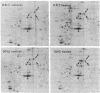
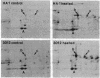
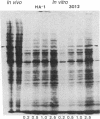
Abstract
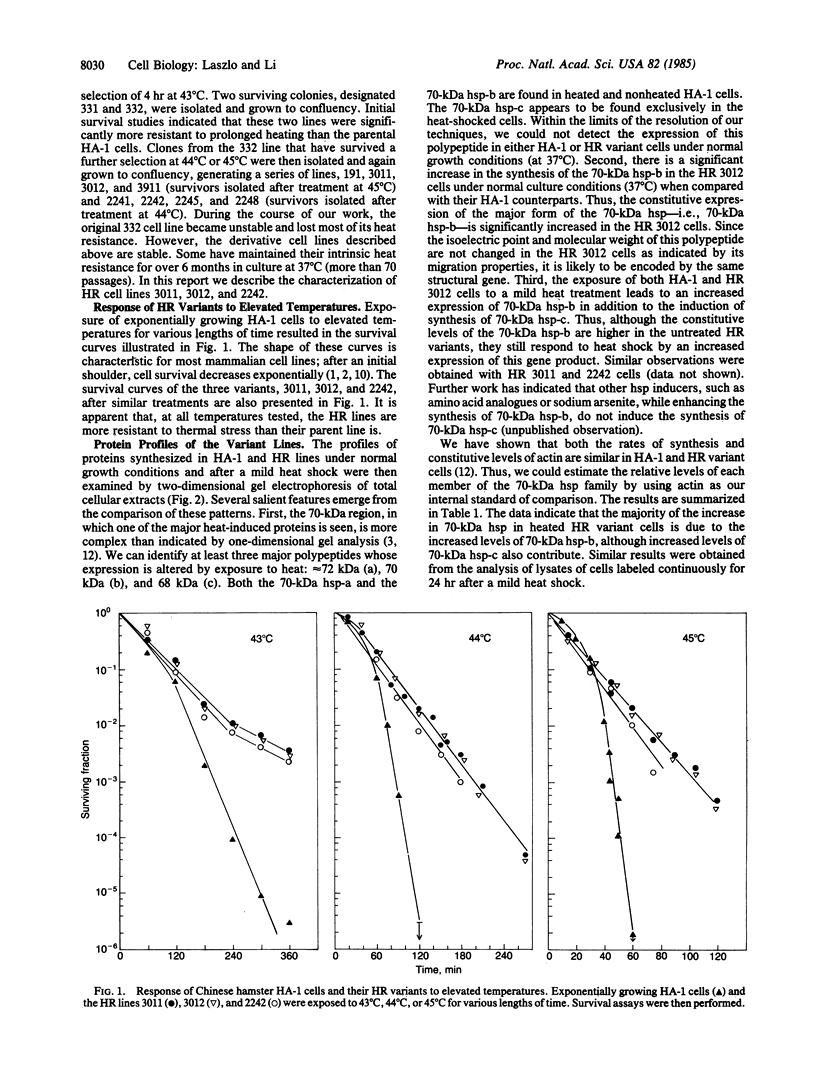
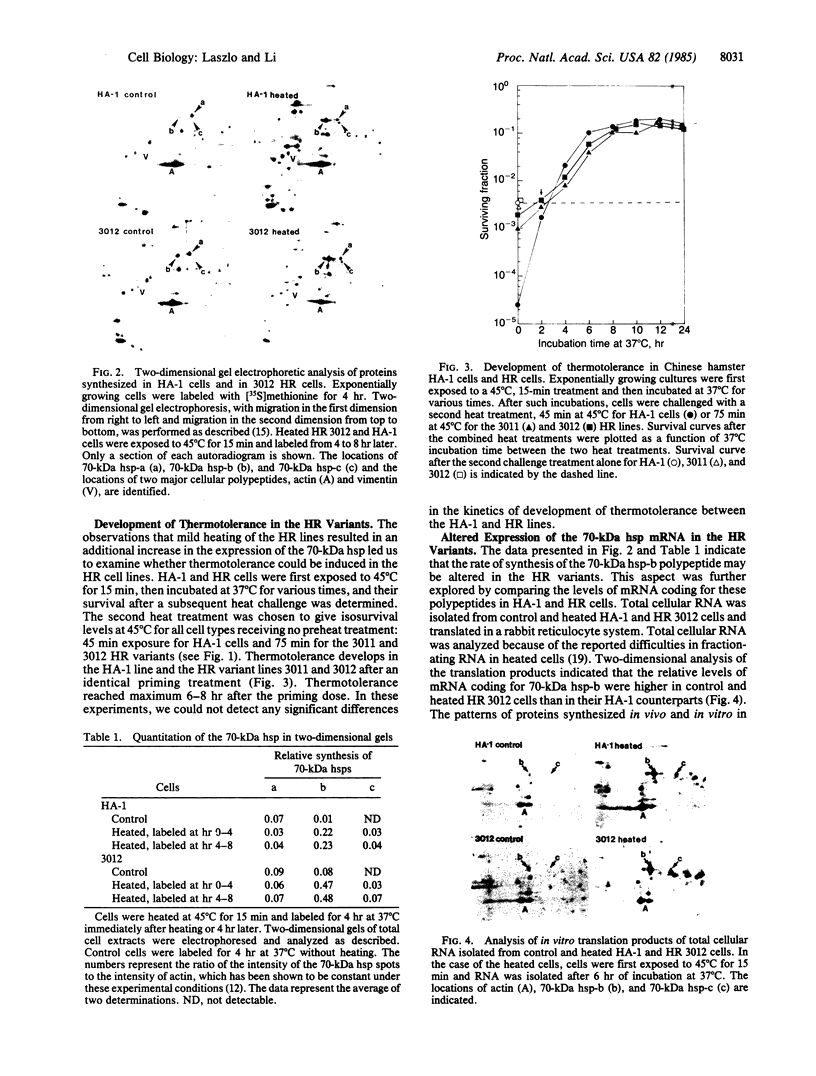
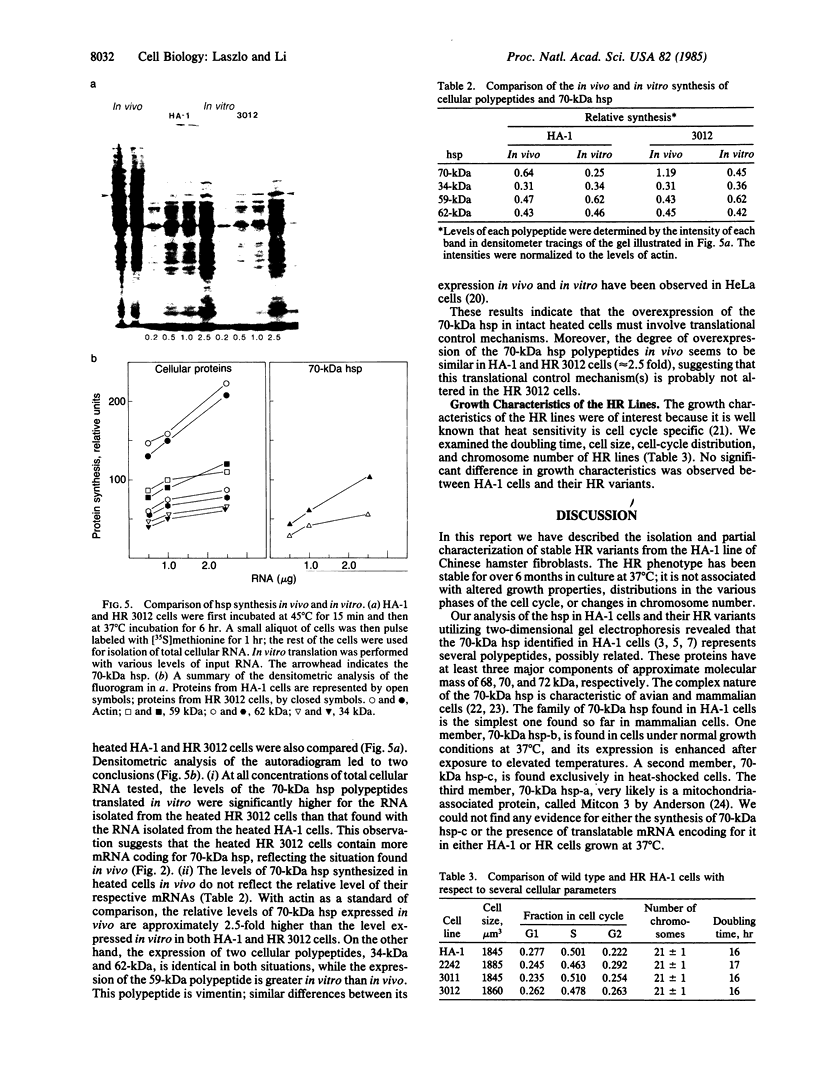
Heat-resistant variants of the Chinese hamster HA-1 line have been isolated after repeated heat treatments. The heat-resistant phenotype has been stable for over 70 passages. One of the members of the 70-kDa heat shock protein family was found to be synthesized at greater levels in the heat-resistant variants under normal growth conditions. Mild heat treatment of the variant lines induced a transient thermotolerance that was accompanied by additional increase in the synthesis of the 70-kDa heat shock proteins. Cell-free translation of total cellular RNA revealed greater amounts of 70-kDa heat shock protein mRNA in both control and heated variant cells. The greater levels of 70-kDa heat shock protein synthesized in the variant cells presumably are a reflection of altered levels of its messenger mRNA. In addition, we found that translational control plays a role in the elevated expression of heat shock proteins in heat-shocked HA-1 cells and their heat-resistant variants. The association of the heat-resistant phenotype with increased levels of a 70-kDa heat shock protein suggests strongly that this gene product plays a role in protecting cells from damage inflicted by elevated temperatures.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. Identification of mitochondrial proteins and some of their precursors in two-dimensional electrophoretic maps of human cells. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2407–2411. doi: 10.1073/pnas.78.4.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Gerner E. W., Schneider M. J. Induced thermal resistance in HeLa cells. Nature. 1975 Aug 7;256(5517):500–502. doi: 10.1038/256500a0. [DOI] [PubMed] [Google Scholar]
- Harris M. Stable heat-resistant variants in populations of Chinese hamster cells. J Natl Cancer Inst. 1980 Jun;64(6):1495–1501. doi: 10.1093/jnci/64.6.1495. [DOI] [PubMed] [Google Scholar]
- Harris M. Temperature-resistant variants in clonal populations of pig kidney cells. Exp Cell Res. 1967 May;46(2):301–314. doi: 10.1016/0014-4827(67)90068-7. [DOI] [PubMed] [Google Scholar]
- Henle K. J., Leeper D. B. Interaction of hyperthermia and radiation in CHO cells: recovery kinetics. Radiat Res. 1976 Jun;66(3):505–518. [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Bernier D., Chrétien P., Nicole L. M., Tanguay R. M., Marceau N. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 1982 Jun;42(6):2457–2461. [PubMed] [Google Scholar]
- Laszlo A., Bissell M. J. TPA induces simultaneous alterations in the synthesis and organization of vimentin. Exp Cell Res. 1983 Oct;148(1):221–234. doi: 10.1016/0014-4827(83)90201-x. [DOI] [PubMed] [Google Scholar]
- Leisti S., Miller W. L., Johnson L. K. Synthesis of growth hormone, prolactin, and proopiomelanocortin by ovine fetal anterior and neurointermediate lobes. Endocrinology. 1982 Oct;111(4):1368–1375. doi: 10.1210/endo-111-4-1368. [DOI] [PubMed] [Google Scholar]
- Li G. C. Elevated levels of 70,000 dalton heat shock protein in transiently thermotolerant Chinese hamster fibroblasts and in their stable heat resistant variants. Int J Radiat Oncol Biol Phys. 1985 Jan;11(1):165–177. doi: 10.1016/0360-3016(85)90376-1. [DOI] [PubMed] [Google Scholar]
- Li G. C., Fisher G. A., Hahn G. M. Induction of thermotolerance and evidence for a well-defined, thermotropic cooperative process. Radiat Res. 1982 Feb;89(2):361–368. [PubMed] [Google Scholar]
- Li G. C., Hahn G. M. A proposed operational model of thermotolerance based on effects of nutrients and the initial treatment temperature. Cancer Res. 1980 Dec;40(12):4501–4508. [PubMed] [Google Scholar]
- Li G. C. Induction of thermotolerance and enhanced heat shock protein synthesis in Chinese hamster fibroblasts by sodium arsenite and by ethanol. J Cell Physiol. 1983 May;115(2):116–122. doi: 10.1002/jcp.1041150203. [DOI] [PubMed] [Google Scholar]
- Li G. C., Werb Z. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3218–3222. doi: 10.1073/pnas.79.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storti R. V., Scott M. P., Rich A., Pardue M. L. Translational control of protein synthesis in response to heat shock in D. melanogaster cells. Cell. 1980 Dec;22(3):825–834. doi: 10.1016/0092-8674(80)90559-0. [DOI] [PubMed] [Google Scholar]
- Tao T. W., Calderwood S., Hahn G. M. Stable heat-resistant clones selected from wild-type and surface variants of B-16 melanoma. Int J Cancer. 1983 Nov 15;32(5):533–535. doi: 10.1002/ijc.2910320502. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Welch W. J., Mathews M. B., Feramisco J. R. Molecular and cellular effects of heat-shock and related treatments of mammalian tissue-culture cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):985–996. doi: 10.1101/sqb.1982.046.01.092. [DOI] [PubMed] [Google Scholar]
- Velazquez J. M., Lindquist S. hsp70: nuclear concentration during environmental stress and cytoplasmic storage during recovery. Cell. 1984 Mar;36(3):655–662. doi: 10.1016/0092-8674(84)90345-3. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Westra A., Dewey W. C. Variation in sensitivity to heat shock during the cell-cycle of Chinese hamster cells in vitro. Int J Radiat Biol Relat Stud Phys Chem Med. 1971;19(5):467–477. doi: 10.1080/09553007114550601. [DOI] [PubMed] [Google Scholar]
- Yang S. J., Hahn G. M., Bagshaw M. A. Chromosome aberrations induced by thymidine. Exp Cell Res. 1966 Apr;42(1):130–135. doi: 10.1016/0014-4827(66)90326-0. [DOI] [PubMed] [Google Scholar]