Abstract
Objectives
To assess the efficacy and safety of varenicline (Chantix®) for the treatment of alcohol use disorders. Varenicline is a partial α4β2 nicotinic acetylcholine agonist approved by the Food and Drug Administration for smoking cessation. It has reduced drinking in animal studies and in small studies of humans who were both heavy drinkers and smokers. This is the first multisite clinical trial of varenicline in a population of smokers and nonsmokers with alcohol use disorders.
Methods
Men and women (n=200) meeting the criteria for alcohol dependence were recruited across 5 clinical sites. Patients received double-blind varenicline or placebo and a computerized behavioral intervention. Varenicline was titrated during the first week to 2 mg/day, which was maintained during weeks 2–13.
Results
The varenicline group had significantly lower weekly percent heavy drinking days (primary outcome) (adjusted mean difference=10.4), drinks per day, drinks per drinking day, and alcohol craving compared with the placebo group (p values < 0.05). The average treatment effect on alcohol use was similar for smokers and nonsmokers. Varenicline was well-tolerated; adverse events were expected and mild.
Conclusions
Varenicline significantly reduced alcohol consumption and craving, making it a potentially viable option for the treatment of alcohol use disorders.
Keywords: Alcohol Dependence, varenicline, Chantix®, Champix®, Alcohol Use Disorder, randomized placebo-controlled clinical trial
Alcohol use disorders (AUD) (abuse and dependence) affect 18 million Americans, causing a wide range of medical, psychological, social, personal, and economic problems (Grant et al., 2004; Rehm et al., 2009). This heterogeneous disorder is characterized by compulsive alcohol use and an inability to stop drinking despite harmful consequences (American Psychiatric Association [APA], 1994). Alcohol use has recently been identified as the third leading risk factor for global burden of disease and injury (Lim et al., 2012). The total economic cost of excessive alcohol consumption in the United States is estimated to be $224 billion annually (Bouchery et al., 2011). Currently, only three medications are approved by the U.S. Food and Drug Administration (FDA) specifically for the treatment of alcohol dependence: disulfiram, naltrexone (oral and injectable), and acamprosate. Though effective for some, these drugs do not work for everyone and they remain under-utilized by clinicians (Litten et al., 2012).
Varenicline (Chantix®) (Pfizer, NY, NY) is a partial α4β2 nicotinic acetylcholine agonist used in aiding smoking cessation. Since being approved by the FDA in 2006, it has been prescribed for 8.9 million people in the United States (SDI, 2011). Converging lines of data suggest that nicotinic acetylcholine receptors may play a significant role in the rewarding effects of both nicotine and alcohol (Le et al., 2000; Tizabi et al., 2002; Ericson et al., 2009), indicating a promising molecular target for the treatment of both disorders.
Alcohol and tobacco use often occur in tandem (Hurley et al., 2012), with interactions occurring at the pharmacologic, genetic, and neurochemical levels. Preclinical studies demonstrated decreases in alcohol consumption in rodents given varenicline (Steensland et al., 2007; Ericson et al., 2009; Wouda et al., 2011). A human laboratory study (McKee et al., 2009) of smokers who also were heavy drinkers reported a reduction in alcohol drinking, craving, and the pleasant subjective and reinforcing effects of alcohol when subjects were given varenicline. More recently, in a preliminary study, 15 heavy drinking smokers treated with varenicline for 3 weeks reported a greater reduction in alcohol craving and fewer heavy drinking days compared with placebo (Fucito et al., 2011). Similarly, in another small study (n=64), varenicline reduced alcohol consumption in heavy drinking smokers (Mitchell et al., 2012).
The study reported here represents the first multisite clinical trial to assess the efficacy and safety of varenicline in an alcohol-dependent population of smokers and nonsmokers. Heavy drinking, alcohol dependent patients who are actively drinking were selected because they are the group that will most likely present at primary care and/or other specialty settings due to alcohol related complications, and thus most likely to be prescribed the medication (Willenbring et al., 2009). Outcomes assessed during the 13-week trial included drinking, alcohol craving, drinking consequences, smoking, and quality of life.
Methods
Study Population
Randomized patients (n=200) included 142 men and 58 women diagnosed with past year alcohol dependence according to the Diagnostic and Statistical Manual, 4th edition Text Revision (DSM–IV–TR) (APA, 1994) as assessed by the MINI International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). Patients were eligible if they were at least 18 years of age; reported drinking an average of at least 28 standard drinks per week for females or 35 drinks per week for males during the 28-day period prior to consent and the 7-day period prior to randomization; did not reduce the total number of drinks per week by more than 50% between the 28-day period prior to consent and the 7-day period prior to randomization; had a blood alcohol content (BAC) of 0.000 upon providing study consent; and agreed to other operational study related requests.
The key exclusion criteria were: past-year DSM–IV–TR dependence on any psychoactive substances other than alcohol and nicotine (MINI); psychiatric disorders including major psychotic disorders (MINI); undergone medical detoxification during the screening phase; previous treatment with varenicline; and history of atherosclerotic cardiovascular disease. In addition, subjects who had ever attempted suicide or had current (past year) suicidality risk (based upon the MINI) were excluded (see Supplemental Digital Content 1, http://links.lww.com/JAM/A9, for full list of inclusion and exclusion criteria).
Study Design and Oversight
The study was a Phase 2, randomized, double-blind, placebo-controlled, parallel-group, multi-site 13-week treatment trial. Interested candidates responded by telephone to advertisements at 5 academic sites in the United States between February 2011 and February 2012.
In addition to screening and baseline visits, 5 in-clinic visits (Weeks 2, 4, 6, 10 and 14) and 8 telephone visits (Weeks 3, 5, 7, 8, 9, 11, 12, 13) were conducted. As follow-up, a telephone interview was conducted at Week 16, approximately 2 weeks after the last in-clinic visit, to assess drug safety and to determine any changes in drinking. Patients were required to have a BAC ≤ 0.02% to complete the in-clinic assessments.
Patients were randomly assigned, in a 1:1 ratio, to receive either varenicline or placebo using a permuted stratified block randomization procedure. The stratification variables were clinical site and regular smoking (≥ 10 versus < 10 cigarettes smoked per day for the past week) (Gonzales et al, 2006). Randomization was implemented via a telephone- or Web-based system.
The medication was dispensed using a double-blind method to patients at scheduled visits over the 13 weeks. Varenicline was supplied in 0.5 mg over-encapsulated tablets with identical matching placebos. For both the varenicline and placebo groups, the amount was titrated from a starting dose of 0.5 mg, taken once a day on Days 1 to 3, to 0.5 mg, taken twice a day on Days 4 to 7. A target dose of 1 mg, taken twice daily, was maintained during Weeks 2–13. Patients who discontinued medication were allowed to remain in the study and participate in study assessments. Dosage compliance was verified by comparing the patient's self report against the number of pills removed from the blister pack. Medication compliance was calculated as the total amount of medication taken divided by the total amount prescribed during the maintenance phase of the study (Weeks 2–13). Varenicline analyte levels were assayed in a subsample of patients to further verify compliance. The varenicline plasma concentrations were determined using a validated liquid chromatography tandem mass spectroscopy method (World Wide Clinical Trials, Austin, TX) with a lower limit of quantitation equaling 0.05 ng/mL.
All patients were required to view Take Control—a novel computerized bibliotherapy platform derived from the National Institute on Alcohol Abuse and Alcoholism's (NIAAA's) self-help approach, Rethinking Drinking (NIAAA, 2009). Take Control consists of 6 modules. Patients were asked to view a single module at each clinic visit.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonization. All patients provided voluntary, written informed consent prior to the initiation of any study procedures. The protocol, consent, and all study-related materials were reviewed and approved by the Institutional Review Board at each participating site, the FDA, and the Data and Safety Monitoring Board.
Measures of Efficacy
Drinking measures were captured via the Time-Line Followback and Form 90 interview methodology and procedures (Sobell and Sobell, 1992; Miller, 1996). One standard drink is 0.5 ounces of absolute alcohol, equivalent to 10 ounces of beer, 4 ounces of wine, or 1 ounce of 100-proof liquor. The a priori primary efficacy endpoint was percent heavy drinking days measured weekly during the maintenance phase of the study (Weeks 2–13). A “heavy drinking day” was defined as 4 or more drinks per drinking day for women and 5 or more drinks per drinking day for men.
A priori secondary efficacy endpoints included other drinking measures (i.e., drinks per day, drinks per drinking day, percent days abstinent, percent very heavy drinking days [8+/10+ drinks per drinking day for women and men, respectively], percent subjects with no heavy drinking days, and percent subjects abstinent), also during Weeks 2–13; as well as alcohol craving (Penn Alcohol Craving Scale [PACS]) (Flannery et al., 1999), alcohol-related consequences (ImBIBe; a revised and abbreviated form of the DrInC [Miller, 1995; Werner et al., 2008]), cigarettes smoked per day (past week), and quality of life (SF-12® Physical and Mental Aggregate Scores) (Szabo, 1996).
Safety Assessments
Safety was assessed via vital signs; blood chemistries and hematology; urine drug tests; BAC; adverse events; concomitant medication use; electrocardiogram (ECG); and neuropsychiatric measures including suicidal ideation (Columbia Suicide Severity Rating Scale) (Posner et al., 2009). Adverse events were assessed both in the clinic and during telephone interviews via an opened ended question: “How have you been feeling since your last visit?” Neuropsychiatric symptoms related to suicidality, mood, and behavior/thinking were assessed every week at the clinic or via telephone. The mood and behavior/thinking questions were adapted from the Brief Psychiatric Rating Scale (Overall and Gorham, 1962).
Statistical Analysis
All efficacy analyses, with the exception of the pre-specified model examining cigarettes smoked per day, were analyzed on a modified intention-to-treat (mITT) population that included all randomized patients who took at least one dose of medication and provided valid post-randomization outcome data (n=197) (see Text, Supplemental Digital Content 2, http://links.lww.com/JAM/A10, for details of analytic sample size). The smoking model included only patients who were smokers at baseline (i.e., smoked at least one cigarette per day in the past week) (n=78). Baseline and safety analyses were performed on patients who took at least one dose of medication (n=198).
Continuous outcomes measured at multiple time points were analyzed using a repeated-measures mixed effects model with all covariates treated as fixed effects except patients treated as the random effect. An unstructured covariance matrix best fit the data and was used to model the correlations between repeated measures among patients. Least-square means (LSMEANs), standard errors (SEs), and 95% confidence intervals (CIs) are presented for each treatment group and were derived from fully adjusted models on untransformed outcomes (to facilitate clinical interpretation) averaged across the maintenance period. For the drinking outcomes, these fully adjusted models included the following covariates: treatment group, week, site, treatment goal (permanent abstinence from alcohol vs. other), alcohol craving (PACS score), baseline value of the outcome (computed during the 28-day period before the first screening visit), and the treatment group by week interaction. Covariates were selected on the basis of their correlation with outcome. Treatment goal and alcohol craving were included as covariates in models of drinking outcomes because they generally were consistently correlated with drinking outcomes in bivariate analyses. However, they were not included as covariates in models of non-drinking outcomes because they were not consistently correlated with these outcomes. For the non-drinking outcomes, the fully adjusted models included the same covariates, minus treatment goal and alcohol craving because these covariates were not consistently correlated with non-drinking outcomes. Cohen's d and p-values are based on the fully adjusted models with the appropriately transformed outcome variables as follows: square root transformations (drinks per day, drinks per drinking day, percent days abstinent, alcohol-related consequences [ImBIBe], and quality of life [SF-12® Physical and Mental Aggregate Scores]); logarithmic transformation (percent very heavy drinking days); and inverse transformation (cigarettes smoked per day). The primary outcome, percent heavy drinking days, and alcohol craving (PACS) were not transformed because they were not skewed. Cohen's d = (μTreatment – μPlacebo)/σ, where μTreatment – μPlacebo is the difference between the means for the treatment and placebo groups, and σ is the pooled standard deviation. The following are offered as cut-offs for interpretive purposes of the effect size: small=.20, medium=.50, and large=.80 (Cohen, 1992).
Continuous outcomes assessed at a single time were evaluated using general linear models (ANCOVAs). For the dichotomous drinking outcomes (i.e., abstinence and no heavy drinking days), unadjusted prevalence rates are presented; odds ratios (ORs) and p-values were derived from unadjusted logistic regression models that included only treatment group; covariates were not included due to insufficient numbers of abstinent and no heavy drinking events (Peduzzi et al., 1996).
As a sensitivity analysis, missing drinking data in the primary efficacy model were handled in two ways a) by imputing missing data as heavy drinking days and b) by using multiple imputation. The multiple imputation model included the same covariates as the primary efficacy model. Twenty-five iterations of this model were run, and model estimates were averaged using PROC MIANALYZE in SAS. An exploratory subgroup analysis was conducted for the primary efficacy outcome to determine if a differential treatment effect existed as a function of baseline smoking status (i.e., smoker vs. non-smoker) during the maintenance period. For this subgroup analysis, a model similar to the primary efficacy model was used, with the additional inclusion of a smoking status covariate and the replacement of the treatment-group-by-week interaction term with a treatment-by-smoking-status interaction term.
Safety measures were assessed as Principal Investigator-reported adverse events (see Supplemental Digital Content 3, http://links.lww.com/JAM/A11, for entire listing of Adverse Events). For descriptive statistics, group mean differences were tested for significance by t-tests for independent samples for normally-distributed variables or Wilcoxon rank-sum tests for skewed variables. Group prevalence rate differences were tested for significance via chi-square or Fisher's exact tests. For all statistical tests, p<0.05 (two-tailed) was considered statistically significant. For the primary outcome, it was estimated that a sample size of 200 patients was required to obtain 170 study completers (85 per treatment group), yielding 80% power to detect a treatment effect (Cohen's d=0.43) with a two-tailed t-test at a .05 significance level. Data were analyzed with SAS version 9.2 (SAS Institute, Inc., Cary, NC).
Results
Study Sample
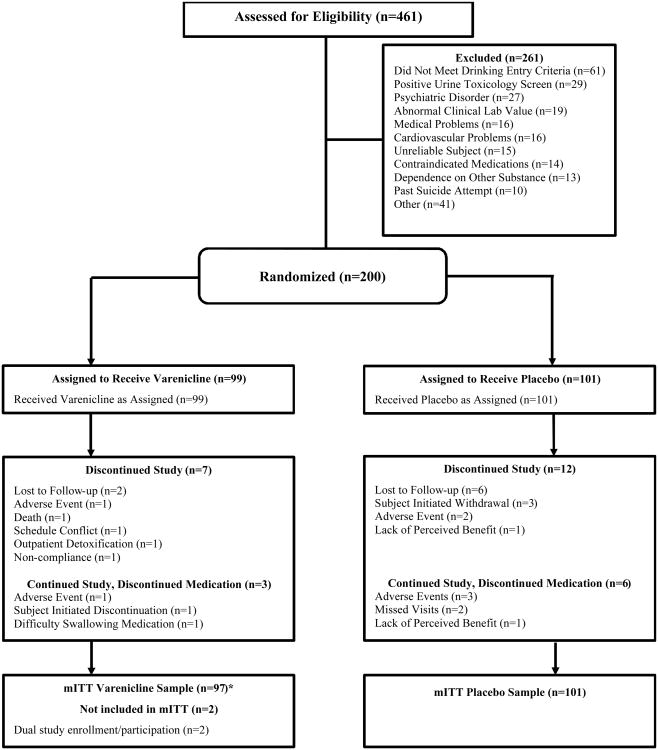
A total of 461 patients consented for the study, 200 of whom were randomized to receive varenicline or a placebo (n=99 and n=101, respectively); 261 were excluded because they did not meet eligibility criteria or they chose not to participate (Figure 1). The main reason for screen failures included: not meeting drinking criteria (23.3%; see Supplemental Digital Content 4, http://links.lww.com/JAM/A12, for details), positive urine toxicology drug screen (11.1%), and exclusionary psychiatric disorder (10.3%). More patients in the placebo than varenicline group withdrew early from the study (12 vs. 7, respectively) and discontinued medication but continued the study (6 vs. 3, respectively).
Figure 1. Subject Disposition.
mITT = modified intention-to-treat
* The mITT sample size of the varenicline group was decreased by n=2 from n=97 to 95 as one patient enrolled twice in the study (at two different study sites), gave invalid data and, consequently, both occurrences of the patient were excluded. The outcome analytic sample size of the varenicline group was further decreased to n=96 as an additional patient discontinued the study prior to reporting outcome data.
Patients in the varenicline and placebo groups had statistically similar values on all baseline characteristics (Table 1). Randomized patients were mostly male, white, employed, unmarried, and middle-aged. On average they drank heavily, consuming approximately 13 drinks per day, and met or exceeded a threshold of 4 drinks (for women) or 5 drinks (for men) per drinking day on approximately 88% of days. With respect to treatment drinking goals, just over a quarter of the patients (28%) desired permanent abstinence, with the majority seeking to drink in a controlled manner (56%). Approximately 39% smoked at least 1 cigarette in the week prior to the screening visit, averaging about 11 cigarettes per day (among the smokers). Patients had near-normal physical and mental functioning (SF-12® physical and mental aggregate scores of approximately 51 and 49, respectively).
Table 1. Baseline Characteristics of Patients.
| Placebo (n=101) | Varenicline (n=97) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| n | Mean or % | SD | n | Mean or % | SD | p-valuea | |
|
|
|||||||
| Demographics | |||||||
| Age | 101 | 45.0 | 12.3 | 97 | 46.0 | 11.0 | 0.571 |
| Male | 69 | 68.3% | 71 | 73.2% | 0.451 | ||
| Employed | 77 | 76.2% | 69 | 71.1% | 0.415 | ||
| Married | 38 | 37.6% | 44 | 45.4% | 0.269 | ||
| Education (≥ high school) | 72 | 71.3% | 60 | 61.9% | 0.159 | ||
| Race/Ethnicity | 0.300 | ||||||
| White | 71 | 70.3% | 60 | 61.9% | 0.210 | ||
| Black | 27 | 26.7% | 30 | 30.9% | 0.515 | ||
| Hispanic | 2 | 2.0% | 2 | 2.1% | 1.000 | ||
| Other | 1 | 1.0% | 5 | 5.2% | 0.113 | ||
| Self-Reported Alcohol Consumptionb | |||||||
| Drinks per day | 101 | 12.5 | 8.9 | 97 | 14.2 | 9.3 | 0.147 |
| Drinks per drinking day | 101 | 13.6 | 9.0 | 97 | 15.3 | 9.6 | 0.118 |
| Percent days abstinent | 101 | 7.9 | 13.6 | 97 | 7.7 | 12.5 | 0.995 |
| Percent heavy drinking days | 101 | 87.2 | 16.4 | 97 | 88.1 | 15.8 | 0.680 |
| Percent very heavy drinking days (8+/10+) | 101 | 57.8 | 35.6 | 97 | 66.2 | 35.0 | 0.132 |
| Other Substance-Related Indicators | |||||||
| Penn Alcohol Craving Scale (PACS) score | 101 | 16.7 | 6.8 | 97 | 17.7 | 6.2 | 0.276 |
| Alcohol-related consequences (ImBIBe) score | 101 | 16.3 | 9.7 | 96 | 17.8 | 9.8 | 0.220 |
| Age of onset of regular drinking | 101 | 19.3 | 5.5 | 97 | 18.7 | 6.1 | 0.418 |
| Alcohol-related treatment goal (abstinence vs. other) | 28 | 27.7% | 27 | 27.8% | 0.986 | ||
| Parental history of alcohol-related problems | 50 | 49.5% | 52 | 53.6% | 0.564 | ||
| Current smoker (any vs. none) | 41 | 41.0%c | 37 | 38.1% | 0.727 | ||
| Cigarettes per day (past-week) among smokers | 41 | 11.3 | 6.7 | 37 | 11.5 | 7.22 | 0.960 |
| Fagerström Test for Nicotine Dependence (FTND) score | 41 | 3.1 | 2.6 | 36 | 3.0 | 2.4 | 0.852 |
| Marijuana used | 12 | 12.0% | 14 | 14.4% | 0.614 | ||
| GGT | 101 | 70.8 | 103.2 | 97 | 72.9 | 123.49 | 0.666 |
| Psychiatric Characteristics | |||||||
| SF-12 Mental Aggregate score | 100 | 50.2 | 9.2 | 97 | 48.1 | 10.4 | 0.195 |
| SF-12 Physical Aggregate score | 100 | 52.2 | 6.0 | 97 | 50.7 | 8.8 | 0.445 |
| Clinical Institute Withdrawal Assessment of Alcohol (CIWA) score | 101 | 1.3 | 1.7 | 97 | 1.3 | 1.5 | 0.561 |
Group mean differences were tested for significance via t-tests of independent samples for normally-distributed variables or Wilcoxon rank-sum tests for skewed variables. Group prevalence rate differences were tested for significance via chi-square or Fisher's exact tests.
Reflects mean values during the 28-day period (Days 1-28) before screening.
The denominator for the placebo group includes 100 patients.
Marijuana use based on positive urine drug screen.
- PACS: 5 questions (0-30)
- ImBIBe: 15 questions (0-60)
- FTND: 6 questions (0-10) (Heatherton et al., 1991)
- SF-12: 7 questions (T-score 0-100), 50 normal functioning
- CIWA: 10 questions (0-67), ≥10 indicative of alcohol withdrawal
Medication Compliance and Participation
Overall medication compliance was 95.5% and was similar between treatment groups (95.1% vs. 96.0% for the placebo and varenicline groups, respectively; p=0.56). Ninety-seven patients consented to provide a single blood sample for pharmacokinetic analysis (placebo n=49; varenicline n=48). Of these patients, 47 patients (97.9%) in the varenicline group had analyte levels that were consistent with their self-report that the medication was taken. The average daily dosage was equivalent to 1.88 mg (or 3.76 of the 4 total pills) in the placebo group and 1.83 mg (or 3.66 of the 4 total pills) in the varenicline group (p = 0.33). The percentage of patients with complete drinking data during the maintenance phase was 85.8% overall and was slightly higher in the varenicline group vs. the placebo group (87.5% vs. 84.2%, respectively), although this difference was not statistically significant (p = 0.50).
Primary Efficacy Outcome
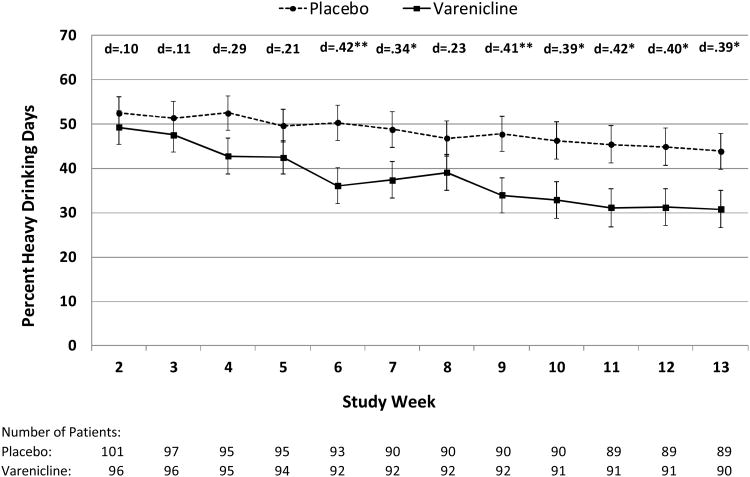
Averaged across the maintenance period (Weeks 2–13), the varenicline group experienced significantly lower levels for the primary outcome, weekly percent heavy drinking days, than the placebo group (37.9 vs. 48.4, respectively; p=0.03; d=0.31) (Table 2). The weekly treatment effects varied significantly (i.e., treatment group by week interaction, p=0.01) and were consistently greatest and most significant during the last 5 weeks of the trial (Weeks 9–13) (d's = 0.39 to 0.42, p's<0.05) (Figure 2). The average treatment effect for the primary outcome was similar using the two methods of handling missing data. For instance, when missing days were imputed as heavy drinking days, the percent heavy drinking days was 39.6 (SE=3.7) for the varenicline group versus 50.2 (SE=3.6) for the placebo group (difference=10.6; p=0.02; d=0.31). When missing days were handled using multiple imputation, the percent heavy drinking days was 38.2 (SE=3.5) for the varenicline group versus 47.4 (SE=3.4) for the placebo group (difference=9.1; p=0.04; d=0.27). There was no substantive difference between this result and that obtained with the main pre-specified mixed model (which does not include imputation) because there were few missing data overall and relatively low differential dropout between treatment groups. The average treatment effect during the maintenance period for the primary outcome did not significantly vary by baseline smoking status (among non-smokers: varenicline = 36.0 vs. placebo = 44.3; among smokers: varenicline = 43.1 vs. placebo = 51.0; treatment group by smoking status interaction, p=0.96).
Table 2. Treatment Outcomes: Differences between Placebo and Varenicline during Study Maintenance Phase (Weeks 2-13).
| Placebo (n=101) | Varenicline (n=96)a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Drinking Outcomes | LSMEANb | SE | 95% CI | LSMEAN | SE | 95% CI | LSMEAN difference | SE | |d| | p-value |
| Percent heavy drinking days (primary outcome) | 48.4 | 3.52 | 41.4 - 55.3 | 37.9 | 3.61 | 30.8 - 45.0 | 10.4 | 4.57 | 0.31 | 0.034 |
| Drinks per day | 5.3 | 0.40 | 4.5 - 6. 1 | 4.4 | 0.41 | 3.6 - 5. 2 | 0.9 | 0.52 | 0.29 | 0.031 |
| Drinks per drinking day | 6.8 | 0.42 | 6.0 - 7. 6 | 5.8 | 0.43 | 4.9 - 6. 6 | 1.0 | 0.54 | 0.26 | 0.032 |
| Percent very heavy drinking days (8+/10+) | 26.1 | 2.92 | 20.3 - 31.8 | 17.6 | 3.00 | 11.7 - 23.1 | 8.5 | 3.83 | 0.25 | 0.047 |
| Percent days abstinent | 35.6 | 3.13 | 29.5 - 41.8 | 40.0 | 3.21 | 33.7 - 46.4 | 4.4 | 4.10 | 0.14 | 0.290 |
|
|
||||||||||
| % | n | denom | % | n | denom | OR (95% CI)c | p-value | |||
|
|
||||||||||
| Percent subjects abstinent | 2.0 | 2 | 101 | 2.1 | 2 | 96 | 1.1 (0.1-7.6) | 0.811 | ||
| Percent subjects with no heavy drinking days | 5.0 | 5 | 101 | 7.3 | 7 | 96 | 1.5 (0.5-4.9) | 0.495 | ||
|
| ||||||||||
| Non-Drinking Outcomesd | LSMEAN | SE | 95% CI | LSMEAN | SE | 95% CI | LSMEAN difference | |d| | p-value | |
|
| ||||||||||
| Cigarettes per day (Weeks 6, 10, 14)e | 11.7 | 0.70 | 10.3 - 13.1 | 7.4 | 0.77 | 5.9 - 9. 0 | 4.3 | 0.99 | 0.73 | 0.002 |
| Penn Alcohol Craving Scale (PACS) score (Weeks 6, 10, 14) | 11.6 | 0.49 | 10.7 - 12.6 | 9.9 | 0.50 | 8.9 - 10.9 | 1.7 | 0.67 | 0.33 | 0.011 |
| Alcohol-related consequences (ImBIBe) score (Weeks 6, 10, 1 4) | 9.0 | 0.73 | 7.5 - 10.4 | 8.2 | 0.75 | 6.7 - 9. 7 | 0.8 | 1.01 | 0.10 | 0.434 |
| SF-12 Physical Aggregate score (Week 14)f | 52.7 | 0.60 | 51.5 - 53.9 | 52.3 | 0.59 | 51.2 - 53.5 | 0.4 | 0.84 | 0.15 | 0.382 |
| SF-12 Mental Aggregate score (Week 14)f | 52.9 | 0.74 | 51.4 - 54.3 | 52.1 | 0.74 | 50.7 - 53.6 | 0.7 | 1.05 | 0.15 | 0.549 |
Abbreviations: LSMEAN = least square means; SE = standard error; 95% CI = 95% confidence interval; |d| = absolute value of Cohen's d; OR = odds ratio; n = numerator sample size; denom = denominator sample size.
Note: Skewed outcomes were transformed as follows: square root transformations (drinks per day, drinks per drinking day, percent days abstinent, ImBIBe, and SF-12); logarithmic transformation (percent very heavy drinking days); and inverse transformation (cigarettes smoked per day).
Outcome datawere not available for one patient who discontinued the study prior to reporting outcome data.
Unless otherwise noted, LSMEANS are based on the outcome variable (untransformed for interprative purposes), averaged across the study maintenance phase (weeks 2-13), and were obtained from a mixed model that includes the treatment group, week, site, treatment goal, alcohol craving, baseline value of the outcome, and the treatment group by week interaction. Corresponding Cohen's d and p-values are based on the same model but with the appropriately transformed outcome variable.
Odds ratios and corresponding p-values are derived from a logistic regression model without covariates. Covariates were not included in order to avoid bias due to the low number of events (Peduzzi et al., 1996).
Unless otherwise noted, LSMEANS for non-drinking outcomes are from models similar to those used for drinking outcomes, but are not additionally adjusted for treatment goal and alcohol craving.
The model for cigarettes per day included only patients who were smokers at baseline (i.e., smoked at least one cigarette per day in the past week) (n=76).
ANCOVA was used to model SF-12 outcomes.
Figure 2. Weekly Differences Between Placebo and Varenicline on the Primary Outcome Measure, Percent Heavy Drinking Days, During Study Maintenance Phase (Weeks 2–13).
* p<.05; ** p<.01
Means are LSMEANS obtained during the maintenance period (Weeks 2-13) from a mixed model that includes treatment group, week, site, treatment goal, craving, baseline percent heavy drinking days, and treatment group by week interaction.
Error bars are standard errors.
Note: the treatment group by week interaction is statistically significant (p=0.011).
Secondary Efficacy Outcomes
On other drinking outcomes, averaged across the maintenance period, the varenicline group had fewer drinks per day than placebo (4.4 vs. 5.3, respectively; p=0.03; d=0.29), drinks per drinking day (5.8 vs. 6.8, respectively; p=0.03; d=0.26), and percent of very heavy drinking days (8+/10+) (17.6 vs. 26.1, respectively; p=0.047; d=0.25) (Table 2). The treatment groups did not differ significantly on percent of subjects who were abstinent (p=0.81), percent of subjects with no heavy drinking days (p=0.50), and percent of days abstinent (p=0.29).
On non-drinking outcomes, averaged across the maintenance period, the varenicline group smoked significantly fewer cigarettes per day (7.4 vs. 11.7, respectively; p=0.002; d=0.73) and scored lower on alcohol craving (PACS score 9.9 vs. 11.6, respectively; p=0.01; d=0.33) (Table 2). Craving scores at weeks 6 and 10 were moderately and positively correlated with the percent of heavy drinking days (r's=0.41 and 0.37, respectively; p's<0.0001). There were no significant differences on alcohol-related consequences (ImBIBe score) (p=0.43) and quality of life (SF-12® Physical and Mental Aggregate Scores, p's=0.48 and 0.55, respectively).
Adverse Events
Twenty-two adverse events occurred in at least 5% of patients from either treatment group (Table 3) (see Table and Text, Supplemental Digital Content 5, http://links.lww.com/JAM/A13, for adverse events stratified by smoking status). Of these, the only adverse events that differed significantly between the varenicline and placebo groups, with higher rates in the varenicline group, included: nausea (37.1% vs. 17.8%, respectively; p=0.002), abnormal dreams (27.8% vs. 11.9%, respectively; p=0.005), and constipation (9.3% vs. 2.0%, respectively; p=0.03). Among patients with these three adverse event types, the majority experienced “mild” symptoms, whereas the remaining subjects experienced “moderate” symptoms; no patients had “severe” symptoms. Four serious adverse events occurred during the treatment phase of the trial; gout and a hernia in the placebo group and back surgery and a shooting death in the varenicline group (the latter which may or may not have been related to taking varenicline as determined the Data and Safety Monitoring Board and the FDA). There were no significant differences between varenicline and placebo groups on the mood and behavior/thinking questions (see Supplemental Digital Content 6, http://links.lww.com/JAM/A14, for assessment items and safety data).
Table 3. Number (%) of Adverse Events Occurring in at least 5% of Patients in a Treatment Groupa.
| MedDRA Preferred Term | Varenicline (n=97) | Placebo (n=101) | p-valueb | ||
|---|---|---|---|---|---|
| Headache | 26 | 26.8% | 30 | 29.7% | 0.651 |
| Nausea | 36 | 37.1% | 18 | 17.8% | 0.002 |
| Abnormal dreams | 27 | 27.8% | 12 | 11.9% | 0.005 |
| Agitation | 12 | 12.4% | 16 | 15.8% | 0.484 |
| Insomnia | 15 | 15.5% | 12 | 11.9% | 0.463 |
| Fatigue | 14 | 14.4% | 11 | 10.9% | 0.453 |
| Vomiting | 12 | 12.4% | 10 | 9.9% | 0.580 |
| Diarrhea | 11 | 11.3% | 10 | 9.9% | 0.742 |
| Somnolence | 6 | 6.2% | 13 | 12.9% | 0.110 |
| Anxiety | 9 | 9.3% | 8 | 7.9% | 0.733 |
| Dizziness | 11 | 11.3% | 6 | 5.9% | 0.175 |
| Arthralgia | 9 | 9.1% | 7 | 6.9% | 0.573 |
| Irritability | 11 | 11.3% | 5 | 5.0% | 0.099 |
| Back pain | 6 | 6.2% | 9 | 8.9% | 0.469 |
| Depression | 7 | 7.2% | 6 | 5.9% | 0.717 |
| Nasopharyngitis | 6 | 6.2% | 7 | 6.9% | 0.832 |
| Constipation | 9 | 9.3% | 2 | 2.0% | 0.031 |
| Hostility | 6 | 6.2% | 4 | 4.0% | 0.531 |
| Rash | 3 | 3.1% | 6 | 5.9% | 0.498 |
| Upper respiratory tract infection | 5 | 5.2% | 4 | 4.0% | 0.744 |
| Dysgeusia | 6 | 6.2% | 1 | 1.0% | 0.061 |
| Chest pain | 0 | 0.0% | 6 | 5.9% | 0.029 |
Multiple occurrences of a specific adverse event for a patient were counted once in the frequency for that adverse event.
Group prevalence rates were tested for significance via chi-square or Fisher's exact tests.
Discussion
This multisite study looked at the effectiveness of varenicline, a medication approved by the FDA for smoking cessation treatment, as a possible therapy for alcohol abuse and dependence. Varenicline significantly reduced measures of alcohol use, including the percent of heavy drinking days, drinks per day, and drinks per drinking day. Varenicline's effects were comparable to the upper end effect sizes that have been reported in naltrexone and acamprosate trials, two medications approved by the FDA for the treatment of alcohol dependence (Feinn and Kranzler, 2005; Mason and Lehert, 2012; Maisel et al., 2013).
Drinking and smoking often co-occur. Prior studies have demonstrated that both alcohol and nicotine can alter the physiological and subjective effects of each other in terms of craving, reinforcement, and self-administration (Ray et al., 2007; McKee et al., 2008). Drinking and smoking also share common genetic components that underlie alcohol and nicotine dependence (Grucza and Bierut, 2006; Schalaepfer et al., 2008). Interestingly, however, the effects of varenicline on alcohol use observed here were independent of smoking status. That is, the positive effects of varenicline on drinking were observed in alcohol-dependent patients from both the smoking and non-smoking groups.
Another outcome measure, craving, also was significantly reduced in the varenicline-treated patients. This is notable because craving is likely to be added as a criterion for a diagnosis of AUD in the upcoming revision of the DSM (O'Brien, 2010). This reduction in craving also suggests a possible mechanism underlying the observed reduction in drinking. Alcohol has been shown to act directly on nicotinic receptors to alter alcohol-seeking and drinking behavior (Davis and de Fiebre, 2006). Furthermore, various drugs acting on nicotinic receptors have been shown to reduce drinking in animal models and the rewarding effects of alcohol in human lab models independent of nicotine administration or smoking (Blomqvist et al., 2002; Farook et al., 2009; Sajja and Rahman, 2011).
Nicotinic receptors exist as pentameric ligand-gated ion channels consisting of various combinations of α2-7 and β2-4 subunits in different regions of the brain (Grady et al., 2010). Varenicline binds to multiple nicotinic receptor subtypes acting as a partial agonist at α4β2, α3β2 and α6β2, and a full agonist at α7 and α3β4, and has the highest affinity for α4β2 subtype (Grady et al., 2010; Mihalak et al., 2006). At this time it is unclear the exact mechanism by which nicotinic receptors modulate drinking behaviors, but the mechanism may differ from smoking since nicotine acts as a direct agonist at all nicotinic receptors (with varying affinities), while alcohol in not a direct agonist but modulates the response of nicotine receptors (Feduccia et al., 2012). In support, there appears to be evidence that nicotinic subunits that are responsible for the reinforcing effects of alcohol and nicotine seem to diverge based on animal studies (Feduccia et al., 2012). More research is needed to further understand the complex interaction of alcohol with the nicotinic system.
No significant differences were found between varenicline and the placebo in the frequency of abstinent days or number of patients who were abstinent. Additionally, varenicline did not increase the number of subjects with no heavy drinking days. This lack of significant differences may be attributed to 1) the small number (approximately one-quarter) of patients who endorsed permanent abstinence as their treatment goal; and 2) the study design feature that allowed patients to continue drinking heavily up to randomization. Thus, patients may not have had sufficient time to establish a pattern of abstinence or a period of non-heavy drinking prior to the start of the study. Although we did not find an association between reductions in drinking and alcohol-related consequences or an improvement in quality of life, these findings may be attributed to the fact that changes in these measures often do not manifest until months after the initial reduction in drinking occurs. Nonetheless reductions in drinking that were observed in this study with varenicline have also been associated with decreases in risk of medical diseases, aggression, suicide, and alcohol-related deaths (Rehm et al., 2010 and 2011). Longer treatment with varenicline and follow-up assessments to determine if there are sustained effects would be a valuable next step in the development of this medication.
Compared with placebo, varenicline did not increase suicidal ideation, mood changes, behavior/thinking changes, hostility, or agitation—all “black box warnings” on the package insert for varenicline (Pfizer, 2009). Consistent with the product label, the most common side-effects of varenicline in this study were nausea, abnormal dreams, and constipation (Table 3) and those effects generally were mild.
Conclusion
Today, varenicline is widely prescribed in primary care settings for smoking cessation (SDI, 2011). Problem drinkers typically visit primary care providers for medical issues that may or may not be related to their drinking (Willenbring, 2009). By routinely assessing patients for both smoking and hazardous alcohol use, clinicians have an opportunity to identify patients at risk for problematic alcohol use. Screening and intervention tools are widely available, including the NIAAA Clinician's Guide (NIAAA, 2005). These resources are designed to help clinicians screen for alcohol problems, administer brief interventions, and provide guidance on the use of medications to treat alcohol dependence (i.e., disulfiram, oral and long-term injectable naltrexone, and acamprosate). Because of the heterogeneity of AUD, however, these medications are not effective for everyone. Results from this proof-of-concept multi-site trial suggest that varenicline may be another promising treatment for patients with AUD. Nonetheless, additional studies are needed to replicate these results, examine if effects are sustained post-treatment, and identify those patients who will benefit the most from this medication.
Supplementary Material
Acknowledgments
The authors thank Barbara Vann of CSR, Incorporated for her excellent editorial comments.
Funding: Supported by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) (Contract HHSN27200900005C); ClinicalTrials.gov NCT 01146613.
Footnotes
*NCIG Study Group: Boston Medical Center – Boston, MA and Quincy, MA: Joanna Piechniczek-Buczek, MD., Chris Streeter, M.D., Eric Devine, Ph.D., Courtney Richambault, Laurie Sickles-Colaneri, R.N.
University of Virginia – Charlottesville, VA: Eva Jenkins-Mendoza, Sean Sembrowich, R.N., Jennifer Kim Penberthy, Ph.D., Amanda Nizam, Nicole Fischer, Shari Steinman, Mindy Borszich
University of Virginia – Richmond, VA: Esther Makanjuola, R.N., Tricia Schirmer, Kathryn Polak, Christina Hill, Kathryn Conley, Alison Eonta, Kasy Serdar, Aubrey Gartner, Amy Madigan
Geisel School of Medicine at Dartmouth– Hanover, NH and Bedford, NH: Audrey Kern, M.D., Christopher O'Keefe, M.S., Mirranda Boshart, Suzanne Miller, Shannon Rondeau R.N., Marjorie Weeks, Pamela Geiger
University of Pennsylvania – Treatment Research Center – Philadelphia, PA: Jennifer Plebani, Ph.D., William Dundon, Ph.D., Elizabeth Mahoney M.A., Gail Kaempf, CRPN, Brenda Beitler, APRN, Cynthia Clark, CPRN, Kelly Griffin, Joshua Lachewitz, Elizabeth Wilson, Margo Hendrickson, Tamara Roth, Laurie Downing, NP
Johns Hopkins University School of Medicine: Joseph Harrison, M.S., Kimberly Nelson, LPN, Ashley Bathgate, Connie Lowery, RN, Elana Schwartz, Torran Claiborne, Sarah Hersh
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, D.C.: American Psychiatric Publishing, Inc.; 1994. DSM–IV. [Google Scholar]
- Blomqvist O, Henandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–31. [PubMed] [Google Scholar]
- Bouchery EE, Henrick MS, Harwood J, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–24. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychol Bull. 1992;112:155–59. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–85. [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Lof E, Stromberg R, Soderpalm B. The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharm Exp Ther. 2009;32:225–30. doi: 10.1124/jpet.108.147058. [DOI] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton J, Barron S. Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology. 2009;83:379–84. doi: 10.1159/000219488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia AA, Chatterjee S, Bartlett S. Neuronal nicotinic acetylcholine receptors: Neuroplastic changes underlying alcohol and nicotine addictions. Front Mol Neurosci. 2012;5:1–18. doi: 10.3389/fnmol.2012.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinn R, Kranzler HR. Does effect size in naltrexone trials for alcohol dependence differ for single-site vs. multi-center studies? Alcohol Clin Exp Res. 2005;29:983–88. doi: 10.1097/01.alc.0000171061.03686.bc. [DOI] [PubMed] [Google Scholar]
- Flannery B, Volpicelli J, Pettinati H. Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res. 1999;23:1289–95. [PubMed] [Google Scholar]
- Fucito LM, Toll BA, Wu R, Romano DM, Tek E, O'Malley SS. A preliminary investigation of varenicline for heavy drinking smokers. Psychopharmacology. 2011;215:655–63. doi: 10.1007/s00213-010-2160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard S, Nides M, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, et al. Structural differences determine the relative selectivity of nicotinic compounds for native α4β2*, α6β2*, α3β4*, and α7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58(1054):10–66. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12 month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Grucza R, Bierut L. Co-occurring risk factors for alcohol dependence and habitual smoking. Alcohol Res Health. 2006;29:172–78. [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hurley L, Taylor R, Tizabi Y. Positive and negative effects of alcohol and nicotine and their interactions: a mechanistic review. Neurotox Res. 2012;21:57–69. doi: 10.1007/s12640-011-9275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of Nicotinic Receptors in Alcohol Self-Administration. Alcohol Clin Exp Res. 2000;24:155–63. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, et al. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litten RZ, Egli M, Heilig M, et al. Medications development to treat alcohol dependence: a vision for the next decade. Addict Biol. 2012;17:513–527. doi: 10.1111/j.1369-1600.2012.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: When are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Lehert P. Acamprosate for alcohol dependence: A sex-specific meta-analysis based on individual patient data. Alcohol Clin Exp Res. 2012;36:497–508. doi: 10.1111/j.1530-0277.2011.01616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Harrison ELR, O'Malley SS, Krishnan-Sarin S, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry. 2009;66:185–95. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, O'Malley SS, Shi J, Mase T, Krishnan-Sarin S. Effect of transdermal nicotine replacement on alcohol responses and alcohol self-administration. Psychopharmacology. 2008;196:189–200. doi: 10.1007/s00213-007-0952-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalak KB, Carroll FI, Luetje CW. Verenicline is ap partial agonist at α4β2 and a full agonist at α7 neuronal nicotinic receptors. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- Miller W. The Drinker Inventory of Consequences (DrInC): an instrument for assessing adverse consequences of alcohol abuse. In: Mattson M, Marshall LA, editors. Vol. 4. National Institute on Alcohol Abuse and Alcoholism; Bethesda, MD: 1995. pp. 1–94. (NIAAA Project MATCH Monograph Series). [Google Scholar]
- Miller W. Form 90: A structured assessment interview for drinking and related behaviors (Test Manual) Bethesda, Md.: National Institute on Alcohol Abuse and Alcoholism; 1996. NIH publication no. 96-4004. [Google Scholar]
- Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology. 2012;223:299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: a clinician's guide. Bethesda, Md.: National Institutes of Health; 2005. NIH publication no. 07–3769. [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Rethinking Drinking. Bethesda, Md.: National Institutes of Health; 2009. NIH publication no. 09-3770. [Google Scholar]
- O'Brien C. Addiction and dependence in DSM-V. Addiction. 2010;106:866–67. doi: 10.1111/j.1360-0443.2010.03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:790–812. [Google Scholar]
- Peduzzi P, Concato J, Kemper E, Holford TR, Feinstein AR. A simulation study of the number of events per variable in logistic regression analysis. J Clin Epidemiol. 1996;49:1373–1379. doi: 10.1016/s0895-4356(96)00236-3. [DOI] [PubMed] [Google Scholar]
- Pfizer. Package Insert. New York: Jul, 2009. Chantix® (varenicline) [Google Scholar]
- Posner K, Brent D, Lucas C, et al. Columbia-Suicide Severity Rating Scale (C-SSRS) [Columbia University web site] [Accessed January 15, 2012];Version. 2009 Jan 14; Available at: http://cssrs.columbia.edu/docs/C-SSRS_1-14-09-Since_Last_Visit_Clinical.pdf.
- Ray LA, Miranda R, Kahler CW, et al. Pharmacological effects of naltrexone and intravenous alcohol on craving for cigarettes among light smokers: a pilot study. Psychopharmacology. 2007;193:449–56. doi: 10.1007/s00213-007-0794-z. [DOI] [PubMed] [Google Scholar]
- Rehm J, Zatonksi, Taylor B, Anderson P. Epidemiology and alcohol policy in Europe. Addiction. 2011;106(Suppl 1):11–19. doi: 10.1111/j.1360-0443.2010.03326.x. [DOI] [PubMed] [Google Scholar]
- Rehm J, Baliunas D, Borges GLG, et al. The relation between different dimensions of alcohol consumption and burden of disease: An overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373:2223–33. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- Sajja RK, Rahman S. Lobeline and cytisine reduce voluntary ethanol drinking behavior in male C57BL/6J mice. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35:257–64. doi: 10.1016/j.pnpbp.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Schalaepfer I, Hoft N, Ehringer M. The genetic components of alcohol and nicotine co-addiction: from genes to behavior. Curr Drug Abuse Rev. 2008;1(2):124–34. doi: 10.2174/1874473710801020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SDI. Vector One®: National (VONA) and Total Patient Tracker (TPT). May 2006—July 2011. Data extracted. 2011 Sep; [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. quiz 34-57. [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline follow-back: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Totowa: Humana Press; 1992. pp. 41–72. [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci. 2007;104:12518–23. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo S. The World Health Organization Quality of Life (WHOQOL) In: Spiker B, editor. Quality of Life and Pharmacoeconomics in Clinical Trials. Philadelphia, PA: Lippincott-Raven Publishers; 1996. pp. 355–62. [Google Scholar]
- Tizabi Y, Copeland R, Louis VA, Taylor R. Effects of combined systemic alcohol and central nicotine administration into ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–99. [PubMed] [Google Scholar]
- Werner M, Rentz A, Frank L, Bowman L, Duhig A, Moss H. Presented at the annual meeting of the Research Society on Alcoholism. Washington, DC: 2008. Participant consequence measures. [Google Scholar]
- Willenbring ML, Huang SW, Gardner MB. Helping patients who drink too much: An evidence-based guide for primary care physicians. Am Fam Physicians. 2009;80:1–7. [PubMed] [Google Scholar]
- Wouda JA, Riga D, De Vries W, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology. 2011;216:267–77. doi: 10.1007/s00213-011-2213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.