Abstract
The subset of patients with late-life onset of bipolar disorder is widely recognized to represent an etiologically diverse group with high rates of neurologic comorbidities.1,2 Efforts to characterize the unique clinical and neurobiological features of this heterogeneous population have yielded inconsistent findings.3,4 Although there is growing evidence that geriatric-onset bipolar disorder is often attributable to a variety of secondary organic etiologies,5 we would like to present a case of bipolar disorder emerging in late life where no clear organic cause was identified. This case highlights the importance of a broad differential diagnosis when approaching new-onset manic symptoms among geriatric patients in an inpatient setting.
Case Report
Ms. C, a 65-year-old Caucasian female professor with a history notable for hypertension and depression presented with a change in mental status in the context of severe hypertension. A psychiatry consultation was requested to evaluate Ms. C for delirium. She had experienced several days of euphoria, insomnia, excessive journaling, pressured speech, inappropriate laughter, and grandiose ideas about inventing a new school of philosophy. When she arrived at the hospital, she was acutely confused, disoriented, and agitated. She had waxing and waning levels of alertness and was non-verbal (communicated with odd gestures; i.e., cupping her hands). She was afebrile with a blood pressure (BP) of 212/119 mmHg. Her other vital signs were unremarkable, as was all initial blood work, which included complete blood count, prothrombin time/partial thromboplastin time, cardiac enzymes, comprehensive metabolic panel, liver function tests, thyroid function tests, urine drug screen, serum toxin screen, vitamin B12, and folate levels. Physical exam was notable for a nonfocal neurologic exam with normal muscle tone and fundi. Her BP was aggressively managed, and by the second hospital day, her confusion and disorientation cleared (mini-mental state examination score was 28/30), though she continued to exhibit grandiosity, mood lability, and loose associations. Extensive medical workup was unremarkable, including magnetic resonance imaging (MRI), electroencephalography (EEG), cerebrospinal fluid (CSF) analysis, porphyrin tests, infectious screens (herpes simplex virus polymerase chain reaction, human immunodeficiency virus antibody, rapid plasma reagin, Lyme antibody), and rheumatologic labs (sedimentation rate, antinuclear antibodies, rheumatoid factor), except for a urinary tract infection (UTI), which was treated with antibiotics. She was started on olanzapine (titrated to a dose of 10 mg) and lorazepam, and had a marked reduction in her manic symptoms after 5 days.
Upon further review of Ms. C’s medical history, it was revealed that she had a remarkably similar episode when she was 61 years old. At that time, she experienced several days of euphoria, decreased need for sleep, excessive productivity, hypergraphia, and grandiose beliefs about seeing “connections” where others could not, ultimately presenting to the hospital with confusion, transient mutism, and fluctuating consciousness in the context of severe hypertension (233/114 mmHg). After medical etiologies were ruled out, she was diagnosed with psychotic disorder not otherwise specified. She was treated with low-dose olanzapine at that time, which was stopped after several days when she quickly returned to her baseline.
Ms. C had no other history of manic symptoms. She has had mild depressive symptoms since her early adulthood, which had been controlled with fluoxetine 40 mg for many years. She had no history of substance abuse and no family history of psychiatric illness.
She was followed on an outpatient basis for a year. After several months, she began experiencing some myalgia and joint pain. Due to concern that these physical symptoms were related to her antipsychotic medication, she was transitioned from olanzapine to lamotrigine. Her mood remained stable during this period. Throughout the following year of follow-up, she displayed no evidence of cognitive decline and no further medical issues arose.
Discussion
Empirical evidence suggests that age of onset for bipolar disorder has a trimodal distribution pattern, with distinct groups represented by onset in late teens, mid 20s, and early 40s.6 Differences in clinical presentations between these groups appear minimal, with several studies failing to separate them on domains such as symptom severity, cognitive function, and genetic vulnerability.1,3,7 Despite being phenotypically similar to the other age of onset groups, the late-onset subtype has historically been viewed as an organic variant of bipolar disorder.8 While it is expected that geriatric populations will carry a higher burden of medical comorbidities than their younger counter-parts, the preponderance of late-onset bipolar patients with cerebrovascular disease and other forms of neurologic illness raises the question of whether the late-onset subtype represents an etiologically distinct entity.
Krauthammer and Klerman (1978) introduced the concept of manic syndromes emerging from organic dys-function.9 Their identification of “secondary mania” highlighted the importance of investigating for potential reversible etiologies when presented with a new-onset manic syndrome, particularly in late life. They suggested that the behavioral symptoms associated with mania may reflect a common final pathway shared by multiple differing pathophysiological processes. What remains unclear, however, is whether the emergence of neuropsychiatric symptoms in the context of physiologic insults represents an independent organic process or rather the triggering of a latent primary mood disorder. More recent investigators have theorized that an underlying affective vulnerability may predict behavioral sequelae of neurologic insults. For example, the emergence of a manic syndrome after a neurologic insult may be more likely in individuals previously thought to have unipolar depression.8 Ongoing investigation in this area offers potential models for the pathogenesis of new-onset manic syndromes in geriatric populations.10
Early work exploring the association between neurologic insults and late presentations of mania emerged from post-stroke patients demonstrating these symptoms. While less common than depression, the emergence of mania following stroke was well described with a strong hemispheric preference for right-sided lesions.11,12 More recent investigation has focused on subtle markers of neurologic disease and their relation to late-onset mania. White matter hyperintensities (WMH) are nonspecific findings on brain MRI often associated with silent cerebral infarcts and un-controlled hypertension. The appearance of WMH on imaging has been associated with bipolar disorder (among other psychiatric disorders), generating hypotheses about the underlying neurocircuitry. It has been proposed that WMH are markers of interference in the function of frontolimbic circuits involving regions implicated in the pathophysiology of bipolar disorder, which may have downstream effects on mood dysregulation.13 Tamashiro et al. explored this association in late-onset bipolar disorder in a comparison study looking at WMH in early versus late-onset patients, finding a greater prevalence in the late-onset group.14
The increased likelihood of neurologic comorbidities in late-onset bipolar disorder calls for a thorough medical workup to rule out reversible organic etiologies. In addition to neurovascular factors, other potentially modifiable processes that may underlie late-onset symptoms of mania include epilepsy, central nervous system infections, head injuries, tumors, endocrine disorders, vitamin deficiencies, dementia, and medication side effects (e.g., corticosteroids, stimulants).5,9 A reasonable diagnostic screen might include complete blood counts, a comprehensive metabolic panel, thyroid function tests, human immunodeficiency virus antibody testing, rapid plasma reagin, urinalysis, serum vitamin B12, and folate. We would also recommend MRI of the brain and EEG on all patients with first-time manic symptoms in late life. If clinically indicated, further investigations may include CSF analysis and an auto-immune workup.
While bipolar disorder in geriatric patients remains an area of active interest among investigators,15 there are relatively few case reports that discuss the approach to symptoms of mania in an elderly patient with no known bipolar history. Arciniegas describes a case of a 60-year-old male with new onset of impulsivity, reduced need for sleep, and labile affect who was given an initial diagnosis of late-onset bipolar disorder. After a thorough neuropsychiatric evaluation correlated with cerebral neuroimaging,he was discovered to have frontotemporal dementia.16 Amaladoss and Le Claire present a 69-year-old male who experienced new symptoms of grandiosity, irritability, and psychosis. He was diagnosed with secondary mania after a medical review revealed multiple comorbid conditions that were thought to have contributed to his mood symptoms: (1) computed tomography evidence of lacunar and thalamic infarcts; (2) history of head injury.17 These cases highlight the value of intensive medical workup when approaching a patient with late-onset mania.
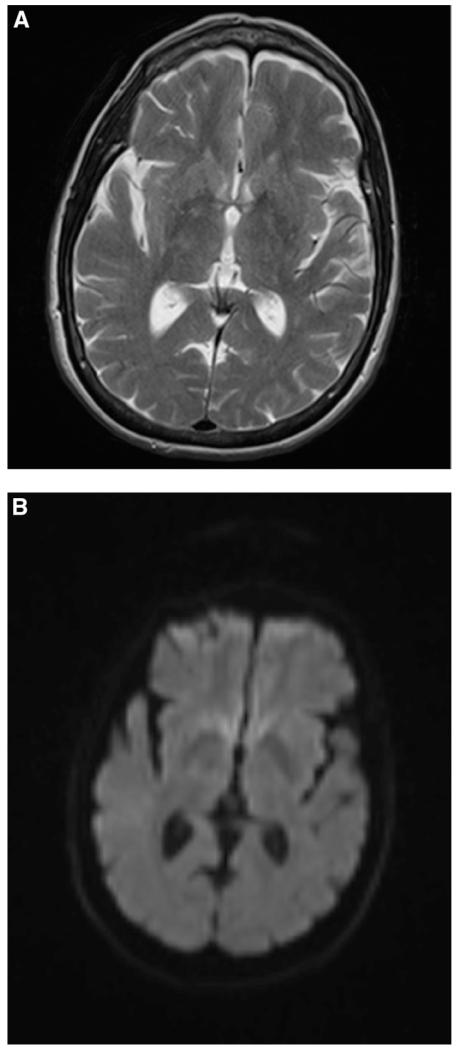
The case of Ms. C illustrates the challenge of diagnosing new-onset bipolar disorder in late life. Several features of her presentation raised suspicion for secondary mania. The co-occurrence of severe hypertension during both of her discrete manic episodes offered a possible avenue for an underlying organic “trigger.” Posterior reversible encephalopathy syndrome (PRES) is associated with headache, mental status alterations, and severe hypertension, and can present with primarily psychiatric symptoms.18 Extensive investigation by the primary neurology team confirmed that the patient did not have the characteristic radiographic features on MRI, ruling out the possibility of PRES (see Figure 1). MRI also failed to reveal evidence of WMH, which might be expected in cases of chronic uncontrolled hypertension. The rapid on-set and resolution of her confusional symptoms may be attributable to a transient hypertensive encephalopathy, though the temporal separation of her manic symptoms indicates that her hypertension alone is not sufficient to explain this episode. Discussions with the patient’s family revealed that her manic symptoms emerged up to a week prior to her acute confusion, and that home BP monitoring was within normal range during this window of time. It is possible that her manic state impaired her ability to comply with her antihypertensive medication regimen.
FIGURE 1.
Magnetic Resonance Imaging of Ms. C. (A) T2-Weighted Axial MRI Lacks Characteristic Findings from PRES (i.e., Symmetric Vasogenic Edema), No Change in Signals in Bilateral Occipital Lobes. (B) Diffusion-Weighted Image of Bilateral Occipital Lobes also Do Not Reveal Any Increased Signaling.
Delirium was also considered, given her altered sensorium on initial presentation, as well as the complicating factor of a UTI. The concept of delirious mania (or “Bell’s Mania”) was described over a century ago and is characterized by an acute onset of confusion, excitement, sleeplessness, perceptual disturbance, often with some symptoms of catatonia including mutism and posturing. It can occur in the context of severe vital sign abnormalities and must be distinguished from malignant catatonia and hyperactive delirium.19 Ms. C shared many of these features in each of her two manic episodes; however, the traditional manic symptoms appeared both before and after her period of altered sensorium and disorientation. When evaluating a patient with psychiatric symptoms co-occurring with signs of delirium, a careful investigation of the temporal relationship of the findings is paramount in guiding diagnosis.
Other potentially modifiable processes were adequately ruled out. Serum and CSF analyses found no evidence of infectious, toxic, endocrinologic, or pathologic metabolic processes. Imaging failed to reveal any structural abnormalities. Twenty-four-hour EEG demonstrated no seizure activity. An emerging neurodegenerative process was considered, since dementia can present initially with disinhibition, lability, and other behavioral disturbances (including classic manic syndromes).5 While this could not be fully ruled out in the initial phase of treatment, her longitudinal course demonstrated a return to baseline cognitive function without evidence of subsequent decline, making dementia unlikely.
While it initially seemed likely that an organic source for her symptoms would be uncovered, a thorough workup coupled with a detailed historical review revealed what appears to have been a primary case of late-onset bipolar disorder. Similarly, Aggarwal et al. offer the case of a 75-year-old male with no prior psychiatric history who presented with grandiosity, elevated mood, hyperverbality, and decreased sleep. A detailed medical history uncovered no comorbid conditions and thorough medical workup failed to establish an organic etiology for the symptoms.20
Our case demonstrates that a new diagnosis of bipolar disorder in geriatric populations is essentially an exclusionary process whereby organic etiologies are systematically ruled out. It draws attention to the fact that bipolar disorder can emerge in late life as a primary psychiatric disorder without a clear organic etiology. This particular group of late-onset bipolar disorder patients warrants further study to explore their response to psychotropic medications and better define the natural progression of illness.
References
- 1.Almeida OP, Fenner S. Bipolar disorder: Similarities and differences between patients with illness onset before and after 65 years of age. Int Psychogeriatr. 2002;14(3):311–322. doi: 10.1017/s1041610202008517. [DOI] [PubMed] [Google Scholar]
- 2.Depp CA, Jeste DV. Bipolar disorder in older adults: a critical review. Bipolar Disord. 2004;6(5):343–367. doi: 10.1111/j.1399-5618.2004.00139.x. [DOI] [PubMed] [Google Scholar]
- 3.Depp CA, Jin H, Mohamed S, Kaskow J, Moore DJ, Jeste DV. Bipolar disorder in middle-aged and elderly adults: is age of onset important? J Nerv Ment Dis. 2004;192(11):796–799. doi: 10.1097/01.nmd.0000145055.45944.d6. [DOI] [PubMed] [Google Scholar]
- 4.Wylie M, Mulsant B, Pollock B, Sweet RA, Zubenko GS, Begley AE, et al. Age at onset in geriatric bipolar disorder. Am J Geriatr Psychiatry. 1999;7(1):77–83. [PubMed] [Google Scholar]
- 5.Almeida OP. Bipolar disorder with late onset: an organic variety of mood disorder? Rev Bras Psychiatry (Rev Bras Psiquiatr) 2004;26(Supl III):27–30. doi: 10.1590/s1516-44462004000700007. [DOI] [PubMed] [Google Scholar]
- 6.Bellivier F, Golmard JL, Rietschel M, Schulze TG, Malafosse A, Preisig M, et al. Age at onset in bipolar I affective disorder: further evidence for three subgroups. Am J Psychiatry. 2003;160(5):999–1001. doi: 10.1176/appi.ajp.160.5.999. [DOI] [PubMed] [Google Scholar]
- 7.Leboyer M, Henry C, Paillere-Martinot ML, Bellivier F. Age at onset in bipolar affective disorders: a review. Bipolar Disord. 2005;7(2):111–118. doi: 10.1111/j.1399-5618.2005.00181.x. [DOI] [PubMed] [Google Scholar]
- 8.Shulman KI. Disinhibition syndromes, secondary mania, and bipolar disorder in old age. J Affect Disord. 1997;46(3):175–182. doi: 10.1016/s0165-0327(97)00156-0. [DOI] [PubMed] [Google Scholar]
- 9.Krauthammer C, Klerman GL. Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry. 1978;35(11):1333–1339. doi: 10.1001/archpsyc.1978.01770350059005. [DOI] [PubMed] [Google Scholar]
- 10.Steffens DC, Krishnan KR. Structural neuroimaging and mood disorders: recent findings, implications for classification, and future directions. Biol Psychiatry. 1998;43:705–712. doi: 10.1016/s0006-3223(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 11.Cummings JL, Mendez MF. Secondary mania with focal cerebrovascular lesions. Am J Psychiatry. 1984;141(9):1084–1087. doi: 10.1176/ajp.141.9.1084. [DOI] [PubMed] [Google Scholar]
- 12.Starkstein SE, Fedoroff JP, Berthier MD, Robinson RG. Manic depressive and pure manic states after brain lesions. Biol Psychiatry. 1991;29(2):149–158. doi: 10.1016/0006-3223(91)90043-l. [DOI] [PubMed] [Google Scholar]
- 13.Zanetti MV, Cordeiro Q, Busatto GF. Late onset bipolar disorder associated with white matter hyperintensities: a pathophysiological hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(2):551–556. doi: 10.1016/j.pnpbp.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Tamashiro JH, Zung S, Zanetti MV, de Castro CC, Vallada H, Busatto GF, et al. Increased rates of white matter hyperintensities in late-onset bipolar disorder. Bipolar Disord. 2008;10(7):765–775. doi: 10.1111/j.1399-5618.2008.00621.x. [DOI] [PubMed] [Google Scholar]
- 15.Benedetti A, Scarpellini P, Casamassima F, Lattanzi L, Liberti M, Musetti L, et al. Bipolar disorder in late life: clinical characteristics in a sample of older adults admitted for manic episode. Clin Pract Epidemiol Ment Health. 2008;4:22. doi: 10.1186/1745-0179-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arciniegas DB. New-onset bipolar disorder in late life: a case of mistaken identity. Am J Psychiatry. 2006;163(2):198–203. doi: 10.1176/appi.ajp.163.2.198. [DOI] [PubMed] [Google Scholar]
- 17.Amaladoss A, Le Claire JK. Late onset mania in the elderly. Int J Geriatr Psychiatry. 2010;25(8):870–872. doi: 10.1002/gps.2408. [DOI] [PubMed] [Google Scholar]
- 18.Kimura R, Yanagida M, Kugo A, Taguchi S, Matsunaga H. Posterior reversible encephalopathy syndrome in chronic alcoholism with acute psychiatric symptoms. Gen Hosp Psychiatry. 2010;32(4):447–e3-5. doi: 10.1016/j.genhosppsych.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Fink M. Delirious mania. Bipolar Disord. 1999;1(1):54–60. doi: 10.1034/j.1399-5618.1999.10112.x. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal A, Kumar R, Sharma RC, Sharma DD. First episode mania at 75 years of age. Indian J Psychol Med. 2010;32(2):144–145. doi: 10.4103/0253-7176.78514. [DOI] [PMC free article] [PubMed] [Google Scholar]