Abstract
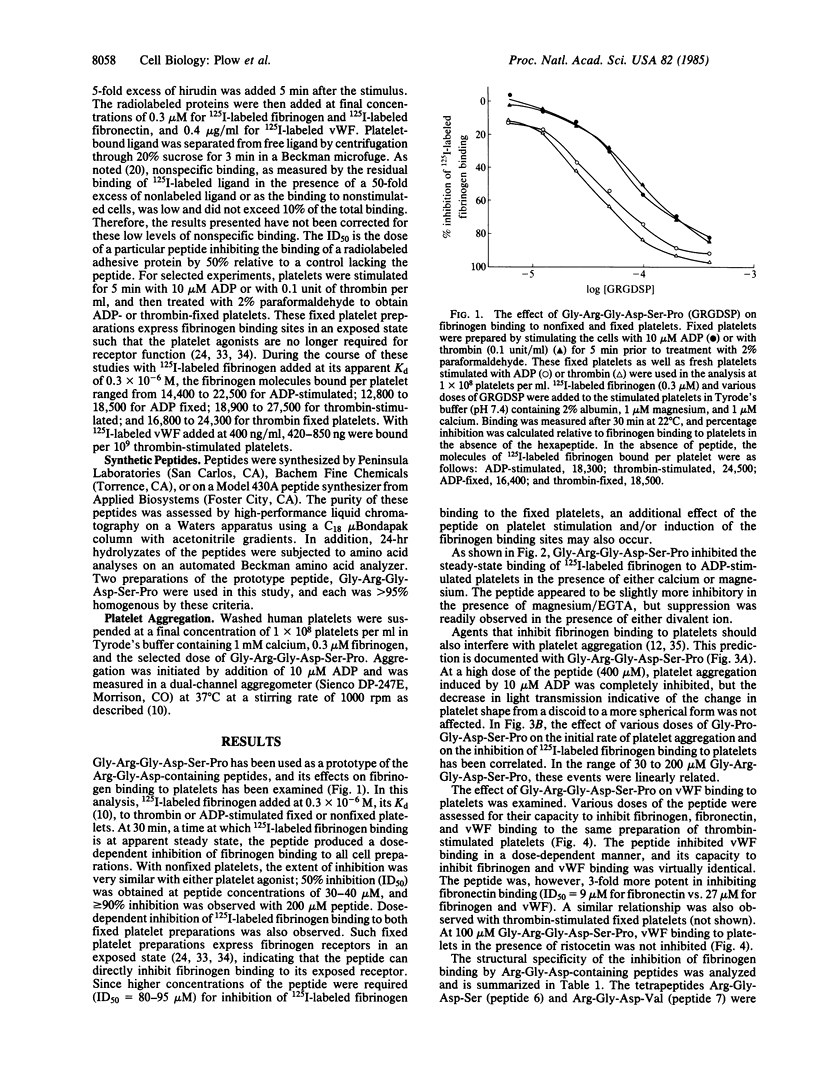
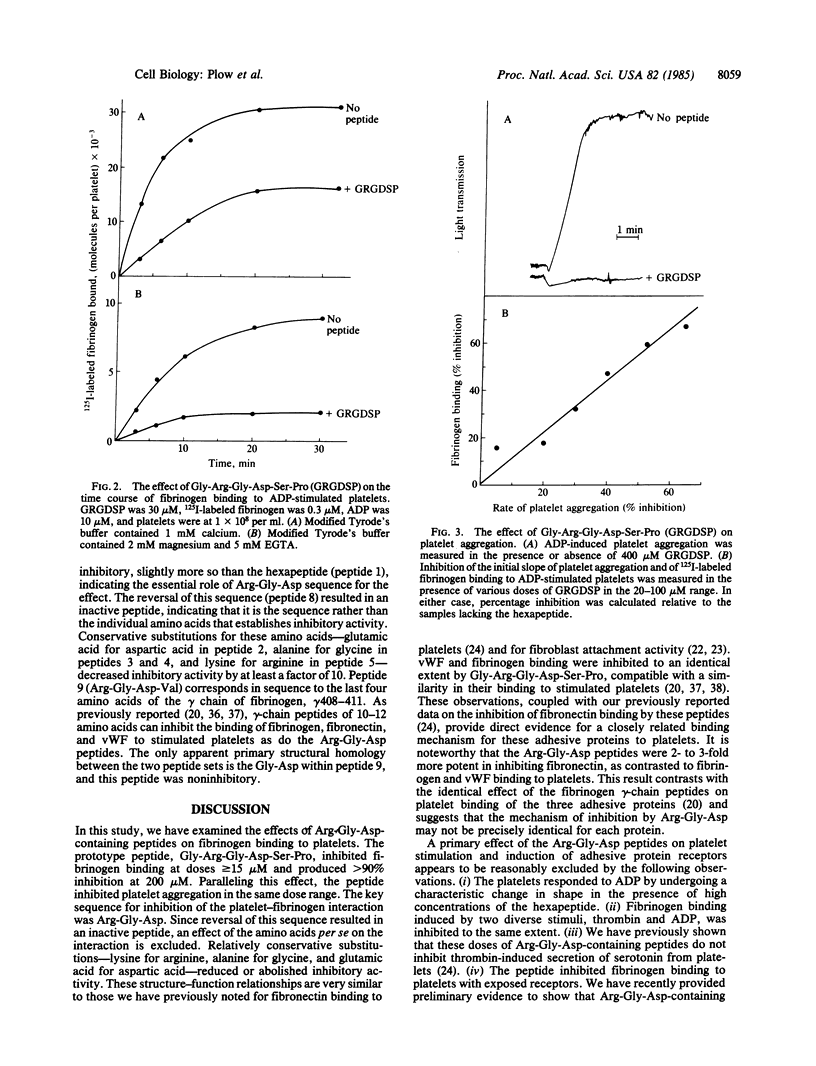
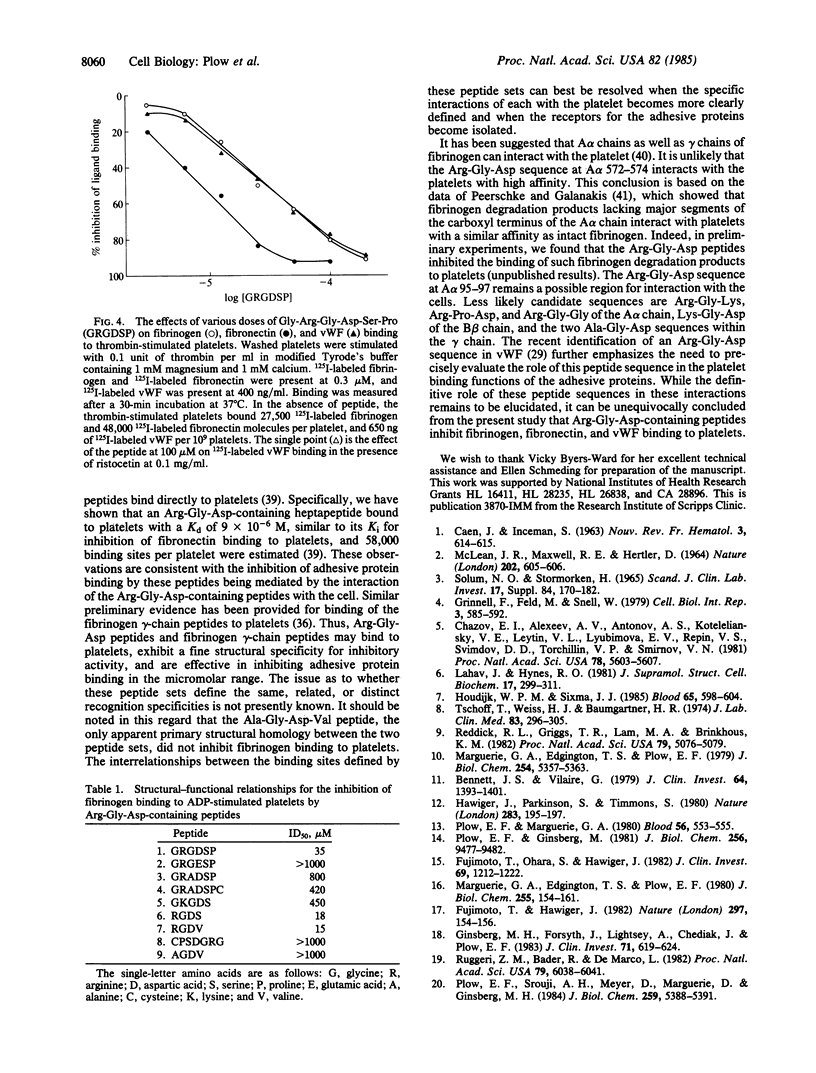
The Arg-Gly-Asp sequence resides in the cell attachment region of fibronectin. Arg-Gly-Asp-containing peptides support fibroblast attachment, inhibit fibroblast adhesion to fibronectin, and inhibit fibronectin binding to thrombin-stimulated platelets. In view of the similarities between the binding of fibronectin, fibrinogen, and von Willebrand factor to stimulated platelets, we have examined the effects of Arg-Gly-Asp-containing peptides on the interaction of these latter two adhesive proteins with platelets. Gly-Arg-Gly-Asp-Ser-Pro was used as a prototype peptide, and this hexapeptide inhibited fibrinogen binding to ADP and thrombin-stimulated platelets in the 10-200 microM range. The inhibition exceeded 90% at high concentrations of peptide and was observed in the presence of either calcium or magnesium. Platelet aggregation was also inhibited by the peptide in this dose range. The hexapeptide inhibited fibrinogen binding to platelets with receptors fixed in an exposed state, indicating direct interference with the ligand-platelet interaction. The peptide was 1/2 to 1/3rd as potent in inhibiting fibrinogen as fibronectin binding to platelets, but fibrinogen and von Willebrand factor binding were inhibited to an identical extent. Conservative amino acid substitutions for the arginine, glycine, or aspartic acid markedly reduced inhibitory activity and the Asp-Gly-Arg sequence was inactive. These results indicate that Arg-Gly-Asp-containing peptides can inhibit the binding of the three adhesive proteins to stimulated platelets, establishing a basic common feature between the interaction of these molecules with platelets.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. S., Vilaire G. Exposure of platelet fibrinogen receptors by ADP and epinephrine. J Clin Invest. 1979 Nov;64(5):1393–1401. doi: 10.1172/JCI109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAEN J., INCEMAN S. CONSID'ERATIONS SUR L'ALLONGEMENT DU TEMPS DE SAIGNEMENT DANS L'AFIBRINOG'EN'EMIE CONG'ENITALE. Nouv Rev Fr Hematol. 1963 Sep-Oct;3:614–615. [PubMed] [Google Scholar]
- Chazov E. I., Alexeev A. V., Antonov A. S., Koteliansky V. E., Leytin V. L., Lyubimova E. V., Repin V. S., Sviridov D. D., Torchilin V. P., Smirnov V. N. Endothelial cell culture on fibrillar collagen: model to study platelet adhesion and liposome targeting to intercellular collagen matrix. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5603–5607. doi: 10.1073/pnas.78.9.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Watt K. W., Cottrell B. A., Strong D. D., Riley M. The amino acid sequence of the alpha-chain of human fibrinogen. Nature. 1979 Aug 9;280(5722):464–468. doi: 10.1038/280464a0. [DOI] [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Hawiger J. Adenosine diphosphate induces binding of von Willebrand factor to human platelets. Nature. 1982 May 13;297(5862):154–156. doi: 10.1038/297154a0. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Ohara S., Hawiger J. Thrombin-induced exposure and prostacyclin inhibition of the receptor for factor VIII/von Willebrand factor on human platelets. J Clin Invest. 1982 Jun;69(6):1212–1222. doi: 10.1172/JCI110560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Forsyth J., Lightsey A., Chediak J., Plow E. F. Reduced surface expression and binding of fibronectin by thrombin-stimulated thrombasthenic platelets. J Clin Invest. 1983 Mar;71(3):619–624. doi: 10.1172/JCI110808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M., Pierschbacher M. D., Ruoslahti E., Marguerie G., Plow E. Inhibition of fibronectin binding to platelets by proteolytic fragments and synthetic peptides which support fibroblast adhesion. J Biol Chem. 1985 Apr 10;260(7):3931–3936. [PubMed] [Google Scholar]
- Grinnell F., Feld M., Snell W. The influence of cold insoluble globulin on platelet morphological response to substrata. Cell Biol Int Rep. 1979 Oct;3(7):585–592. doi: 10.1016/0309-1651(79)90056-0. [DOI] [PubMed] [Google Scholar]
- Harfenist E. J., Packham M. A., Kinlough-Rathbone R. L., Mustard J. F. Inhibitors of ADP-induced platelet aggregation prevent fibrinogen binding to rabbit platelets and cause rapid deaggregation and dissociation of bound fibrinogen. J Lab Clin Med. 1981 May;97(5):680–688. [PubMed] [Google Scholar]
- Hawiger J., Parkinson S., Timmons S. Prostacyclin inhibits mobilisation of fibrinogen-binding sites on human ADP- and thrombin-treated platelets. Nature. 1980 Jan 10;283(5743):195–197. doi: 10.1038/283195a0. [DOI] [PubMed] [Google Scholar]
- Hawiger J., Timmons S., Kloczewiak M., Strong D. D., Doolittle R. F. gamma and alpha chains of human fibrinogen possess sites reactive with human platelet receptors. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2068–2071. doi: 10.1073/pnas.79.6.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman E. G., Pierschbacher M. D., Ruoslahti E. Detachment of cells from culture substrate by soluble fibronectin peptides. J Cell Biol. 1985 Jun;100(6):1948–1954. doi: 10.1083/jcb.100.6.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdijk W. P., Sixma J. J. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985 Mar;65(3):598–604. [PubMed] [Google Scholar]
- Kloczewiak M., Timmons S., Lukas T. J., Hawiger J. Platelet receptor recognition site on human fibrinogen. Synthesis and structure-function relationship of peptides corresponding to the carboxy-terminal segment of the gamma chain. Biochemistry. 1984 Apr 10;23(8):1767–1774. doi: 10.1021/bi00303a028. [DOI] [PubMed] [Google Scholar]
- Lahav J., Hynes R. O. Involvement of fibronectin, Von Willebrand factor, and fibrinogen in platelet interaction with solid substrata. J Supramol Struct Cell Biochem. 1981;17(4):299–311. doi: 10.1002/jsscb.380170402. [DOI] [PubMed] [Google Scholar]
- MCLEAN J. R., MAXWELL R. E., HERTLER D. FIBRINOGEN AND ADENOSINE DIPHOSPHATE-INDUCED AGGREGATION OF PLATELETS. Nature. 1964 May 9;202:605–606. doi: 10.1038/202605a0. [DOI] [PubMed] [Google Scholar]
- Marguerie G. A., Edgington T. S., Plow E. F. Interaction of fibrinogen with its platelet receptor as part of a multistep reaction in ADP-induced platelet aggregation. J Biol Chem. 1980 Jan 10;255(1):154–161. [PubMed] [Google Scholar]
- Marguerie G. A., Plow E. F., Edgington T. S. Human platelets possess an inducible and saturable receptor specific for fibrinogen. J Biol Chem. 1979 Jun 25;254(12):5357–5363. [PubMed] [Google Scholar]
- Meyer D., Obert B., Pietu G., Lavergne J. M., Zimmerman T. S. Multimeric structure of factor VIII/von Willebrand factor in von Willebrand's disease. J Lab Clin Med. 1980 Apr;95(4):590–602. [PubMed] [Google Scholar]
- Peerschke E. I., Galanakis D. K. Binding of fibrinogen to ADP-treated platelets. Comparison of plasma fibrinogen fractions and early plasmic fibrinogen derivatives. J Lab Clin Med. 1983 Mar;101(3):453–460. [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984 May 3;309(5963):30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- Pierschbacher M. D., Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M., Hayman E. G., Ruoslahti E. Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1224–1227. doi: 10.1073/pnas.80.5.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piétu G., Cherel G., Marguerie G., Meyer D. Inhibition of von Willebrand factor-platelet interaction by fibrinogen. Nature. 1984 Apr 12;308(5960):648–649. doi: 10.1038/308648a0. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Ginsberg M. H. Specific and saturable binding of plasma fibronectin to thrombin-stimulated human platelets. J Biol Chem. 1981 Sep 25;256(18):9477–9482. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A., Ginsberg M. H. Fibronectin binding to thrombin-stimulated platelets: evidence for fibrin(ogen) independent and dependent pathways. Blood. 1985 Jul;66(1):26–32. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. A. Participation of ADP in the binding of fibrinogen to thrombin-stimulated platelets. Blood. 1980 Sep;56(3):553–555. [PubMed] [Google Scholar]
- Plow E. F., Marguerie G. Inhibition of fibrinogen binding to human platelets by the tetrapeptide glycyl-L-prolyl-L-arginyl-L-proline. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3711–3715. doi: 10.1073/pnas.79.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Srouji A. H., Meyer D., Marguerie G., Ginsberg M. H. Evidence that three adhesive proteins interact with a common recognition site on activated platelets. J Biol Chem. 1984 May 10;259(9):5388–5391. [PubMed] [Google Scholar]
- Reddick R. L., Griggs T. R., Lamb M. A., Brinkhous K. M. Platelet adhesion to damaged coronary arteries: Comparison in normal and von Willebrand disease swine. Proc Natl Acad Sci U S A. 1982 Aug;79(16):5076–5079. doi: 10.1073/pnas.79.16.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rixon M. W., Chan W. Y., Davie E. W., Chung D. W. Characterization of a complementary deoxyribonucleic acid coding for the alpha chain of human fibrinogen. Biochemistry. 1983 Jun 21;22(13):3237–3244. doi: 10.1021/bi00282a031. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Bader R., de Marco L. Glanzmann thrombasthenia: deficient binding of von Willebrand factor to thrombin-stimulated platelets. Proc Natl Acad Sci U S A. 1982 Oct;79(19):6038–6041. doi: 10.1073/pnas.79.19.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. E., Shelton-Inloes B. B., Sorace J. M., Harlan J. M., Titani K., Davie E. W. Cloning and characterization of two cDNAs coding for human von Willebrand factor. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6394–6398. doi: 10.1073/pnas.82.19.6394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solum N. O., Stormorken H. Influence of fibrinogen on the aggregation of washed human blood platelets induced by adenosine diphosphate, thrombin, collagen, and adrenaline. Scand J Clin Lab Invest. 1965;17(Suppl):170+–170+. [PubMed] [Google Scholar]
- Springer W. R., Cooper D. N., Barondes S. H. Discoidin I is implicated in cell-substratum attachment and ordered cell migration of Dictyostelium discoideum and resembles fibronectin. Cell. 1984 Dec;39(3 Pt 2):557–564. doi: 10.1016/0092-8674(84)90462-8. [DOI] [PubMed] [Google Scholar]
- Timmons S., Kloczewiak M., Hawiger J. ADP-dependent common receptor mechanism for binding of von Willebrand factor and fibrinogen to human platelets. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4935–4939. doi: 10.1073/pnas.81.15.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp T. B., Weiss H. J., Baumgartner H. R. Decreased adhesion of platelets to subendothelium in von Willebrand's disease. J Lab Clin Med. 1974 Feb;83(2):296–300. [PubMed] [Google Scholar]


