Abstract
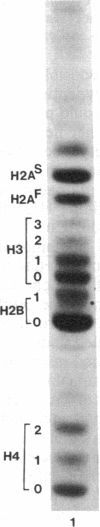
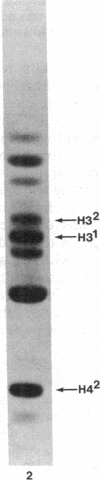
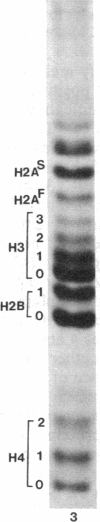
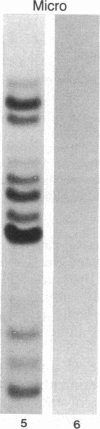
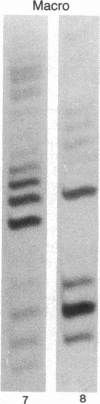
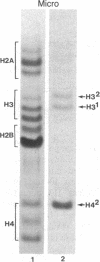
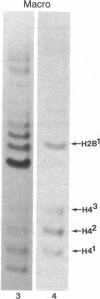
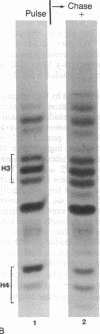
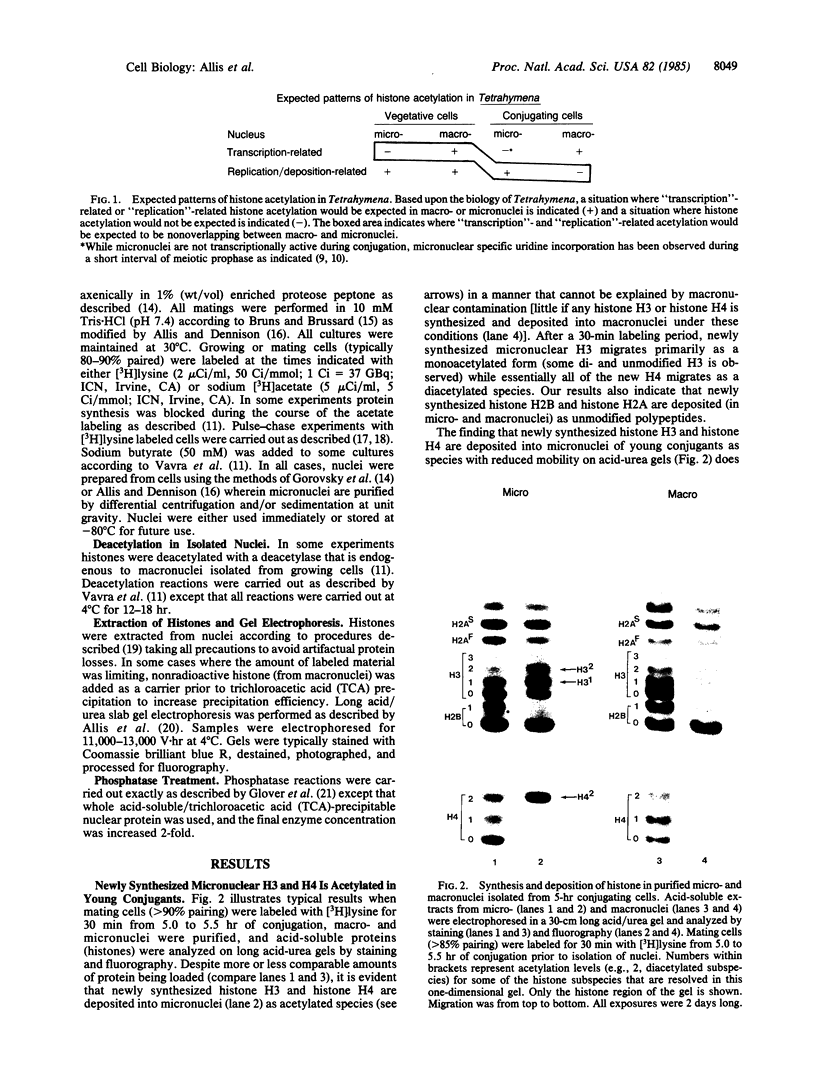
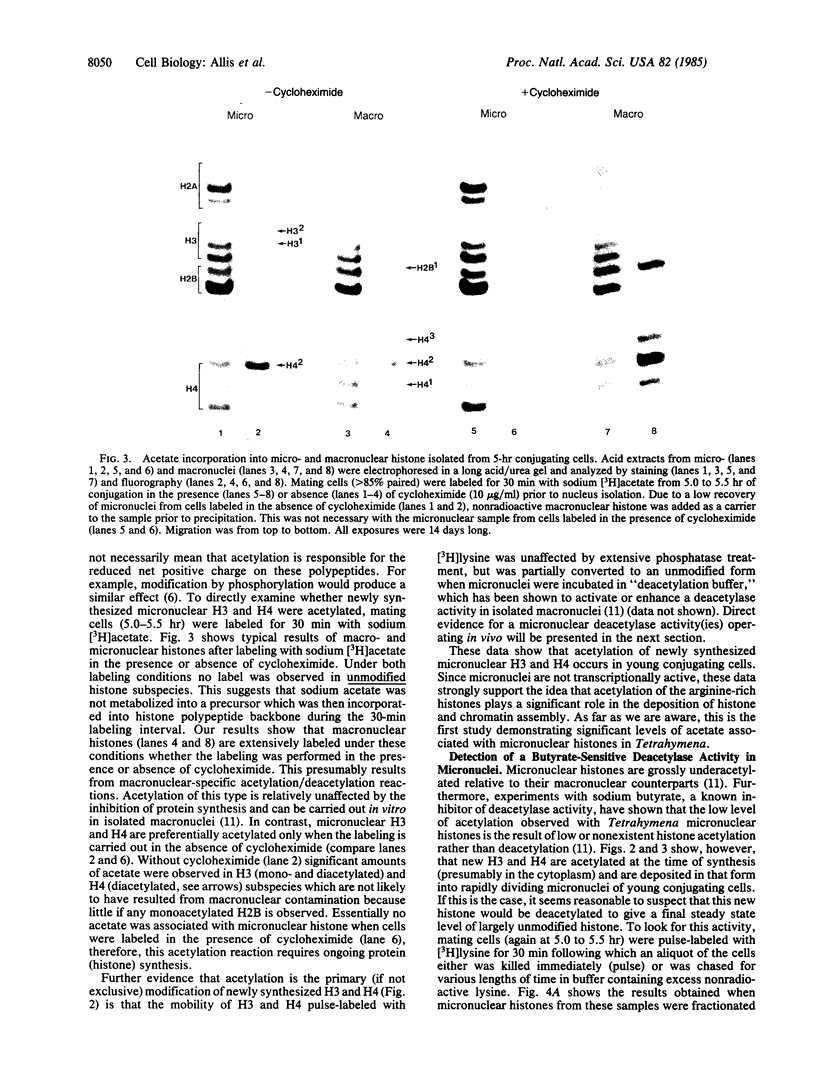
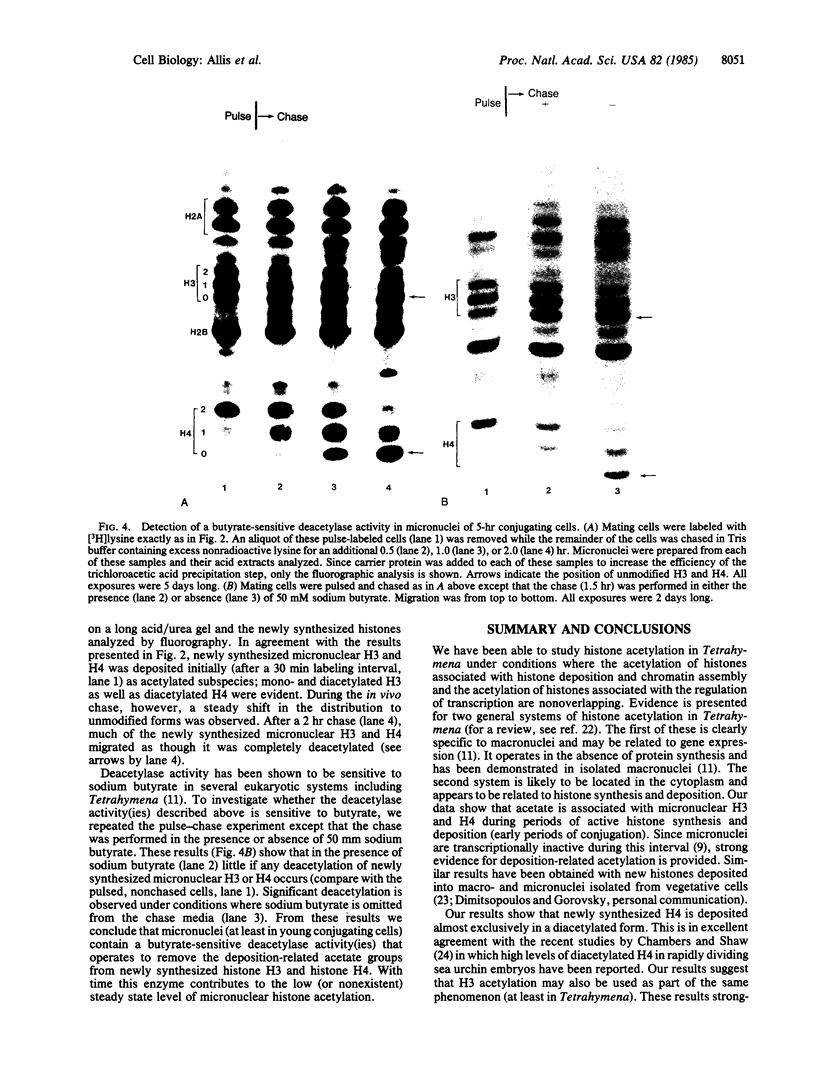
Macro- and micronuclei of the ciliated protozoan, Tetrahymena thermophila, afford a unique opportunity to study histone acetylation under conditions where acetylation associated with the regulation of transcription and acetylation associated with the deposition of histones on the DNA are separable. In this study we demonstrate that histone H3 and histone H4 synthesized in young (5 hr) conjugating Tetrahymena are deposited into micronuclei in acetylated forms. Most of the newly synthesized histone H3 migrates as a monoacetylated form while essentially all of the new histone H4 is deposited as a diacetylated species. Since micronuclei replicate rapidly during this stage of the life cycle, but are transcriptionally inactive, these data suggest that histone acetylation is related functionally to histone deposition and chromatin assembly. Pulse-chase experiments show that micronuclei also contain a butyrate-sensitive deacetylase activity(ies) which operates to remove the deposition-related acetate groups from newly synthesized and deposited H3 and H4. This enzymatic activity probably contributes to the steady state level of micronuclear histone acetylation that is low or nonexistent. Thus, evidence is emerging for at least two independent systems of histone acetylation in Tetrahymena. The first system is specific to macronuclei and may be related to gene expression. The second system is common to macro- or micronuclear histones (H3 and H4) and may be related to histone deposition during DNA replication.
Full text
PDF

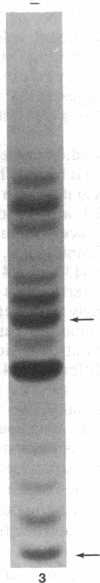
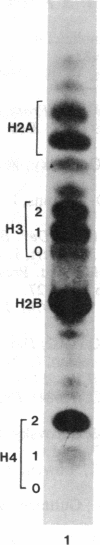
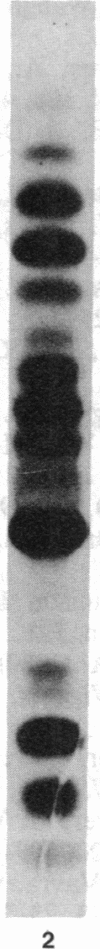
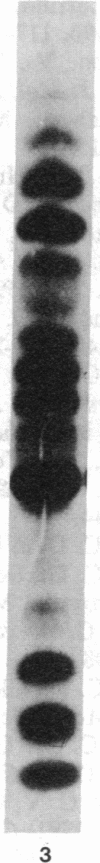
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allis C. D., Bowen J. K., Abraham G. N., Glover C. V., Gorovsky M. A. Proteolytic processing of histone H3 in chromatin: a physiologically regulated event in Tetrahymena micronuclei. Cell. 1980 May;20(1):55–64. doi: 10.1016/0092-8674(80)90234-2. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Dennison D. K. Identification and purification of young macronuclear anlagen from conjugating cells of Tetrahymena thermophila. Dev Biol. 1982 Oct;93(2):519–533. doi: 10.1016/0012-1606(82)90139-7. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Glover C. V., Bowen J. K., Gorovsky M. A. Histone variants specific to the transcriptionally active, amitotically dividing macronucleus of the unicellular eucaryote, Tetrahymena thermophila. Cell. 1980 Jul;20(3):609–617. doi: 10.1016/0092-8674(80)90307-4. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Glover C. V., Gorovsky M. A. Micronuclei of Tetrahymena contain two types of histone H3. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4857–4861. doi: 10.1073/pnas.76.10.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis C. D., Wiggins J. C. Histone rearrangements accompany nuclear differentiation and dedifferentiation in Tetrahymena. Dev Biol. 1984 Feb;101(2):282–294. doi: 10.1016/0012-1606(84)90142-8. [DOI] [PubMed] [Google Scholar]
- Allis C. D., Wiggins J. C. Proteolytic processing of micronuclear H3 and histone phosphorylation during conjugation in Tetrahymena thermophila. Exp Cell Res. 1984 Aug;153(2):287–298. doi: 10.1016/0014-4827(84)90601-3. [DOI] [PubMed] [Google Scholar]
- Bina-Stein M., Simpson R. T. Specific folding and contraction of DNA by histones H3 and H4. Cell. 1977 Jul;11(3):609–618. doi: 10.1016/0092-8674(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Bruns P. J., Brussard T. B. Pair formation in tetrahymena pyriformis, an inducible developmental system. J Exp Zool. 1974 Jun;188(3):337–344. doi: 10.1002/jez.1401880309. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Sollner-Webb B., Felsenfeld G. The organization of histones and DNA in chromatin: evidence for an arginine-rich histone kernel. Cell. 1976 Jul;8(3):333–347. doi: 10.1016/0092-8674(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Chambers S. A., Shaw B. R. Levels of histone H4 diacetylation decrease dramatically during sea urchin embryonic development and correlate with cell doubling rate. J Biol Chem. 1984 Nov 10;259(21):13458–13463. [PubMed] [Google Scholar]
- Christensen M. E., Dixon G. H. Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev Biol. 1982 Oct;93(2):404–415. doi: 10.1016/0012-1606(82)90127-0. [DOI] [PubMed] [Google Scholar]
- Glover C. V., Vavra K. J., Guttman S. D., Gorovsky M. A. Heat shock and deciliation induce phosphorylation of histone H1 in T. pyriformis. Cell. 1981 Jan;23(1):73–77. doi: 10.1016/0092-8674(81)90271-3. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Jackson V., Shires A., Tanphaichitr N., Chalkley R. Modifications to histones immediately after synthesis. J Mol Biol. 1976 Jun 25;104(2):471–483. doi: 10.1016/0022-2836(76)90282-5. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. Conjugation in Tetrahymena thermophila. A temporal analysis of cytological stages. Exp Cell Res. 1982 Jul;140(1):227–236. doi: 10.1016/0014-4827(82)90172-0. [DOI] [PubMed] [Google Scholar]
- Martindale D. W., Allis C. D., Bruns P. J. RNA and protein synthesis during meiotic prophase in Tetrahymena thermophila. J Protozool. 1985 Nov;32(4):644–649. doi: 10.1111/j.1550-7408.1985.tb03094.x. [DOI] [PubMed] [Google Scholar]
- Mathis D., Oudet P., Chambon P. Structure of transcribing chromatin. Prog Nucleic Acid Res Mol Biol. 1980;24:1–55. doi: 10.1016/s0079-6603(08)60670-4. [DOI] [PubMed] [Google Scholar]
- Perry M., Chalkley R. Histone acetylation increases the solubility of chromatin and occurs sequentially over most of the chromatin. A novel model for the biological role of histone acetylation. J Biol Chem. 1982 Jul 10;257(13):7336–7347. [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Wangh L. J., Allfrey V. G. Processing of newly synthesized histone molecules. Science. 1975 Oct 10;190(4210):117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- Sugai T., Hiwatashi K. Cytologic and autoradiographic studies of the micronucleus at meiotic prophase in Tetrahymena pyriformis. J Protozool. 1974 Oct;21(4):542–548. doi: 10.1111/j.1550-7408.1974.tb03695.x. [DOI] [PubMed] [Google Scholar]
- Travis G. H., Colavito-Shepanski M., Grunstein M. Extensive purification and characterization of chromatin-bound histone acetyltransferase from Saccharomyces cerevisiae. J Biol Chem. 1984 Dec 10;259(23):14406–14412. [PubMed] [Google Scholar]
- Vavra K. J., Allis C. D., Gorovsky M. A. Regulation of histone acetylation in Tetrahymena macro- and micronuclei. J Biol Chem. 1982 Mar 10;257(5):2591–2598. [PubMed] [Google Scholar]
- Wallis J. W., Rykowski M., Grunstein M. Yeast histone H2B containing large amino terminus deletions can function in vivo. Cell. 1983 Dec;35(3 Pt 2):711–719. doi: 10.1016/0092-8674(83)90104-6. [DOI] [PubMed] [Google Scholar]