Abstract
Coronary spasm is an important and often overlooked etiology of chest pain. While coronary spasm, or Prinzmetal’s angina, has been thought of as benign, contemporary studies have shown serious associated adverse outcomes including acute coronary syndrome, arrhythmia, and death. Definitive diagnosis of coronary spasm can at times be difficult given the transience of symptoms. Numerous agents have been historically described for provocative testing. We provide a review of literature for the role of provocation testing in the diagnosis of coronary spasm.
Keywords: Prinzmetal’s angina, Coronary spasm, provocation testing, Acetylcholine, Ergonovine
Introduction
Coronary spasm (CS) is an important etiology of angina that often goes undiagnosed. While older literature suggests that the prognosis for patients with coronary spasm is relatively benign (1), contemporary reports indicate that CS has been associated with ischemia, acute coronary syndrome, arrhythmia, and sudden cardiac arrest (SCA) (2–4), with a worse prognosis reported in those with even trivial coronary stenosis (5). Given the transience of CS, diagnosis can be difficult and may require more sophisticated provocative diagnostic approaches. In current United States (US) practice, it seems provocation testing in the cardiac catheterization laboratory is performed less frequently, although quantitative data is not available. Numerous agents have been described for spasm provocation testing including ergonovine (ER), acetylcholine (ACH), neuropeptide Y, and dopamine (6–9), however a relatively larger body of evidence supports ER and ACH for clinical practice. We herein review provocative testing for the diagnosis of CS.
Pharmacology
The pharmacologic agents most often used clinically in provocation testing for the diagnosis of CS are ER (6,10,11) (12–17) (18) (19,20) and ACH (1,8,21–23). ER acts on smooth muscle mainly via activation of serotonergic (5-HT2) receptors to produce vasoconstriction (24). Activation of the endothelium in response to ER also causes release of inhibitory prostanoid substances; those with endothelial dysfunction may have more pronounced contraction (24). ER is predominantly metabolized by the liver, and serves as a major substrate of CYP3A4 hepatic enzymes. Adverse reactions to ergot alkaloids are diverse and include angina, ischemia, MI, arrhythmia, nausea, allergic reaction and ergotism (18,25).
ACH acts on the endothelium and smooth muscle via muscarinic receptors. In healthy endothelium, ACH activation results in vasodilation. However in the setting of endothelial dysfunction, endothelial cells insufficiently produce nitric oxide, a potent smooth muscle relaxant (26) resulting in blood vessel contraction rather than vasodilation. Adverse reactions to ACH include hypotension, bradycardia, dyspnea, flushing (27). When using IC ACH, the risk of brady-arrhythmia is often circumvented with temporary ventricular pacing. Serious reactions include ventricular tachycardia, shock, and cardiac tamponade (28).
Both ACH and ER are not US FDA approved for the indication of coronary vasospasm diagnosis. Various testing protocols utilizing intracoronary (IC) and intravenous (IV) administration have been described (Table 1). Importantly, induction of spasm with IV ER can produce multivessel spasm and hemodynamic instability, making arteriograms difficult to obtain. Furthermore, IC nitroglycerin may be required to relieve spasm. For these reasons, Hackett el al demonstrated that induction of CS with IC ER may be safer than IV administration (6). Additionally, IC (ER or ACH) administration allows provocation of the right and left coronaries separately. Furthermore, while IV ER provocation testing has good sensitivity (100% using angina as part of the diagnostic criterion and 94% using ST elevation) (17), reports show frequency of provoked CS with IC ER to be 2.2–2.6 times higher than IV testing (23). Specificity of IV and IC ER provocation testing are similarly high >90% (6,11). Despite high sensitivity, false negatives have been reported (29), thus a negative test cannot always exclude CS.
Table 1.
Provocation Testing Dosing Protocols
| Study | Ergot Derivative | Acetylcholine |
|---|---|---|
| Invasive | ||
| Akasaka et al (10) | ER 100 ug IV (up to 200 ug) | N/A |
| Bertrand et al (11) | Methergine 400 ug IV | N/A |
| Hackett et al (6) | ER 6–50 ug IC | N/A |
| Harding et al (12) | ER 50–150 ug IV | N/A |
| Japanese Circulation Society (45) | ER 20–60 ug (LCA, IC); ER 20–60 ug (RCA, IC) | 20–100 ug (LCA, IC); 20–50ug (RCA, IC) |
| Okumura et al (8,22) | 200 ug IV | 20–100 ug (LCA, IC); 20–50 ug (RCA, IC) |
| Song et al (19) | ER 1–30 ug IC | 10–100 ug IC |
| Sueda et al (13–16,23) | ER 40ug (RCA, IC); 64 ug (LCA, IC) | 20–100 ug (LCA, IC); 20–80 ug (RCA, IC) |
| Takagi et al (18) | ER 20–60 ug (LCA, IC); ER 20–60 ug (RCA, IC) | 20–100 ug (LCA, IC); 20–50 ug (RCA, IC) |
| Waters et al (17) | ER 12.5–400 ug IV | N/A |
| Yasue et al (21) | N/A | suspected vessel: 10–100 ug IC; Contralateral artery:20–100 ug (LCA, IC); 20–50 ug (RCA, IC) |
| Noninvasive | ||
| Song et al (20) | ER 25–50 ug IV (up to 350 ug total) | N/A |
ER= ergonovine maleate, IC= intracoronary, IV=intravenous, LCA= left coronary artery, ml= milliliter, NSS= normal saline solution, RCA= right coronary artery, ug= micrograms, N/A= not applicable.
Pathogenesis of Coronary Spasm
The role of CS in variant angina, or Prinzmetal’s angina, is well documented (30). Patients have spontaneous angina episodes associated with reversible constriction of a focal segment or segments of coronary artery leading to restriction of coronary blood flow and myocardial ischemia. These episodes are often associated with ST segment elevation (31). Spasm can involve the epicardial coronary vessels but coronary microvascular spasm can also occur and may be associated with cardiac syndrome X (32).
The pathogenesis of CS is likely multifactorial and heterogeneous among different populations. Coronary vascular smooth muscle hyper-reactivity (33) has been described and is thought to be a consequence of loss of balance between vascular myosin light chain kinase and phosphatase activity, leading to a predominance of myosin light chain phosphorylation and resultant excessive vascular smooth muscle contraction (34). Endothelial cell dysfunction also contributes as these cells act as paracrine regulators of vascular tone and respond to changes in shear stress, myogenic constriction, and vasoactive substances by releasing various vasorelaxant substances (35,36). Prior work has demonstrated that ACH induced dilation is lost in the presence of atherosclerosis in the coronaries of human transplanted hearts (37).
Interestingly, differing pathophysiology has been proposed for focal and diffuse vasospasm. Atherosclerotic lesions have been identified at the site of focal spasm using intravascular ultrasound (38). Akasaka et al compared coronary flow reserve (CFR) of patients with focal versus diffuse spasm and found that patients with ER-induced diffuse spasm had significantly reduced CFR compared with control (normal coronaries, no spasm). In contrast, those with focal ER-induced spasm maintained normal CFR. They suggested that focal spasm might be related to localized epithelial dysfunction of the epicardial coronaries without significant effect on coronary microvascular function (10).
Variant angina episodes occur most from midnight to early morning when vagal tone is highest. Increased vagal tone and hyperreactivity to sympathetic stimulation have been described in the mechanism, with some even reporting surgical sympathetic denervation as a therapeutic option for medically refractory patients (39).
Environmental factors such as smoking (1,40), metabolic abnormalities (41), and alcohol consumption (1) may also be pathogenic contributors. Racial variations in incidence have been reported (42) with a higher prevalence found in Japanese than Western individuals (11,23,43), suggesting genetic differences in addition to differences in environmental exposures. Several single nucleotide polymorphisms have been identified (44) that are thought to be related to CS.
Provocation Testing
Invasive
A positive response to ACH or ER spasm provocation testing is defined as transient occlusion (>90% narrowing) of a coronary artery with signs and symptoms of myocardial ischemia (angina/ST changes) (45).
The incidence of positive testing depends on the population. Bertrand et al conducted a large French cohort study with 1089 patients who underwent ER provocation testing during routine coronary angiography for suspected ischemia (11). They found that 12.3% of patients developed CS. Provoked spasm was most common in patients with rest angina (38%) and recent MI (20%), and less in those with exertional symptoms (4.3%) and atypical angina (1.2%). Notably, 59% of vasospasm episodes occurred on pre-existing fixed stenoses. Harding et al conducted a large North American study evaluating 3447 patients with non-obstructive coronary disease (<50% stenosis) and without previously documented Prinzmetal’s angina (12). These investigators reported 4% positive invasive ER testing. In comparison to the study by Bertrand et al, lower doses of ER were used. After multivariate analysis, degree of coronary disease on angiography and smoking were statistically significant predictors of spasm (12).
Yasue et al evaluated the sensitivity of IC ACH in provoking CS (21). All 27 patients studied had CS based on rest angina symptoms with associated ST changes. Injection of ACH into the suspected coronary artery induced spasm in 30 of the 32 (94%) arteries in 25 of the 27 (93%) patients. Half of the coronary arteries tested not suspected to be responsible for the attacks showed 25–75% luminal narrowing, without chest pain or ST changes, suggestive of good sensitivity and specificity of the testing (21). Okumura et al examined the effect of IC ACH testing in 70 patients with variant angina and 93 patients without variant or rest angina and reported a 90% sensitivity and 99% specificity (22). In a larger study, Sueda el al explored the incidence of spasm with IC ACH testing in 685 patients undergoing angiography and found CS in 221 patients (32.3%)(23). Similar to ER provocation studies, more provoked spasm was seen in patients with rest angina (83 of 125, 66.9%) and least in those with atypical angina (4 of 83, 4.8%). Spasm was also more common in patients with prior MI and atherosclerosis, although those with advanced atherosclerosis were excluded (23).
ER testing is often compared with or used in combination with ACH. Sueda et al compared performed IC administration of both ACH and ER in the same 171 patients, all of whom had <50% stenosis (13). They found no significant differences in provoked spasm between the two agents (ACH: 33% versus ER: 32%, NS). Notably, ACH provoked more diffuse and distal spasm whereas ER induced more focal spasm. No serious or irreversible complications were observed. In a subsequent study in 2004, the same Japanese investigators retrospectively analyzed 1508 selective spasm provocation tests (873 ACH, 635 ER) (14). They found no difference between the agents in patients with ischemic heart disease, but found significantly higher provoked spasm with ACH than ER in patients with no CAD (11.0% vs 6.4%, p<0.05 respectively). Additionally, multiple spasm as well as spasm not associated with focal stenosis was provoked more with ACH. More complications were seen with ACH compared with ER (1.4% vs 0.2%) however none were serious (death, MI) or irreversible.
Sueda et al also investigated the usefulness of combined provocation testing with ACH and ER (15). Three sequential provocation tests were performed using ACH, then ER, followed by a combination test using sequential IC injections of both agents, eliminating patients with positive spasm between tests. In this study, significantly more spasm was induced in patients with rest angina and ischemia (98% with provoked spasm) compared with patients with atypical angina and no ischemia (8% with provoked spasm). Ischemia was defined as ECG changes or abnormal scintigraphy during exercise. In those with rest angina, ACH provoked spasm in 55%, ER provoked spasm in 33%, and the combination test provoked spasm in 92% of the remaining patients. No major complications were reported.
Sueda et al. additionally studied whether the CS induced by provocation testing correlated with the angina provoking artery responsible for the sites of ST elevation during ischemic attacks (16). They evaluated 42 patients, predominantly men, with variant angina and history of a recent STEMI attributed to spasm. The correlation with the ACH test was 78.6% for all patients and 80.0% for all sites of ST segment elevation. By adding the ER test after the ACH test, the correlation increased to 95.2% for all patients and 95.6% for all sites of ST segment elevation.
A particularly important population in which diagnostic testing for CS may be critical are survivors of SCA who have no apparent cardiac disease. Studies demonstrate that CS may trigger lethal arrhythmias and lead to SCA (3,46). Igarashi et al studied 14 survivors of SCA without apparent heart disease (47). Overall, 4 were found to have angina with ST elevation during observation, 5 of the remaining 9 had positive ER provocation testing. Survivors who underwent subsequent diagnostic spasm provocation testing and appropriate therapy appear to have a good prognosis. Chevalier et al, followed 7 survivors of SCA (with absence of known heart disease) who underwent ER provocation testing that was positive (48). Treatment with a calcium channel blocker was initiated at a dose determined by titration until a negative provocation test resulted. All were habitual smokers. In 58 months of follow up, only the patient who did not abstain from tobacco had a recurrent event, highlighting treatment efficacy and importance of risk management (48). Studies indicate that severe multivessel spasm, daytime ST-segment changes, and younger age are predictors of SCA (3). Additionally, Togashi et al have reported differing circadian variance in patients with SCA and syncope triggered by CS relative to patients with typical CS (nocturnal symptoms, angina only), suggesting possible differences in pathogenesis and need for provocation testing even in those without typical variant angina (4).
Noninvasive
Noninvasive, non-pharmacologic evaluation for the diagnosis of CS includes standard 12-lead ECG (during attack), holter monitoring (45), exercise testing (49), and hyperventilation testing (50). Waters et al reported better sensitivity with pharmacologic testing (IV ER) compared with non-invasive non-pharmacologic measures in patients with untreated variant angina (17). ER testing induced angina in all 34 patients and ST elevation in 32 (94%), exercise testing induced angina in 17 (50%) and ST elevation in 10 (29%), and cold pressor testing provoked angina in 5 (15%) and ST elevation in 3 (9%) (17). Conversely, Okumura report 93% sensitivity using exercise myocardial scintigraphy and echocardiography during the hyperventilation provocation test in predicting IC ACH spasm (8). Sueda et al assessed the usefulness of a combined hyperventilation and exercise test and reported 64.9% sensitivity and 100% specificity (51).
There is a relative paucity of literature regarding the safety of bedside ER provocation testing for CS. Protocols using continuous monitoring of wall motion by echocardiography to detect spasm induced ischemia in patients with near normal angiographic findings are described (20). The safety of bedside ER stress echocardiography is reported by Song et al in a retrospective analysis of 1372 patients without significant myocardial ischemia (most evaluated by exercise stress testing) (19). Overall, 31% of the patients had positive results, arrhythmia developed in 1.9%, all episodes were transient and reversible. No mortality or MI was reported. Of the 16% patients who also underwent invasive CS testing, the investigators reported agreement between tests in 93% of the patients, suggesting similar diagnostic accuracy. Specificity was found to be 91% (19). The accuracy of bedside ER testing among patients without prior coronary angiography may be less diagnostic for spasm, due to provocation of occult obstructive coronary disease-related ischemia.
Safety and practice guidelines
Contemporary reports suggest that provocation testing is relatively safe (18), however there are older reports of refractory spasm and recurrent spasm resulting in prolonged ischemia, MI, and death (52,53). A recent observational study evaluated 1244 patients with vasospastic angina who underwent IC provocation tests (40% ER, 57% ACH, 2% ER+ACH, 1% other) (18). The overall incidence of arrhythmic complications was 6.8%, which is comparable to 7.0% during spontaneous angina events. They reported a 5.5% MACE rate during the 32 month follow up period. After multivariable analysis, mixed (focal and diffuse) multi-vessel spasm predicted MACE (adjusted HR 2.84; 95% CI [1.43–6.03], p<0.01), but provocation-related arrhythmias, defined as ventricular tachycardia, ventricular fibrillation and brady-arrhythmias did not (18).
Prior ACC/AHA guidelines support limited use of provocative testing for spasm (54), however current guidelines do not address this generally or specifically e.g. invasive vs noninvasive, ER vs ACH, IC vs IV administration, while other international practice guidelines do (Table 2).
Table 2.
Practice guidelines for coronary spasm provocation testing
| Guidelines | Classification | Level of evidence | Recommendations |
|---|---|---|---|
| 2006 European Society of Cardiology; stable angina (55) | Class IIa | B | IC Provocation testing
|
| 2008 Japanese Circulation Society; vasospastic angina (45) | Class I | - | IC Provocation testing during angiography
|
| 2011 ACCF/SHS; unstable angina/non ST-elevation MI (54) | Class IIb | C | Provocation testing indicated:
|
| 2011 ACCF/AHA/SCAI; percutaneous coronary intervention (56) | - | - | None |
| 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS; stable ischemic heart disease (57) | - | - | None |
| 2012 ACCF/AHA; unstable angina/non-ST elevation MI (58) | - | - | None |
CAD= coronary artery disease, ECG=electrocardiogram, IC= intracoronary, MI=myocardial infarction.
Conclusions
Provocative testing is effective for diagnosis of CS. Testing may be appropriate in properly equipped facilities with experienced physicians for patients without obstructive coronary artery disease presenting with suspected variant angina, as outlined in our recommended algorithm (Figure 2). Both ACH and ER appear to have comparable diagnostic yield. With ER provocation testing, IC testing rather than IV is useful in identifying the culprit vasospastic vessel and allows for treatment of refractory spasm. There is a paucity of safety data on the use of bedside ER provocation testing with echocardiography monitoring. Larger, ethnically diverse studies that include more women are needed to generate evidence-based guidelines regarding the effectiveness and safety of ER and ACH provocation testing for the diagnosis of CS.
Figure 2. Variant angina algorithm.
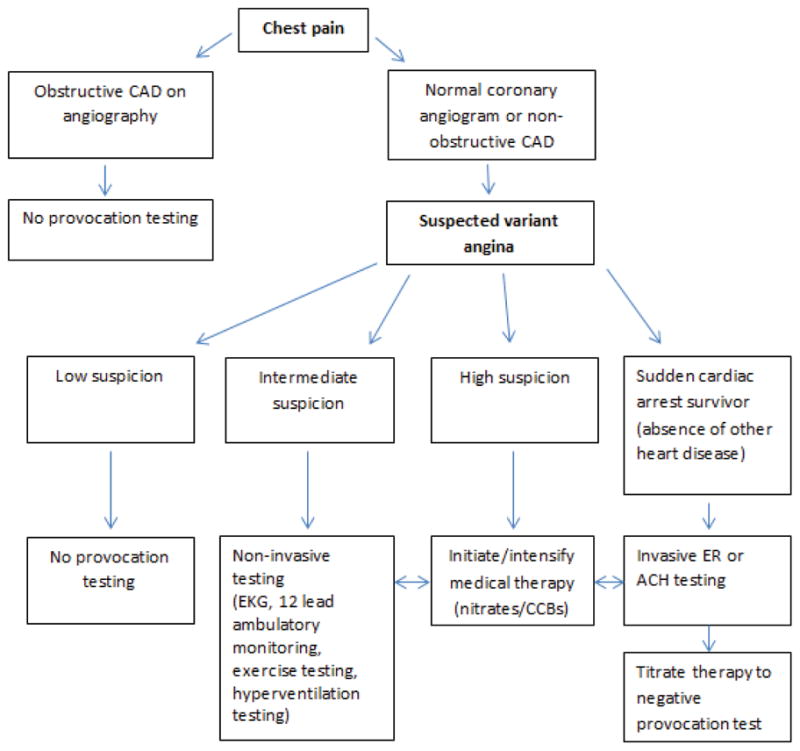
Suspicion is based on clinical factors: Spontaneous episodes of angina occurring at rest and between night and early morning hours, marked diurnal variation in exercise tolerance (reduced during early morning hours), quick relief of angina with nitrates, suppression of attacks with calcium channel blockers (CCBs), smoking, Asian descent. CAD=coronary artery disease, ER=Ergonovine, ACH=Acetylcholine, EKG= electrocardiogram.
Figure 1. Coronary spasm.
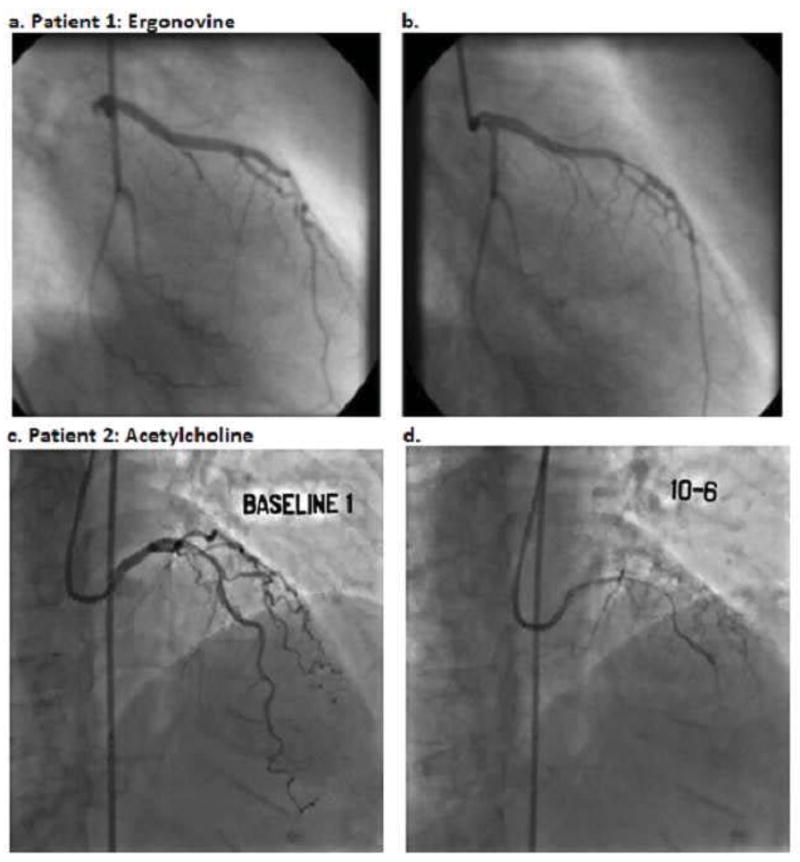
Angiogram before (a) and after (b) administration of 200 micrograms IV ergonovine showing focal spasm of the left anterior descending artery (courtesy of Adlam, et al (59), reprint permission requested). Angiogram before (c) and after (d) intracoronary infusion of acetylcholine at a concentration of 0.182 micrograms/milliliter (2 milliliters over 3 minutes) showing diffuse spasm of the left anterior descending artery.
Acknowledgments
Funding: This work was funded by NHLBI N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, NIA 1R03AG032631 and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Los Angeles, CA.
Abbreviations
- ACH
Acetylcholine
- CFR
coronary flow reserve
- CS
coronary spasm
- ECG
Electrocardiogram
- ER
Ergonovine
- IC
intracoronary
- MACE
Major Adverse Cardiovascular Events
- MI
Myocardial Infarction
- SCA
sudden cardiac arrest
- STEMI
ST elevation MI
Footnotes
Disclosures: Dr. Zaya has no relationships to disclose. Dr. Mehta discloses a relationship for research support with Gilead Sciences. Dr. Bairey Merz discloses the following relationships: Mayo Foundation (lectures), Sutter West Bay Hospital (lectures), Practice Point Communications (lectures), Bristol Meyers Squibb (DSMB service), Amgen (consulting, lectures), Gilead (grant review committee), Duke (consulting), Pri-Med (lectures), Cardiovascular Inst. San Diego (lectures), Vox Media (lectures), Ohio State University (visiting professor), BGB Communications (lectures), Slocum Dickson Educational Inst (lectures), Annual DeStevens Lectureship (Northwestern) - Honorarium and Consulting,24th Annual Dan May Lectureship (Vanderbilt) - Honorarium and Consulting, California Society for Cardiac Rehabiltation (lecture) - Honorarium and Consulting, NIH-SEP (grant review study section) - Honorarium and Consulting, ACC-SAP (self-assessment panel writing) -Honorarium and Consulting.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yasue H, Takizawa A, Nagao M, et al. Long-term prognosis for patients with variant angina and influential factors. Circulation. 1988;78:1–9. doi: 10.1161/01.cir.78.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Wang CH, Kuo LT, Hung MJ, Cherng WJ. Coronary vasospasm as a possible cause of elevated cardiac troponin I in patients with acute coronary syndrome and insignificant coronary artery disease. Am Heart J. 2002;144:275–81. doi: 10.1067/mhj.2002.123843. [DOI] [PubMed] [Google Scholar]
- 3.MacAlpin RN. Cardiac arrest and sudden unexpected death in variant angina: complications of coronary spasm that can occur in the absence of severe organic coronary stenosis. Am Heart J. 1993;125:1011–7. doi: 10.1016/0002-8703(93)90108-l. [DOI] [PubMed] [Google Scholar]
- 4.Togashi I, Sato T, Soejima K, et al. Sudden cardiac arrest and syncope triggered by coronary spasm. Int J Cardiol. 2013;163:56–60. doi: 10.1016/j.ijcard.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 5.Takatsu F, Watarai M. Mild stenosis makes prognosis of vasospastic angina worse. Coron Artery Dis. 2011;22:1–5. doi: 10.1097/MCA.0b013e3283402313. [DOI] [PubMed] [Google Scholar]
- 6.Hackett D, Larkin S, Chierchia S, Davies G, Kaski JC, Maseri A. Induction of coronary artery spasm by a direct local action of ergonovine. Circulation. 1987;75:577–82. doi: 10.1161/01.cir.75.3.577. [DOI] [PubMed] [Google Scholar]
- 7.Clarke JG, Davies GJ, Kerwin R, et al. Coronary artery infusion of neuropeptide Y in patients with angina pectoris. Lancet. 1987;1:1057–9. doi: 10.1016/s0140-6736(87)90483-1. [DOI] [PubMed] [Google Scholar]
- 8.Okumura K, Yasue H, Horio Y, et al. Multivessel coronary spasm in patients with variant angina: a study with intracoronary injection of acetylcholine. Circulation. 1988;77:535–42. doi: 10.1161/01.cir.77.3.535. [DOI] [PubMed] [Google Scholar]
- 9.Crea F, Chierchia S, Kaski JC, et al. Provocation of coronary spasm by dopamine in patients with active variant angina pectoris. Circulation. 1986;74:262–9. doi: 10.1161/01.cir.74.2.262. [DOI] [PubMed] [Google Scholar]
- 10.Akasaka T, Yoshida K, Hozumi T, et al. Comparison of coronary flow reserve between focal and diffuse vasoconstriction induced by ergonovine in patients with vasospastic angina. Am J Cardiol. 1997;80:705–10. doi: 10.1016/s0002-9149(97)00499-2. [DOI] [PubMed] [Google Scholar]
- 11.Bertrand ME, LaBlanche JM, Tilmant PY, et al. Frequency of provoked coronary arterial spasm in 1089 consecutive patients undergoing coronary arteriography. Circulation. 1982;65:1299–306. doi: 10.1161/01.cir.65.7.1299. [DOI] [PubMed] [Google Scholar]
- 12.Harding MB, Leithe ME, Mark DB, et al. Ergonovine maleate testing during cardiac catheterization: a 10-year perspective in 3,447 patients without significant coronary artery disease or Prinzmetal’s variant angina. J Am Coll Cardiol. 1992;20:107–11. doi: 10.1016/0735-1097(92)90145-d. [DOI] [PubMed] [Google Scholar]
- 13.Sueda S, Kohno H, Fukuda H, et al. Induction of coronary artery spasm by two pharmacological agents: comparison between intracoronary injection of acetylcholine and ergonovine. Coron Artery Dis. 2003;14:451–7. doi: 10.1097/00019501-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Sueda S, Kohno H, Fukuda H, et al. Clinical impact of selective spasm provocation tests: comparisons between acetylcholine and ergonovine in 1508 examinations. Coron Artery Dis. 2004;15:491–7. doi: 10.1097/00019501-200412000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Sueda S, Ochi T, Yano K, et al. New combined spasm provocation test in patients with rest angina: intracoronary injection of acetylcholine after intracoronary administration of ergonovine. Jpn Circ J. 2000;64:559–65. doi: 10.1253/jcj.64.559. [DOI] [PubMed] [Google Scholar]
- 16.Sueda S, Ochi N, Kawada H, Uraoka T. Usefulness of intracoronary injection of acetylcholine and ergonovine in patients with variant angina. J Cardiol. 1998;31:145–50. [PubMed] [Google Scholar]
- 17.Waters DD, Szlachcic J, Bonan R, Miller DD, Dauwe F, Theroux P. Comparative sensitivity of exercise, cold pressor and ergonovine testing in provoking attacks of variant angina in patients with active disease. Circulation. 1983;67:310–5. doi: 10.1161/01.cir.67.2.310. [DOI] [PubMed] [Google Scholar]
- 18.Takagi Y, Yasuda S, Takahashi J, et al. Clinical implications of provocation tests for coronary artery spasm: safety, arrhythmic complications, and prognostic impact: multicentre registry study of the Japanese Coronary Spasm Association. Eur Heart J. 2013;34:258–67. doi: 10.1093/eurheartj/ehs199. [DOI] [PubMed] [Google Scholar]
- 19.Song JK, Park SW, Kang DH, et al. Safety and clinical impact of ergonovine stress echocardiography for diagnosis of coronary vasospasm. J Am Coll Cardiol. 2000;35:1850–6. doi: 10.1016/s0735-1097(00)00646-x. [DOI] [PubMed] [Google Scholar]
- 20.Song JK, Lee SJ, Kang DH, et al. Ergonovine echocardiography as a screening test for diagnosis of vasospastic angina before coronary angiography. J Am Coll Cardiol. 1996;27:1156–61. doi: 10.1016/0735-1097(95)00590-0. [DOI] [PubMed] [Google Scholar]
- 21.Yasue H, Horio Y, Nakamura N, et al. Induction of coronary artery spasm by acetylcholine in patients with variant angina: possible role of the parasympathetic nervous system in the pathogenesis of coronary artery spasm. Circulation. 1986;74:955–63. doi: 10.1161/01.cir.74.5.955. [DOI] [PubMed] [Google Scholar]
- 22.Okumura K, Yasue H, Matsuyama K, Goto K, Miyagi H, Ogawa H. Sensitivity and specificity of intracoronary injection of acetylcholine for the induction of coronary artery spasm. J Am Coll Cardiol. 1988;12:883–8. doi: 10.1016/0735-1097(88)90449-4. [DOI] [PubMed] [Google Scholar]
- 23.Sueda S, Ochi N, Kawada H, et al. Frequency of provoked coronary vasospasm in patients undergoing coronary arteriography with spasm provocation test of acetylcholine. Am J Cardiol. 1999;83:1186–90. doi: 10.1016/s0002-9149(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 24.Suyama A, Kuriyama H. Mechanisms of the ergonovine-induced vasoconstriction in the rabbit main coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1984;326:357–63. doi: 10.1007/BF00501443. [DOI] [PubMed] [Google Scholar]
- 25.Lacy CFAL, Goldman MP, Lance LL, editors. Drug Information Handbook. 17. Hudson: Lexi-Comp Inc; 2008. [Google Scholar]
- 26.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–6. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 27.Eshaghpour E, Mattioli L, Williams ML, Moghadam AN. Acetylcholine in the treatment of idiopathic respiratory distress syndrome. J Pediatr. 1967;71:243–6. doi: 10.1016/s0022-3476(67)80080-5. [DOI] [PubMed] [Google Scholar]
- 28.Sueda S, Saeki H, Otani T, et al. Major complications during spasm provocation tests with an intracoronary injection of acetylcholine. Am J Cardiol. 2000;85:391–4. A10. doi: 10.1016/s0002-9149(99)00754-7. [DOI] [PubMed] [Google Scholar]
- 29.Kim YG, Kim HJ, Choi WS, et al. Does a negative ergonovine provocation test truly predict freedom from variant angina? Korean Circ J. 2013;43:199–203. doi: 10.4070/kcj.2013.43.3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliva PB, Potts DE, Pluss RG. Coronary arterial spasm in Prinzmetal angina. Documentation by coronary arteriography. N Engl J Med. 1973;288:745–51. doi: 10.1056/NEJM197304122881501. [DOI] [PubMed] [Google Scholar]
- 31.Prinzmetal M, Kennamer R, Merliss R, Wada T, Bor N. Angina pectoris. I. A variant form of angina pectoris; preliminary report. Am J Med. 1959;27:375–88. doi: 10.1016/0002-9343(59)90003-8. [DOI] [PubMed] [Google Scholar]
- 32.Sun H, Mohri M, Shimokawa H, Usui M, Urakami L, Takeshita A. Coronary microvascular spasm causes myocardial ischemia in patients with vasospastic angina. J Am Coll Cardiol. 2002;39:847–51. doi: 10.1016/s0735-1097(02)01690-x. [DOI] [PubMed] [Google Scholar]
- 33.Kaski JC, Maseri A, Vejar M, Crea F, Hackett D. Spontaneous coronary artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. 1989;14:1456–63. doi: 10.1016/0735-1097(89)90382-3. [DOI] [PubMed] [Google Scholar]
- 34.Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–9. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 35.Chataigneau T, Feletou M, Huang PL, Fishman MC, Duhault J, Vanhoutte PM. Acetylcholine-induced relaxation in blood vessels from endothelial nitric oxide synthase knockout mice. Br J Pharmacol. 1999;126:219–26. doi: 10.1038/sj.bjp.0702300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Popp R, Fleming I, Busse R. Pulsatile stretch in coronary arteries elicits release of endothelium-derived hyperpolarizing factor: a modulator of arterial compliance. Circ Res. 1998;82:696–703. doi: 10.1161/01.res.82.6.696. [DOI] [PubMed] [Google Scholar]
- 37.Bossaller C, Habib GB, Yamamoto H, Williams C, Wells S, Henry PD. Impaired muscarinic endothelium-dependent relaxation and cyclic guanosine 5′-monophosphate formation in atherosclerotic human coronary artery and rabbit aorta. The Journal of clinical investigation. 1987;79:170–4. doi: 10.1172/JCI112779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamagishi M, Miyatake K, Tamai J, Nakatani S, Koyama J, Nissen SE. Intravascular ultrasound detection of atherosclerosis at the site of focal vasospasm in angiographically normal or minimally narrowed coronary segments. J Am Coll Cardiol. 1994;23:352–7. doi: 10.1016/0735-1097(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand ME, Lablanche JM, Tilmant PY, Ducloux G, Warembourg H, Jr, Soots G. Complete denervation of the heart (autotransplantation) for treatment of severe, refractory coronary spasm. Am J Cardiol. 1981;47:1375–8. doi: 10.1016/0002-9149(81)90271-x. [DOI] [PubMed] [Google Scholar]
- 40.Sugiishi M, Takatsu F. Cigarette smoking is a major risk factor for coronary spasm. Circulation. 1993;87:76–9. doi: 10.1161/01.cir.87.1.76. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki M, Nishizaki M, Arita M, Kakuta T, Numano F. Impaired glucose tolerance with late hypersecretion of insulin during oral glucose tolerance test in patients with vasospastic angina. J Am Coll Cardiol. 1996;27:1458–63. doi: 10.1016/0735-1097(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 42.Beltrame JF, Sasayama S, Maseri A. Racial heterogeneity in coronary artery vasomotor reactivity: differences between Japanese and Caucasian patients. J Am Coll Cardiol. 1999;33:1442–52. doi: 10.1016/s0735-1097(99)00073-x. [DOI] [PubMed] [Google Scholar]
- 43.Sueda S, Kohno H, Fukuda H, et al. Frequency of provoked coronary spasms in patients undergoing coronary arteriography using a spasm provocation test via intracoronary administration of ergonovine. Angiology. 2004;55:403–11. doi: 10.1177/000331970405500407. [DOI] [PubMed] [Google Scholar]
- 44.Murase Y, Yamada Y, Hirashiki A, et al. Genetic risk and gene-environment interaction in coronary artery spasm in Japanese men and women. Eur Heart J. 2004;25:970–7. doi: 10.1016/j.ehj.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 45.Guidelines for diagnosis and treatment of patients with vasospastic angina (coronary spastic angina) (JCS 2008): digest version. Circ J. 2010;74:1745–62. doi: 10.1253/circj.cj-10-74-0802. [DOI] [PubMed] [Google Scholar]
- 46.Krahn AD, Healey JS, Chauhan V, et al. Systematic assessment of patients with unexplained cardiac arrest: Cardiac Arrest Survivors With Preserved Ejection Fraction Registry (CASPER) Circulation. 2009;120:278–85. doi: 10.1161/CIRCULATIONAHA.109.853143. [DOI] [PubMed] [Google Scholar]
- 47.Igarashi Y, Tamura Y, Suzuki K, et al. High prevalence of coronary artery spasm in survivors of cardiac arrest with no apparent heart disease. Jpn Heart J. 1992;33:653–63. doi: 10.1536/ihj.33.653. [DOI] [PubMed] [Google Scholar]
- 48.Chevalier P, Dacosta A, Defaye P, et al. Arrhythmic cardiac arrest due to isolated coronary artery spasm: long-term outcome of seven resuscitated patients. J Am Coll Cardiol. 1998;31:57–61. doi: 10.1016/s0735-1097(97)00442-7. [DOI] [PubMed] [Google Scholar]
- 49.Scardi S, Pivotti F, Pandullo C, Ceschia G, Salvi A. Exercise-induced intermittent angina and ST-segment elevation in Prinzmetal’s angina. Eur Heart J. 1988;9:102–5. [PubMed] [Google Scholar]
- 50.Nakao K, Ohgushi M, Yoshimura M, et al. Hyperventilation as a specific test for diagnosis of coronary artery spasm. Am J Cardiol. 1997;80:545–9. doi: 10.1016/s0002-9149(97)00419-0. [DOI] [PubMed] [Google Scholar]
- 51.Sueda S, Fukuda H, Watanabe K, et al. Usefulness of accelerated exercise following mild hyperventilation for the induction of coronary artery spasm : comparison with an acetylcholine Test. Chest. 2001;119:155–62. doi: 10.1378/chest.119.1.155. [DOI] [PubMed] [Google Scholar]
- 52.Pepine CJ, Feldman RL, Conti CR. Action of intracoronary nitroglycerin in refractory coronary artery spasm. Circulation. 1982;65:411–4. doi: 10.1161/01.cir.65.2.411. [DOI] [PubMed] [Google Scholar]
- 53.Pepine CJ. Ergonovine echocardiography for coronary spasm: facts and wishful thinking. J Am Coll Cardiol. 1996;27:1162–3. doi: 10.1016/0735-1097(96)00015-0. [DOI] [PubMed] [Google Scholar]
- 54.Anderson JL, Adams CD, Antman EM, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;123:e426–579. doi: 10.1161/CIR.0b013e318212bb8b. [DOI] [PubMed] [Google Scholar]
- 55.Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: The Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27:1341–81. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 56.Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012;60:e44–e164. doi: 10.1016/j.jacc.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 58.Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–81. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Adlam D, Azeem T, Ali T, Gershlick A. Is there a role for provocation testing to diagnose coronary artery spasm? Int J Cardiol. 2005;102(1):1–7. doi: 10.1016/j.ijcard.2004.07.016. [DOI] [PubMed] [Google Scholar]


