Abstract
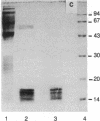
A myogenic growth factor has been purified from a skeletal muscle, the anterior latissimus dorsi, of adult chickens. In the range of 1-10 ng, this factor stimulates DNA synthesis as well as protein and muscle-specific myosin accumulation in myogenic cell cultures. Purification is achieved through binding of the factor to heparin. The factor is distinct from transferrin and works synergistically with transferrin in stimulating myogenesis in vitro.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. E., Dodson M. V., Luiten L. S. Regulation of skeletal muscle satellite cell proliferation by bovine pituitary fibroblast growth factor. Exp Cell Res. 1984 May;152(1):154–160. doi: 10.1016/0014-4827(84)90239-8. [DOI] [PubMed] [Google Scholar]
- Bandman E., Strohman R. C. Increased K+ inhibits spontaneous contractions reduces myosin accumulation in cultured chick myotubes. J Cell Biol. 1982 Jun;93(3):698–704. doi: 10.1083/jcb.93.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hall H. G., Parry G. How does the extracellular matrix direct gene expression? J Theor Biol. 1982 Nov 7;99(1):31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Ewton D. Z., Florini J. R. Relative effects of the somatomedins, multiplication-stimulating activity, and growth hormone on myoblasts and myotubes in culture. Endocrinology. 1980 Feb;106(2):577–583. doi: 10.1210/endo-106-2-577. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Bialecki H., Greenburg G. Purification of the fibroblast growth factor activity from bovine brain. J Biol Chem. 1978 May 25;253(10):3736–3743. [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D., Weseman J., Moran J. S., Lindstrom J. Effect of fibroblast growth factor on the division and fusion of bovine myoblasts. J Cell Biol. 1976 Aug;70(2 Pt 1):395–405. doi: 10.1083/jcb.70.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray S. D., McDonagh P. F., Gore R. W. Comparison of functional and total capillary densities in fast and slow muscles of the chicken. Pflugers Arch. 1983 May;397(3):209–213. doi: 10.1007/BF00584359. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Huchet M., Changeux J. P. Denervation increases a neurite-promoting activity in extracts of skeletal muscle. Nature. 1983 Apr 14;302(5909):609–611. doi: 10.1038/302609a0. [DOI] [PubMed] [Google Scholar]
- Ii I., Kimura I., Ozawa E. A myotrophic protein from chick embryo extract: its purification, identity to transferrin, and indispensability for avian myogenesis. Dev Biol. 1982 Dec;94(2):366–377. doi: 10.1016/0012-1606(82)90354-2. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci U S A. 1985 Feb;82(3):805–809. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lobb R. R., Fett J. W. Purification of two distinct growth factors from bovine neural tissue by heparin affinity chromatography. Biochemistry. 1984 Dec 18;23(26):6295–6299. doi: 10.1021/bi00321a001. [DOI] [PubMed] [Google Scholar]
- MAURO A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961 Feb;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Markelonis G., Tae Hwan O. H. A sciatic nerve protein has a trophic effect on development and maintenance of skeletal muscle cells in culture. Proc Natl Acad Sci U S A. 1979 May;76(5):2470–2474. doi: 10.1073/pnas.76.5.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda R., Spector D., Micou-Eastwood J., Strohman R. C. There is selective accumulation of a growth factor in chicken skeletal muscle. II. Transferrin accumulation in dystrophic fast muscle. Dev Biol. 1984 Jun;103(2):276–284. doi: 10.1016/0012-1606(84)90315-4. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Spector D., Strohman R. C. There is selective accumulation of a growth factor in chicken skeletal muscle. I. Transferrin accumulation in adult anterior latissimus dorsi. Dev Biol. 1984 Jun;103(2):267–275. doi: 10.1016/0012-1606(84)90314-2. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Cohen I. R., Fuks Z., Vlodavsky I. Activated T lymphocytes produce a matrix-degrading heparan sulphate endoglycosidase. Nature. 1984 Jul 19;310(5974):241–244. doi: 10.1038/310241a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Schmid C., Steiner T., Froesch E. R. Preferential enhancement of myoblast differentiation by insulin-like growth factors (IGF I and IGF II) in primary cultures of chicken embryonic cells. FEBS Lett. 1983 Sep 5;161(1):117–121. doi: 10.1016/0014-5793(83)80742-x. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Bandman E., Walker C. R. Regulation of myosin accumulation by muscle activity in cell culture. J Muscle Res Cell Motil. 1981 Sep;2(3):269–282. doi: 10.1007/BF00713266. [DOI] [PubMed] [Google Scholar]
- Varon S. S., Bunge R. P. Trophic mechanisms in the peripheral nervous system. Annu Rev Neurosci. 1978;1:327–361. doi: 10.1146/annurev.ne.01.030178.001551. [DOI] [PubMed] [Google Scholar]
- Younkin S. G., Brett R. S., Davey B., Younkin L. Substances moved by axonal transport and released by nerve stimulation have an innervation-like effect on muscle. Science. 1978 Jun 16;200(4347):1292–1295. doi: 10.1126/science.78522. [DOI] [PubMed] [Google Scholar]