demonstrated that conjugation of collagen mimetic peptides to the termini of 20 kDa PEG-NHS via amidation still allowed for temperature induced self-assembly of the peptides [29]. The larger fiber diameter for NHS-PEG system is likely due the PEG cross-linking multiple protein fibers together while the network is self-assembling since the gelation rate of the myocardial matrix and both PEG-NHS systems is similar, 30 minutes at 37 °C. The increased fiber diameter also for the radical system is likely due to the rapid formation of the radically cross-linked network (4 minutes) in which the PEG is cross-linking to itself and multiple fibers. Increase in fiber diameter has been seen with other methods of cross-linking collagen based materials [13].
PEG incorporation into the myocardial matrix network also had a dramatic effect on in vitro enzymatic degradation rate. The decrease in enzymatic degradation rate upon cross-linking the myocardial matrix with glutaraldehyde has been previously reported [26]. Decrease in enzymatic degradation rate also been observed when incorporating PEG into collagen [23] and gelatin hydrogels [14]. The method and amount of PEG incorporation into the myocardial matrix can alter the degradation rate over time. This is an important design feature to be able to adjust since the myocardial matrix only material is degraded within 2–3 weeks in vivo and rate of enzymatic degradation has been shown to be important for cell encapsulation and viability [7].
The method of PEG incorporation into the myocardial matrix also has an effect on its end application. Cross-linking of the myocardial matrix with PEG-NHS allows the material to still be injectable. The PEG-NHS/myocardial matrix had similar mechanical properties and tunable rate of degradation to the glutaraldehyde cross-linked myocardial matrix. It was demonstrated that cells easily migrated through the glutaraldehyde cross-linked hydrogels [26]. As a result we investigated if cells migrated through the PEG-NHS hybrids. Interestingly, when cells are seeded on top of preformed MM, N12, and N24 hydrogel they migrate through the materials to the bottom of the well, thus limiting the application of this method of PEG incorporation for in vitro 3D culture studies. However, this type of material could have positive results when applied in vivo since the material is still injectable and allow cells to migrate through it. A slower degradation rate of the myocardial matrix could potentially prolong the recruitment of endogenous progenitors cells. In contrast, a photo-cross-linked radical PEG-myocardial matrix has limited application as an injectable material in vivo due to the limited ability of light to penetrate tissue. Radical cross-linked PEG hydrogels have however been used extensively to encapsulate cells for in vitro applications [19, 20]. Here we demonstrate that this is also possible with PEG/myocardial matrix hydrogels. Viable cells are evenly distributed throughout the hydrogels after photo-induced polymerization. Alamar blue was added to cells 24 hours after encapsulation and metabolic activity increased over the course of four days indicating that cells were still viable five days after encapsulation. This provides promise for cell studies that rely on 3-D systems with cardiac extracellular matrix specific cues to better mimic the in vivo environment.
5. Conclusion
A new approach for expanding the properties of ECM based hydrogels has been described. We demonstrate that incorporation of PEG into the ECM network of myocardial matrix hydrogels can vary the properties of the material, and that the method of PEG incorporation can have a direct impact on the end application of the hybrid hydrogel. All hybrid investigated had decreased rates of enzymatic degradation compared to the unmodified myocardial matrix, while maintaining a nanofibrous structure. Myocardial matrix cross-linked with four-arm PEG-NHS resulted in slowed degradation time, yet still allowed cells to quickly migrate through these materials. Radical cross-linking the myocardial matrix with multi-armed PEG acrylates resulted in hybrid hydrogels with range of mechanical properties dependent upon PEG content and structure. Radical cross-linking also allowed for cells to be encapsulated within the hydrogels, which were viable and metabolically active five days after encapsulation. This demonstrates both an in vitro 3-D hybrid system for cell culture and promise for an in vivo hybrid injectable material for repairing the heart.
Acknowledgments
This work was funded by the National Heart Lung Blood Institute (NHLBI) (5R01HL113468).. GNG acknowledges the American Heart Association for a postdoctoral fellowship (12POST9750018). Dr. James A. J. Fitzpatrick and Matthew Joens from the Salk Institute for Biological Studies are thanked for scanning electron microscopy acquisition. Prof. Michael J. Sailor is thanked for access to an infrared spectrometer. Dr. Yu Suk Choi is thanked for guidance with cellular labeling protocols. Dr. Adam J. Engler and Ludovic Vincent are thanked for assistance with confocal microscopy.
References
- 1.Alcantar NA, Aydil ES, Israelachvili JN. Polyethylene glycol-coated biocompatible surfaces. J Biomed Mater Res. 2000;51:343–51. doi: 10.1002/1097-4636(20000905)51:3<343::aid-jbm7>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 2.Browning MB, Russell B, Rivera J, Höök M, Cosgriff-Hernandez EM. Bioactive Hydrogels with Enhanced Initial and Sustained Cell Interactions. Biomacromolecules. 2013 doi: 10.1021/bm400634j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi YS, Dusting GJ, Stubbs S, Arunothayaraj S, Han XL, Collas P, Morrison WA, Dilley RJ. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J Cell Mol Med. 2010;14:878–89. doi: 10.1111/j.1582-4934.2010.01009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeQuach J, Lin JE, Cam C, Hu D, Salvatore M, Sheikh F, Christman KL. Injectable skeletal muscle matrix hydrogel promotes neovascularization and muscle cell infiltration in a hindlimb ischemia model. Eur Cell Mater. 2012;23:400–12. doi: 10.22203/ecm.v023a31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. Simple and high yielding method for preparing tissue specific extracellular matrix coatings for cell culture. PLoS One. 2010;5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeQuach JA, Yuan SH, Goldstein LS, Christman KL. Decellularized porcine brain matrix for cell culture and tissue engineering scaffolds. Tissue Eng Part A. 2011;17:2583–92. doi: 10.1089/ten.tea.2010.0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–51. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 8.Duan Y, Liu Z, O’Neill J, Wan LQ, Freytes DO, Vunjak-Novakovic G. Hybrid Gel Composed of Native Heart Matrix and Collagen Induces Cardiac Differentiation of Human Embryonic Stem Cells without Supplemental Growth Factors. Journal of Cardiovascular Translational Research. 2011;4:605–15. doi: 10.1007/s12265-011-9304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.French KM, Boopathy AV, DeQuach JA, Chingozha L, Lu H, Christman KL, Davis ME. A naturally derived cardiac extracellular matrix enhances cardiac progenitor cell behavior in vitro. Acta Biomater. 2012;8:4357–64. doi: 10.1016/j.actbio.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–7. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Gonen-Wadmany M, Goldshmid R, Seliktar D. Biological and mechanical implications of PEGylating proteins into hydrogel biomaterials. Biomaterials. 2011;32:6025–33. doi: 10.1016/j.biomaterials.2011.04.055. [DOI] [PubMed] [Google Scholar]
- 12.Hillwest JL, Chowdhury SM, Sawhney AS, Pathak CP, Dunn RC, Hubbell JA. Prevention of Postoperative Adhesions in the Rat by in-Situ Photopolymerization of Bioresorbable Hydrogel Barriers. Obstet Gynecol. 1994;83:59–64. [PubMed] [Google Scholar]
- 13.Hovakimyan M, Guthoff RF, Stachs O. Collagen Cross-Linking: Current Status and Future Directions. J Ophthalmol. 2012 doi: 10.1155/2012/406850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutson CB, Nichol JW, Aubin H, Bae H, Yamanlar S, Al-Haque S, Koshy ST, Khademhosseini A. Synthesis and Characterization of Tunable Poly(Ethylene Glycol): Gelatin Methacrylate Composite Hydrogels. Tissue Eng Pt A. 2011;17:1713–23. doi: 10.1089/ten.tea.2010.0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia XQ, Kiick KL. Hybrid Multicomponent Hydrogels for Tissue Engineering. Macromol Biosci. 2009;9:140–56. doi: 10.1002/mabi.200800284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson TD, Lin SY, Christman KL. Tailoring material properties of a nanofibrous extracellular matrix derived hydrogel. Nanotechnology. 2011;22:494015. doi: 10.1088/0957-4484/22/49/494015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudson MJ, Cooper CS, Block CA, Hawtrey CE, Austin JC. Calcification of glutaraldehyde cross-linked collagen in bladder neck injections in children with incontinence: A long-term complication. J Urology. 2006;176:1143–6. doi: 10.1016/j.juro.2006.04.059. [DOI] [PubMed] [Google Scholar]
- 18.Li ZQ, Guan JJ. Hydrogels for Cardiac Tissue Engineering. Polymers-Basel. 2011;3:740–61. [Google Scholar]
- 19.Nguyen KT, West JL. Photopolymerizable hydrogels for tissue engineering applications. Biomaterials. 2002;23:4307–14. doi: 10.1016/s0142-9612(02)00175-8. [DOI] [PubMed] [Google Scholar]
- 20.Nuttelman CR, Rice MA, Rydholm AE, Salinas CN, Shah DN, Anseth KS. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33:167–79. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18:1345–60. [Google Scholar]
- 22.Rao N, Evans S, Stewart D, Spencer KH, Sheikh F, Hui EE, Christman KL. Fibroblasts influence muscle progenitor differentiation and alignment in contact independent and dependent manners in organized co-culture devices. Biomed Microdevices. 2013;15:161–9. doi: 10.1007/s10544-012-9709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sargeant TD, Desai AP, Banerjee S, Agawu A, Stopek JB. An in situ forming collagen-PEG hydrogel for tissue regeneration. Acta Biomaterialia. 2012;8:124–32. doi: 10.1016/j.actbio.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 24.Seif-Naraghi SB, Singelyn JM, Salvatore MA, Osborn KG, Wang JJ, Sampat U, LKO, Strachan GM, Wong J, Schup-Magoffin PJ, Braden RL, Bartels K, DeQuach J, Preul M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Science Translational Medicine. 2013;5:173ra25. doi: 10.1126/scitranslmed.3005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sellaro TL, Ranade A, Faulk DM, McCabe GP, Dorko K, Badylak SF, Strom SC. Maintenance of human hepatocyte function in vitro by liver-derived extracellular matrix gels. Tissue Eng Part A. 2010;16:1075–82. doi: 10.1089/ten.tea.2008.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singelyn JM, Christman KL. Modulation of Material Properties of a Decellularized Myocardial Matrix Scaffold. Macromol Biosci. 2011;11:731–8. doi: 10.1002/mabi.201000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009;30:5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singelyn JM, Sundaramurthy P, Johnson TD, Schup-Magoffin PJ, Hu DP, Faulk DM, Wang J, Mayle KM, Bartels K, Salvatore M, Kinsey AM, DeMaria AN, Dib N, Christman KL. Catheter-Deliverable Hydrogel Derived From Decellularized Ventricular Extracellular Matrix Increases Endogenous Cardiomyocytes and Preserves Cardiac Function Post-Myocardial Infarction. Journal of the American College of Cardiology. 2012;59:751–63. doi: 10.1016/j.jacc.2011.10.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stahl PJ, Romano NH, Wirtz D, Yu SM. PEG-Based Hydrogels with Collagen Mimetic Peptide-Mediated and Tunable Physical Cross-Links. Biomacromolecules. 2010;11:2336–44. doi: 10.1021/bm100465q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tibbitt MW, Anseth KS. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol Bioeng. 2009;103:655–63. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandermeulen GWM, Klok HA. Peptide/protein hybrid materials: Enhanced control of structure and improved performance through conjugation of biological and synthetic polymers. Macromol Biosci. 2004;4:383–98. doi: 10.1002/mabi.200300079. [DOI] [PubMed] [Google Scholar]
- 32.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomaterialia. 2011;7:1040–9. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu JM. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639–56. doi: 10.1016/j.biomaterials.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]