Abstract
The endocannabinoid system (ECS) is an evolutionarily conserved master system deeply involved in the central and local control of reproductive functions in both sexes. The tone of these lipid mediators—deeply modulated by the activity of biosynthetic and hydrolyzing machineries—regulates reproductive functions from gonadotropin discharge and steroid biosynthesis to the formation of high quality gametes and successful pregnancy. This review provides an overview on ECS and reproduction and focuses on the insights in the regulation of endocannabinoid production by steroids, in the regulation of male reproductive activity, and in placentation and parturition. Taken all together, evidences emerge that the activity of the ECS is crucial for procreation and may represent a target for the therapeutic exploitation of infertility.
1. Introduction
Reproductive functions are under a fine regulation exerted at multiple levels along the hypothalamic-pituitary-gonadal axis. The formation of high quality gametes, followed by a successful pregnancy event, is the result of deep cell to cell communications. In this respect, the list of potential modulators of reproductive activity is still growing. In the last two decades the upcoming role of lipid mediators that share some of the effects with delta-9-tetrahydrocannabinol (Δ9-THC), the active principle of marijuana plant, Cannabis sativa, emerged. These bioregulators, collectively named endocannabinoids (eCBs), are amides, esters, and ethers of long-chain polyunsaturated fatty acid and have been detected in most reproductive tissues and fluids [1–3].Besides ligands, a wide range of receptors, biosynthetic and hydrolyzing enzymes, and putative membrane transporters (EMT) all together form the endocannabinoid system (ECS) (Figure 1), a master system that is deeply involved in the central and local control of male and female reproduction. Since their discovery, research made giant strides in the comprehension of physiological, cellular, and molecular events in reproduction driven by eCBs. Many inputs in the field came from studies conducted in invertebrates and nonmammalian vertebrates, indicating that ECS is an evolutionarily conserved master system deeply involved in the control of reproductive functions. Thus, the aim of this review is just to provide new insights into the complex field of eCBs and reproduction.
Figure 1.
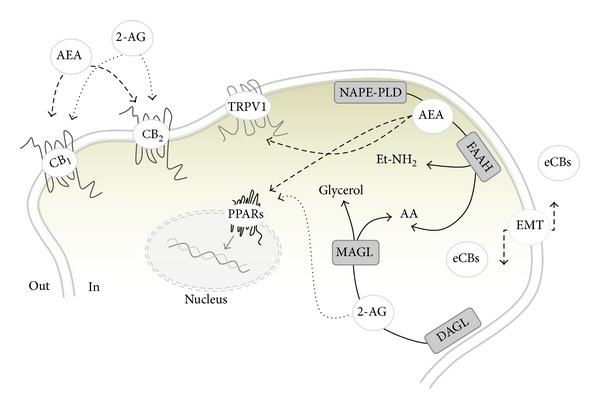
Schematic representation of the ECS. N-arachidonoyl-ethanolamine (AEA) is mainly produced by the activity of an N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD), whereas its degradation is due to a fatty acid amide hydrolase (FAAH), which releases ethanolamine (Et-NH2) and arachidonic acid (AA). 2-Arachidonoylglycerol (2-AG) is also released from membrane lipids through the activity of diacylglycerol lipase (DAGL) and can be hydrolyzed by a cytosolic monoacylglycerol lipase (MAGL) that releases glycerol and AA. The cellular uptake from the extracellular to the intracellular space is ascribed to a purported “endocannabinoid membrane transporter (EMT)” that is likely to take up both AEA and 2-AG. Both eCBs trigger several signal transduction pathways by acting at their targets, CB1, CB2, GPR55, and nuclear peroxisome proliferator-activated receptors (PPARs). AEA, but not 2-AG, binds intracellularly also Transient Receptor Potential Cation Channel type 1 (TRPV1).
2. ECS and Reproduction: An Overview
Smoking marijuana has always represented a warning for the long lasting effects not only on physical and mental performances but also on the reproductive events. The effects of Δ9-THC on pregnancy was highlighted for the first time at the beginning of 1970 [4] and, since then, numerous papers have been focused on the potential aversive effects caused by the use of recreational drugs during gestation and in offspring born from cannabis users.
In 1992, N-arachidonoylethanolamine (also known as anandamide, AEA), a cannabinoid-like compound, was identified to compete with exocannabinoid ligands for type 1 and type 2 cannabinoid receptors (CB1 and CB2, resp.) [5, 6] and, few years later, the group of Dr. Schuel and Dr. Das reported the ability of AEA to affect negatively both male and female fertility [7, 8]. In the next years, the endocannabinoid signaling was demonstrated to play a key role in the preimplantation embryo development [9–12] and it was supposed that AEA content could be critical for a timely embryo implantation [13]. Nowadays, it is clear that, in order to guarantee a receptive uterine environment, AEA levels must be kept low [14], and this is accomplished through a tight regulation mediated by an N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD), the enzyme responsible for its synthesis, and fatty acid amide hydrolase (FAAH), in charge of its degradation to arachidonic acid and ethanolamine [15–18]. Further confirmations on the harmful effects caused by high AEA levels for a normal pregnancy outcome were obtained from measurements of AEA levels in plasma samples from women with normal menstrual cycle and laboring patients [19] and NAPE-PLD and FAAH analysis performed on human placenta [20].
In the same timeframe, several experimental studies highlighted the ability of AEA in regulating sperm functions required for fertilization [7, 21], by reducing sperm motility, inhibiting capacitation-induced acrosome reaction and mitochondrial activity [21, 22]. Cannabinoid and vanilloid (in particular transient receptor potential cation channel type 1, TRPV1) receptors mediate the physiological action of AEA with double effects. On the one hand, AEA binding to CB1 affects the capacitation process in mammals [23–25]; on the other hand activation of the intracellular site of TRPV1 inhibits spontaneous acrosome reaction in porcine [23] and human sperm cells [26]. Lately, TRPV1-mediated activities of AEA were also reported in capacitated mouse spermatozoa (SPZ), where elevated intracellular levels of AEA are due to a reduced FAAH activity [27]. In the last decade, many studies have been focused on the involvement of the CB1/CB2-signaling in follicle maturation, oviductal-uterine embryo migration, implantation and (neuro)development, placentation, and parturition onset, showing that any aberration of endocannabinoid signaling can severely affect these processes (for a review see [28]). Specific and selective antagonists of CB1/CB2 and/or CB1/CB2 knockout mice (CB 1 −/− and CB 2 −/−, resp.) have always been useful tools that allowed researchers to better understand which target is critical to achieving correctly all reproductive events from sperm-oocyte fusion to the birth of offspring. In this context, we should recall that short and long term exposure to HU210, a selective agonist for CB1 and CB2, showed how the deregulation of the ECS markedly reduces total sperm count, depletes spermatogenic efficiency, and impairs sperm motility [29]. A recent paper by Fonseca and workers proposed a functional role of GPR55 receptor in the uterine remodeling and in immune processes activated during fetoplacental development [30]. The differential spatiotemporal expression pattern of GPR55 found in decidual and natural killer (NK) cells might implicate possible interactions of this target with other endocannabinoid-like compounds (i.e., N-palmitoylethanolamide), since the main eCBs lack affinity for this receptor [31].
Besides AEA, 2-arachidonoylglycerol (2-AG) is the other main representative of this family of bioactive lipids and its role in fertility seemed unknown up to few years ago, when its impact on mouse spermatogenesis [32], fetoplacental development [33], epididymal start-up [34], and mouse sperm capacitation [27] has been remarked. In fact, it has been reported that transcriptional and translational levels of 2-AG synthesizing (diacylglycerol lipase, DAGL) and hydrolyzing enzymes (monoacylglycerol lipase, MAGL) are finely tuned in various processes of male and female reproduction. This metabolic equilibrium is required in order to guarantee an appropriate 2-AG tone in reproductive stages; in this context, low 2-AG levels were detected in seminal plasma of infertile men, suggesting a reduced sperm fertilizing capacity through a mechanism yet to be explored [35].
To date, we have good knowledge about the existence of a definite network, including eCBs, hormones, prostaglandins, and cytokines that warrant a successful pregnancy in animals and humans. In particular, the involvement of the eCBs in lymphocyte-mediated control of the hormone-cytokine crosstalk at the fetal-maternal interface was reported for the first time by the group of Dr. Maccarrone, showing that FAAH activity and protein were lower in women who miscarried and who underwent IVF treatment [36–38], whereas cannabinoid receptor binding and AEA-carrier were not altered during gestation [36, 39, 40]. Moreover, it seems that steroid hormones primarily regulate AEA levels, with estradiol (E2) increasing the levels and progesterone suppressing them, and that an equilibrium between profertility Th2 cytokines and antifertility Th1 cytokines is requested to establish blastocyst implantation, trophoblast growth, and pregnancy maintenance. On the male side, follicle stimulating hormone (FSH) regulates the expression of FAAH in Sertoli cells through an estrogen-mediated pathway [41], and, in turn, E2 levels induce, directly or indirectly, epigenetic modifications at the FAAH promoter site [42] and influence, via CB1 [43], chromatin remodeling of spermatids with a clear impact on spermatogenesis [44, 45]. A schematic chronological overview of local activity of eCBs in male and female reproduction is depicted in Figure 2.
Figure 2.
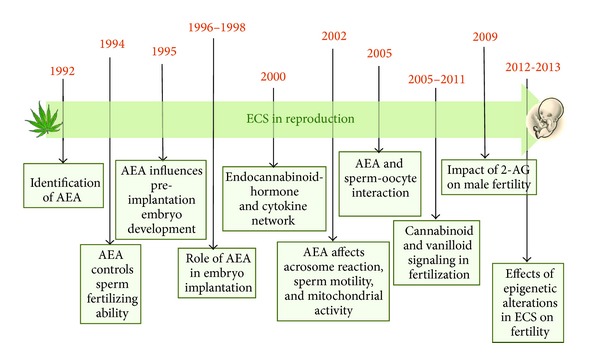
Major breakthroughs in male and fertility reproduction.
3. Evolutionary Aspects of ECS
The study of physiological mechanisms by comparative approach is a fundamental tool to build general models. Key events in reproduction such as the activity of estrogen—classical female hormone—in spermatogonial proliferation [46] or nongenomic action of steroids themselves have been firstly discovered in nonmammalian species and then confirmed in mammals [47, 48].
In this respect, enzymes involved in endocannabinoid biosynthesis and/or degradation occur throughout the animal kingdom including deuterostomian (i.e., sea urchin), protostomian (i.e., crustaceans and nematodes), and basal (i.e., cnidarians and placozoans) invertebrates [49]. Conversely, molecular cloning of CB 1 /CB 2 receptor orthologs has produced positive results only in urochordates (the sea squirt, Ciona intestinalis), in cephalochordata (the amphioxus, Branchiostoma floridae), in nonmammalian vertebrates (fish, amphibians, reptiles, and birds), and in mammals, with duplication of CB 1 or CB 2 genes found in fish [49–51]. Thus, given that CB1/CB2 are unique to chordates, the molecular nature of endocannabinoid signaling in noncordate invertebrate is currently under investigation and may be related to primitive neuronal functions; conversely, the appearance of multiple receptors and receptor splicing forms coming from invertebrates to humans may indicate the subsequent occurrence of functional partitioning. However, the recent identification of candidate TRPV1 orthologs in the genome of the sea urchin Strongylocentrotus purpuratus [52] and of the annelid, the leech Hirudo medicinalis [53], confirms the existence of an ancient non-CB1/CB2-mediated endocannabinoid signaling.
The functional conservation of ECS is not limited to the central nervous system (CNS) but also extends to the modulation of gonadal functions. The first direct evidence of endocannabinoid activity on male reproduction came from studies conducted in sea urchin to assess the mechanisms of acrosome reaction and polyspermy ([54] for review). In this respect, the endocannabinoid-signaling similarity in neurotransmitter release and acrosomic granule exocytosis let Meizel in 2004 speculate that the sperm may be a “neuron with a tail” adapted to fertilize egg cell [55]. However, in the last 10 years, evidences of endocannabinoid activity have been provided in testis and/or sperm of both invertebrates and vertebrates, including sea urchin, fish, frogs, mice, rats, boars, bulls, and humans [7, 21, 23, 26, 56–63]. AEA inhibitory effects on sperm motility and acrosome reaction have been conserved from sea urchin to mammals and elsewhere properly reviewed [54, 56]. A retrograde AEA signaling is involved in sperm-egg interaction in sea urchin [54], whereas CB1 and/or CB2 are differentially expressed in fish [64, 65] and frog ovary [60]. Interestingly, CB1 signaling is likely involved in the process of testicular regression in the gilthead seabream, Sparus aurata, a hermaphrodite species in which the gonadal tissues first develop as testes and then as functional ovary [66]. As described in paragraph 6, in mammals—human included—most female reproductive events, from ovogenesis and fertilization to successful pregnancy and parturition, require a functional endocannabinoid signaling, once again confirming the conservation of functions related to reproduction.
4. eCBs, Hypothalamic GnRH, and Steroids
Three main lines of evidence suggest that the eCBs and gonadal hormone signaling systems interact. (1) eCBs and their receptors are present throughout the hypothalamic-pituitary-gonadal (HPG) axis, (2) changes to the ECS cause changes in the HPG and changes in the HPG axis can affect the expression of eCBs, and (3) the ECS mediates behaviors, which are also mediated by gonadal hormones, such as motivation or reproduction (reviewed in [67]).
The CB1 partial agonist, Δ9-THC, has been implicated in negative reproductive outcomes, including the inhibition of ovulation in women [68] and lower serum luteinizing hormone (LH) and testosterone (T) in men [69]. The inhibitory effects of eCBs on gonadal hormone production suggest that eCBs help regulate this circuitry. Leydig cells in the testes contain CB1. CB 1 −/− mice show reduced serum T levels and abnormal Leydig cell development. These results suggest that endocannabinoid signaling is essential for the organization of the reproductive system [70]. Upcoming observations in the hypothalamic control of reproductive functions and gonadal sex steroid production are described below.
4.1. Insights into the Hypothalamic Control of GnRH Activity
In the CNS eCBs are well known retrograde signals that modulate neuronal communications inhibiting presynaptic release of neurotransmitters including γ-aminobutyric acid (GABA) and glutamate. Postsynaptic synthesis of 2-AG or AEA is a phylogenetically widespread phenomenon described from mammals to annelids [49] which modulates neural activity through cannabinoid or vanilloid receptors. Brain maps of CB1, CB2, and TRPV1 have been provided in fish, amphibians, and mammals [57, 58, 71–73], with CB1/TRPV1 colocalization in specific hypothalamic nuclei in mammalian brain [72]. A master system in the central control of reproductive activity is the gonadotropin releasing hormone (GnRH), a hypothalamic decapeptide responsible for gonadotropin discharge and steroid biosynthesis [47, 48, 74]. Inhibitory effects of phytocannabinoids, cannabinoids and eCBs upon the endocrine control of reproduction have been largely described in the literature [51, 75]. Immortalized neuronal cell lines (GT1) possess a complete ECS and are themselves targets of endocannabinoid signaling, since the in vitro activation of cannabinoid receptors suppresses the pulsatile release of GnRH [76]. Furthermore, in the mediobasal hypothalamus of male rats, AEA intracerebroventricular injection suppresses GnRH release [77]. The importance of CB1 in negative modulation of reproductive axis has been demonstrated by altered GnRH signalling in CB 1 −/− mice [44]. However, 2-AG is able to suppress LH secretion in wild-type but not in CB 1 −/− mice [78], whereas AEA decreases LH levels also in CB 1 −/− [78]. Thus, receptors other than CB1—that is, TRPV1—might be involved in such a modulation.
Despite these observations, only recently has the mechanism involving direct/indirect endocannabinoid activity on the hypothalamic GnRH secreting neurons been provided. From fish to mammals GABA is a modulator of GnRH secreting neurons in the adult ([79, 80] and references inside). Metabolic, sex steroid, and circadian cues are usually conveyed to the GnRH system; involvement of metabotropic glutamate receptor located on astrocytes [81], eCBs [80] or GnRH itself [82] has been described in these routes. A 2-AG dependent inhibitory activity on the release of GnRH has been recently proposed in male mice [80]. At the molecular level, GnRH secreting neurons release 2-AG that directly acts as a retrograde signal on CB1 receptor located on GABAergic presynaptic neurons and inhibits the release of GABA (Figure 3(a)). As a consequence, GABA receptors located on GnRH secreting neurons are not activated and GnRH is not released [80]. Since astrocytes express CB1 [83] and eCBs can alter astrocyte transmitter uptake [84], a simplified alternative mechanism involving endocannabinoid-dependent modulation of glial cell functions (i.e., prostaglandin production) has been postulated [85]. In such a model, glutamate release by GnRH neurons may stimulate astrocyte to produce prostaglandins; these, in turn, may induce the synthesis of eCBs and/or the exposure of presynaptic CB1, thus modulating GABA release (Figure 3(b)).
Figure 3.
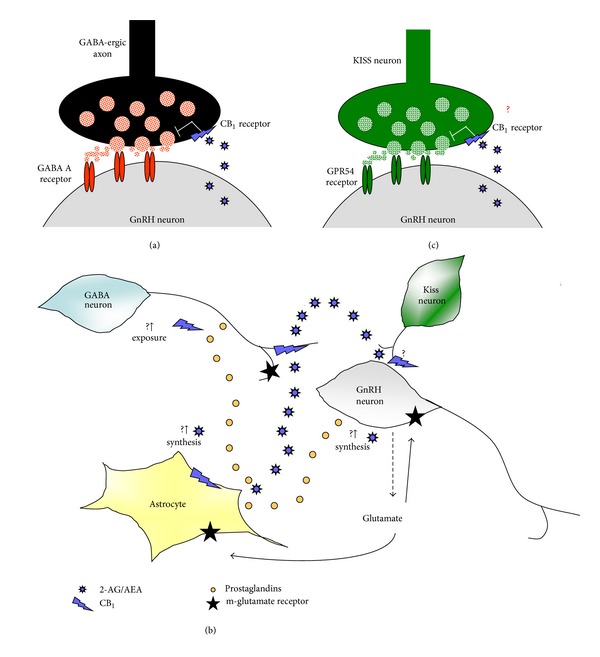
Possible mechanisms for the modulation of GnRH secreting neurons by eCBs. (a) GABAergic modulation of GnRH secreting neurons. GnRH secreting neurons release eCBs that, as retrograde signals, directly act on CB1 located on GABAergic presynaptic neurons and inhibit the release of GABA; as a consequence, GnRH secreting neurons do not receive GABAergic input and do not release the GnRH. (b) Possible involvement of glial cells in eCBs/GABA/GnRH circuitry. Glutamate release by GnRH neurons may stimulate astrocytes to produce prostaglandins which in turn induce the synthesis of eCBs and/or the exposure of presynaptic CB1, thus modulating GABA release. (c) Hypothesis: are there neuronal systems other than GABAergic able to modulate endocannabinoid/GnRH crosstalk? Kisspeptins stimulate gonadotropin discharge in several species modulating the activity of GnRH secreting neurons via the activation of GPR54 receptor located on GnRH neurons. Might AEA also act as retrograde signal upon kisspeptin neurons in order to suppress GnRH secretion?
Functional crosstalk among eCBs and GnRH neuronal systems has been described also in fish and amphibians [51, 57, 86–88], indicating that this is an evolutionarily conserved master system. In nonmammalian vertebrates, GnRH saga is more intricate since, as in humans, at least two distinct GnRH molecular forms (i.e., GnRH-I and GnRH-II) and one GnRH receptor (GnRH-R) have been described [47]. New insights in the central control of male reproduction emerged from nonmammalian vertebrates. CB1 has been localized in fish forebrain—the encephalic area mainly involved in the control of GnRH secretion and gonadotropin discharge— but in teleosts colocalization was observed in GnRH-III secreting neurons [57]. In the diencephalon of the anuran amphibian the frog Rana esculenta, CB1 dependent modulation of GnRH system expression rate (both ligands and receptors) has been reported [87, 88]. In particular, in the basal hypothalamus, via CB1, AEA significantly decreases GnRH-I and GnRH-II expression and upregulates GnRH-RI and GnRH-RII mRNA without any effect upon GnRH-RIII [87]. Twenty percent of hypothalamic GnRH-I secreting neurons possess CB1, and buserelin, a long acting GnRH analog, increases CB 1 expression and inhibits those of GnRH-I [88]. The opposite profiles of CB1 and GnRH-I proteins [86] seem to confirm such AEA-dependent self-modulation route in which GnRH secreting neurons might produce eCBs to suppress the production of GnRH.
Conversely, as in the mouse, most frog GnRH-I secreting neurons are surrounded by CB1 immunopositive fibers, [76, 88] confirming the conservation of endocannabinoid-dependent retrograde signalling. GABAergic transmission, however, is not the only neuronal system that might be involved in the modulation of endocannabinoid/GnRH crosstalk. In this respect, one of the possible candidates is the kisspeptin signaling system. Kisspeptins, RFamide peptides encoded by the kiss1 gene, stimulate LH and, to a lesser extent, FSH secretion in several species modulating the activity of GnRH secreting neurons via the activation of GPR54 receptor located on GnRH neurons [89]. Preliminary observations in male frogs indicate that in vivo administration of AEA suppresses the expression of diencephalic GPR54, turning off the GnRH system and steroidogenesis (Meccariello R., personal communication). Thus, it is not excluded that AEA might also act as retrograde signal upon kisspeptin neurons (Figure 3(c)). Interestingly, in both male and female, kisspeptin neuronal activities are strongly involved in steroid-dependent feedback mechanisms [90, 91].
As described in the next paragraph, AEA-dependent suppression of GnRH release is reversed by E2 administration in female rats [77] whereas, steroids represent the major factor in negative feedback mechanisms in males. Thus, in addition to E2-dependent modulation of endocannabinoid tone via FAAH modulation [42], the investigations concerning the possible crosstalk between kiss/GnRH/cannabinergic neurons [92] might open new insights in the molecular mechanisms of gonadal steroid feedback.
4.2. Interplay between Sex Steroids and eCBs
In the HPG axis, CB1 regulates sex hormone production. Intracerebroventricular injection of AEA reduced GnRH release in male and ovariectomized (OVX) female rats. However, in the same experiment, estrogen treated females experienced increased plasma LH after AEA injection. Therefore, estrogen possibly reverses the inhibitory effects of AEA [77]. Steroid hormones regulate CB1 expression in the pituitary [93]. In the rat pituitary there are sex differences; however the same may not be the case for humans. Therefore, in humans, nonsteroid signaling molecules may influence CB 1 expression in the pituitary [94]. The possible direct activity of eCBs upon pituitary gland—evaluated in terms of ECS characterization as well as of eCBs dependent secretion of anterior pituitary hormones—has been suggested in vertebrates, but this issue is still controversial since species specific activities have been observed (for a recent review see [51]). In addition to the pituitary, cannabinoids and gonadal steroid function are linked in the hypothalamus. GnRH neurons in the medial preoptic area can synthesize endogenous cannabinoids, which exhibit negative feedback on GnRH release. However, it has also been noted that relatively few GnRH neurons contain CB 1 mRNA, so eCBs must be exerting influence over neighboring cells [76]. Providing further evidence for a link between eCBs and sex hormones, endocannabinoid levels in the rat hypothalamus have been shown to fluctuate over the hormonal cycle. AEA levels reached a maximum during diestrous in the hypothalamus. Also, males showed significantly lower levels of 2-AG than females [95].
In a relationship critical to female reproductive success, an estrogen response element exists in the FAAH gene sequence. When estrogen binds to this response element, FAAH gene transcription is downregulated and AEA levels should remain elevated [96]. High doses of estrogen can have an anxiolytic effect. Hill et al. proposed that the anxiolytic effect is mediated by alterations in FAAH [97]. It may be that eCBs regulate the onset of puberty. eCBs may contribute to the peripubertal inhibition of GnRH neurons. Lopez hypothesizes that estrogen release from the ovaries at the time of puberty helps remove the endocannabinoid “brake” on reproductive functioning [69]. The overall relationship between estrogen and eCBs can be described as “bidirectional.” In one direction, endocannabinoid activity downregulates HPG axis activity, leading to reduced estrogen levels. In contrast, decreasing FAAH activity and modulating CB1 expression, estrogen up- regulates AEA production [67].
In addition to estrogen interactions, endocannabinoid activity attenuates progesterone release from the corpus luteum. Administration of AEA to pregnant rats caused a decrease in serum progesterone, as well as serum LH. Therefore, it appears that eCBs regulate the release of progesterone in two ways: (1) by directly binding onto receptor sites in the corpus luteum and (2) by directly controlling LH release in the CNS [98]. Like estrogen, progesterone can interact with a promoter region in the FAAH gene in that progesterone has been shown to increase FAAH expression in T-cells and human lymphoma U937 cells. In contrast, progesterone had no effect on FAAH expression in human neuroblastoma CPH100 cells [99]. Blocking progesterone receptors with antisense oligonucleotides eliminated the facilitating effect of Δ9-THC on female rodent mating behavior. In addition, blocking CB1, using SR141716, and blocking dopamine (DA) D1 receptors, using antisense nucleotides, also eliminated the effects of Δ9-THC on mating behavior [100]. Therefore, an interaction between progesterone, DA signaling, and cannabinoid signaling is necessary for female reproductive behavior. It is not known whether this could apply to human females.
As an example of how the ECS is involved in sexual motivation that is driven by sex steroids, studies with phytocannabinoids have been shown to affect sexual motivation. For example, exogenous CB1 agonist treatment in male rodents attenuates both appetitive and consummatory aspects of sexual behavior. However, studies in humans have been less conclusive. Men who use marijuana show great variation in sexual response [67]. For females, the effects of cannabinoids on sexual motivation and performance are much less clear. It appears that acute blocking Δ9-THC administration in female rats increased sexual receptivity at lower doses but decreased sexual receptivity at higher doses [101]. These findings are similar to Δ9-THC's effects on anxiety. Estrogen and DA have a complex relationship. Estrogen enhances dopaminergic activity in the nucleus accumbens via enhanced DA release and downregulates autoreceptor inhibition. Thus, eCBs could elicit a strong DA response in the nucleus accumbens and striatum. The effect could possibly overpower the motivational value of sex steroids and increase the likelihood of mate-seeking behavior [67].
On average, Δ9-THC affects males and females differently. This is not to say that there is not a large variation in response within the sexes, but there have been enough differences shown to suggest gonadal steroid modulation of exogenous cannabinoid reward. After showing that CB1 agonists induce stronger analgesic and motor suppressing effects in female rats than in male rats, Craft and colleagues investigated whether activational effects of gonadal hormones were responsible for these differences. In males, T attenuated the motor effects of Δ9-THC. In females, estrogen was linked to increased antinociception. OVX females showed less analgesia in response to Δ9-THC than OVX females given estrogen. In addition, intact estrous females showed more antinociception than diestrous females [102]. Likewise, Fattore and colleagues determined that female rats found the CB1 agonist WIN55,212-2 (WIN) more rewarding than male rats. Compared to male rats and OVX females, female rats showed faster acquisition of WIN self-administration and higher overall drug intake. However, gonad-intact female rats showed faster extinction for WIN self-administration. One explanation for Fattore's work is that there is a higher hedonistic value on cannabinoids for females [103]. On the other hand, estrogen may attenuate the disruptive effects of Δ9-THC on learning, leaving female rats less affected by a negative side effect [104]. It is possible that the greater response to Δ9-THC seen in female rats is due to estrogen modulation of DA signaling in the ventral tegmental area and nucleus accumbens. However, precise interactions between cannabinoids and estrogen are not well understood [67].
5. Insights in the Progression of the Spermatogenesis and the Acquisition of Sperm Functions
The suppression of LH levels in marijuana smokers as well as in animal models is related to the impairment of hypothalamic GnRH secretion ([51, 105, 106] for reviews). However, the presence of eCBs in reproductive fluids [1, 107, 108] and the ubiquity of testicular endocannabinoid activity are critical for the activity of Sertoli and Leydig cells, for germ cells progression and sperm quality [51, 105, 106].
Interstitial Leydig cells were the first target of CB1 activity to be identified [70, 109]. Such CB1 dependent modulation has been confirmed in CB 1 −/− mice, where a decreased number of Leydig cells [110] and low estrogen levels [44] have been observed. Consistingly, also in nonmammalian vertebrates CB 1 mRNA [111], but not CB1 protein [59], has been localized in interstitial compartment. In the germinal compartment AEA reduces the spermatogenetic output by inducing apoptosis of Sertoli cells [41], a process reversed by FSH-dependent activation of aromatase and by E2-dependent activation of FAAH [41, 42]. The involvement of endocannabinoid signaling in the progression of spermatogenetic stages has been only recently elucidated. In Rana esculenta increasing levels of CB1 and FAAH have been detected in postmeiotic stages [59, 60], whereas NAPE- PLD has been detected by in-situ hybridization in Leydig cells and mitotic and early-meiotic stages [111]. In mice, CB1, CB2, and TRPV1 fluctuate in a stage specific manner [32, 109]. During the first spermatogenetic wave transcriptional downregulation of CB 1 has been observed as soon as meiotic events occur [109] whereas the expression peak has been observed in postmeiotic stages [32, 109]. Besides the control of sperm function required for the fertilization (i.e., sperm motility, capacitation, and acrosome reaction) CB1 activity in chromatin remodeling during the spermiogenesis has been recently reported [43–45]. Interestingly, CB2, the receptor with higher affinity to 2-AG than CB1, is highly expressed in mitotic/meiotic stages and the protein is retained in residual body at the end of the spermiogenesis [32], indicating CB2 participation in meiotic progression. Consistently to the above observations, the levels of eCBs, especially 2-AG, decrease throughout the progression of spermatogenesis, being higher in the spermatogonia and reaching minimal level in spermatids [32]. Lastly, an intriguing matter of debate is the high expression of TRPV1 observed in meiotic stages [32] and the massive germ cell depletion observed in mice lacking the receptor [112]. Thus, a possible role in the protection of meiotic stages has been postulated for TRPV1.
In such a context, the gonadal activity of neurohormones such as GnRH might be critical. In human testes two GnRH molecular forms and two GnRH-Rs have been detected [47, 113, 114], with GnRH-RII gene postmeiotically expressed in round and elongating spermatids. Beside a central query to be resolved is whether these transcripts are functional in sperm the mRNA levels of GnRH-I, GnRH-II, GnRH-R, cytochrome P450 side-chain cleavage (CYP11A1), and 3beta-hydroxy-steroid dehydrogenase type 2 enzyme (HSD3B2) as well as the intratesticular T levels are significantly increased in patients with spermatogenic failure [115] indicating that testicular GnRH may locally act to regulate spermatogenesis and steroidogenesis in humans. Once again, data obtained in nonmammalian vertebrates as well as in mollusks confirmed the involvement of local GnRH in processes such as Sertoli-Leydig cells communication, estradiol dependent spermatogonia proliferation, and sperm release [47, 48, 74, 116, 117] whereas evidences in frogs and rats suggest the participation in sperm functions related to fertilization [111, 118]. Only recently has AEA-dependent modulation of local GnRH system been provided in amphibian testes. In fact, during the annual sexual cycle eCBs, via CB1 activation, modulate GnRH activity in frog testes in a stage dependent manner [111]. When the upsurge of a new spermatogenetic wave occurs (February), in vitro AEA treatment specifically upregulates GnRH-II and GnRH-RIII mRNA and downregulates GnRH-RII. Conversely, in postreproductive period (June), in vitro AEA treatment significantly decreases GnRH-I and GnRH-RII mRNA, whereas it stimulates the transcription of GnRH-II and GnRH-RI. GnRH/GnRH-R localization in frog testes clearly indicates a functional distribution with a GnRH-I/GnRH-RII system mainly involved in the control of germ cell progression and Leydig/Sertoli cell communication and a GnRH-II/GnRH-RII system mainly involved in the control of sperm functions [111]. Thus, the differential AEA-dependent modulation of hypothalamic and testicular GnRH systems may reflect the functional divergence of GnRH molecular forms in testes. In such a picture, TRPV1 signaling should be also considered since, in postreproductive period, the activation of TRPV1 modulates the transcription of testicular GnRHs and of GnRH-RI and GnRH-RII, but in an opposite way compared to that of AEA ([119] in this issue).
Focusing on sperm functions, recently, a fertilization strategy adapted mechanism (external or internal fertilization) has been characterized for the control of sperm motility. In amphibians, exhibiting fertilization in aquatic environment, endocannabinoid activity in cloacal fluid may keep SPZ in a quiescent stage; the addition of CB1 antagonist SR141716A [120] and/or the dilution of cloacal fluid soon increase SPZ motility, in a fashion that mimics the quick activation of SPZ in the aquatic environment during the mating [59]. Such a “dilution-activating mechanism,” in mammals adapted into a 2-AG functional gradient inside the epididymus, the anatomical structure in which SPZ acquire the motility. High 2-AG level has been measured in the caput where SPZ are immotile whereas low level has been detected in the cauda, where SPZ acquire the ability to become motile [34, 121]. Accordingly, (1) the SPZ of CB 1 −/− mice early acquire the motility in the caput epididymus [62], (2) the pharmacological inactivation of CB1 drives the same effects observed in knockout animals, and (3) the administration of EMT inhibitors results in the falling down of cauda motile SPZ in normal mice [34].
A tight control of eCBs levels in SPZ and seminal plasma is required to assure the correct progression of multiple steps involved in the fertilization process. In fact, it has been reported that in FAAH null mice (FAAH −/−) elevated AEA levels [122] impair the sperm fertilizing ability and motility, and the administration of HU-210, a synthetic analogue of Δ9-THC, to rats has adverse effects on both spermatogenesis and sperm motility, suggesting that heightened AEA signaling in the male reproductive tract compromises some sperm cells features [29]. Recently, low 2-AG or AEA levels were measured in seminal plasma of infertile men [35, 123], thus suggesting a key role of eCBs in the acquisition of sperm functions and opening new perspectives in the treatment of male infertility.
The importance to keep AEA content at physiological concentrations in cells, tissues, and fluids involved in male and female reproductive events might be related to the existence of an eCBs gradient. In this context, several papers highlighted the involvement of eCBs signaling in the spatiotemporal control of sperm-egg fusion [26, 63, 108, 124]. Analogously to human menstrual cycle phases [125, 126], fluctuations of AEA levels, in combination with sex hormones oscillations, were detected in the various stages of bovine oestrus cycle [108], strengthening the idea that oviductal AEA content is crucial to avoid impairments in the normal sperm-oocyte interaction.
6. eCBs and Pregnancy: A Focus on Placentation and Parturition
In the past few decades, a large amount of evidence has demonstrated that endocannabinoid signaling via cannabinoid receptors is an important player in various female reproductive events, including sperm-egg fusion as fertilization, preimplantation development of embryos and their timely transport from the oviduct into the uterus, attainment of uterine receptivity, embryo-uterine crosstalk during implantation and decidualization, trophoblast differentiation and placental development, and initiation of parturition. In this section, we will briefly introduce the involvement of endocannabinoid signaling in early pregnancy events, with a focus on its pathophysiological significance during trophoblast development and placental formation as well as the labor onset.
6.1. Endocannabinoid Signaling in Early Pregnancy Events
In mammals, the beginning of a new life is seeded at fertilization. The fertilized egg undergoes serial cell divisions to form the 2-cell embryo, 4-cell embryo, 8-cell embryo, morula, and eventually the blastocyst with the first two differential cell lineages, the inner cell mass (ICM), and the trophectoderm [127–129]. During the past two decades, molecular and genetic studies have demonstrated that the ECS is tightly associated with early pregnancy events [130]. For example, cannabinoid receptors are expressed in the preimplantation mouse embryo, as well as in the oviduct and uterus. In mice, CB 1 mRNA is primarily detected from the four-cell embryo through the blastocyst stages, while CB 2 mRNA is present from the zygote through the blastocyst stages [8, 9, 131]. These results indicate that preimplantation embryo is a potential target for endocannabinoid signaling. Activation of CB1 by cannabinoid ligands interferes with preimplantation embryo development in culture [9]. On the other hand, asynchronous preimplantation embryo development is also observed in mice lacking CB1 [131]. This pharmacological and genetic evidence pointed toward a tightly regulated endocannabinoid signaling during preimplantation embryo development [9, 11, 131].
During early pregnancy, another critical event occurring in parallel with preimplantation embryo development is the timely transport of preimplantation embryos from the oviduct into the uterus. In mice, embryos at the late morula or early blastocyst stage enter the uterus, where they develop and differentiate to gain implantation competency, escape from the zona pellucida, and implant into the receptive uterus. Therefore, normal oviductal embryo transport is one of the prerequisites for on-time implantation. In CB 1 −/−, a large portion of embryos are retained in the oviduct on day 4 of pregnancy and thus fail to initiate on-time implantation [132]. Moreover, wild-type mice treated with methanandamide, a CB1 agonist, also exhibit a similar phenomenon, collectively suggesting that a tonic endocannabinoid signaling is essential for normal embryo transport from the oviduct into the uterus prior to blastocyst implantation. The endogenous levels of AEA, one of the primary endocannabinoid, are maintained by its synthesis and degradation activity. In this respect, FAAH −/− mice exhibit an elevated level of AEA in the oviduct during early pregnancy, accompanied with a derailed oviductal embryo transport [133]. Thus, an aberrant cannabinoid signaling impairs the oviductal transport of embryos, preventing on-time implantation [132, 133]. This finding is clinically relevant to human ectopic pregnancy, since high AEA levels and aberrant expression of FAAH and CB1 in fallopian tubes have been observed in women with ectopic pregnancy [130, 134, 135]. Synchronized embryo development to blastocyst and uterine differentiation to receptive state are important for successful implantation. In the mouse, at pregnant day 1 to day 4 (day 1 = vaginal plug), the ovarian hormones estrogen and progesterone control the uterine undergoing from prereceptive to receptive stage. In this respect, lower levels of AEA in the receptive uterus and at the implantation site have been observed in contrast to its high levels in the nonreceptive uterus [13, 131]. Moreover, the CB1 expression in activated blastocyst is significantly lower than that in dormant blastocysts [12, 131]. These observations suggest a biphasic role of endocannabinoid signaling in synchronizing trophoblast differentiation and uterine preparation to the receptive state for implantation. Also in female rats, ovarian hormones operate in conjunction with the blastocyst intrinsic programme, in order to regulate the synthesis of AEA in a specific manner during the crucial reproductive events that may compromise pregnancy outcome [136]. However, the interaction between lysophosphatidic acid, prostaglandins, and ECS during the window of implantation in the rat uterus has also been reported [137]. Indeed, employing delayed implantation model, previous studies have further demonstrated that AEA at low level renders the blastocyst competent for implantation viaactivating mitogen-activated protein kinase (MAPK) signaling, whereas at a higher concentration it inhibits calcium channel activity and blastocyst reactivation for implantation [12]. This finding has high clinical relevance, since the circulating level of AEA is well associated with pregnancy outcome in women with threatened miscarriage [36, 138]. Taken together, endocannabinoid signaling is an important player directing the normal preimplantation embryo development, activation, and uterine differentiation during the peri-implantation embryo-uterine dialogue.
6.2. Endocannabinoid Signaling Regulates Trophoblast Development and Placentation
With the initiation and progression of implantation and decidualization, trophectodermal epithelium, the wall of spherical blastocyst, will further develop into the extraembryonic tissues and eventually form the placenta. In mice, while the mural trophectoderm penetrates the uterine stromal, forming primary trophoblast giant cells, the polar trophectoderm, adjacent to the ICM, continues to proliferate and forms the ectoplacental cone (EPC) of the early conceptus and the extraembryonic ectoderm [129, 139]. Thereafter, the extraembryonic ectoderm develops to form the chorionic epithelium, which will be further fused with the allantois. Soon after, the chorionic trophoblast and its associated fetal blood vessels undergo extensive villous branching to create a functional mature placenta [140, 141]. Placenta serves as an interface for the exchange of nutrients, gases, and wastes between the maternal and fetal compartments. Moreover, placenta can secrete many hormones and growth factors conducive to the success of pregnancy establishment and maintenance [140, 141].
Increasing evidence suggests that the placenta is also a target of endocannabinoid signaling. In mice, CB1 and FAAH are expressed in the EPC, and later in the spongiotrophoblast cells [142]. CB 1 −/− placentas exhibit compromised spongiotrophoblast development with reduced expression of Mash2 and trophoblast-specific protein α (Tpbpa). This reduced population of Tpbpa positive trophoblast cells is due to an attenuated proliferation of spongiotrophoblast cells in the absence of CB1 receptors [142]. This is consistent with the observations that CB1/CB2 null mutant trophoblast stem (TS) cells show remarkably slower cell proliferation compared with that in wild-type TS cells [142, 143]. It has been further demonstrated that endocannabinoid signaling regulates trophoblast cell proliferation via PI3 K/AKT signaling pathway [142]. Endocannabinoid signaling is also operative during human placental development, since CB1, FAAH, and NAPE-PLD have been demonstrated to be expressed in human placentas [144–147]. For example, CB1 receptors are present in all layers of the membrane, with particularly strong expression in the amniotic epithelium and reticular cells. Moderate expression is observed in the chorionic cytotrophoblasts. Moreover, FAAH is highly expressed in the amniotic epithelial cells, chorionic cytotrophoblast, and maternal decidua layer [145]. Besides, emerging evidence suggests that the levels of CB1, FAAH, and NAPE-PLD in first trimester placentas are highly associated with the term pregnancy outcomes. The expression levels of CB1 and FAAH are significantly lower or even absent, whereas the NAPE-PLD mRNA expression is aberrantly higher in spontaneous miscarriage women [20]. Higher level of AEA is also detected in plasma of nonviable pregnancies than in viable pregnancies [147]. Most recent study further demonstrates that aberrant endocannabinoid signaling plays an important role in the pathophysiology of preeclampsia. The placental expression of NAPE-PLD is significantly higher in preeclamptic pregnancies, while FAAH exhibits an opposite result [148]. Moreover, AEA and Δ9-THC have been shown to be able to inhibit human trophoblast BeWo cell proliferation and the transcription of genes involved in growth and apoptosis [138, 149]. These findings reinforce the notion that a tightly regulated endocannabinoid signaling is conducive to normal trophoblast development and placentation in humans.
6.3. Endocannabinoid Signaling Is Operative during Labor Onset
Preterm birth is defined as the birth of a baby which is less than 37 weeks of gestational age in humans [150]. In the world, 15 million babies are born prematurely [151]. Preterm birth is among the top causes of death in infants worldwide, which is the greatest health burden associated with pregnancy and childbirth [152]. Preterm labor may be caused by many factors, for example, genetics, infection, chemical substances, environmental contaminant or other factors [153–157], but the cause of preterm birth in many situations is elusive and unknown.
Progesterone and corticotropin-releasing hormone (CRH) are the most important mediators of labor. Progesterone has an essential and multifaceted role in the maintenance of myometrial quiescence during pregnancy and its withdrawal induces labor. The functions of progesterone are mediated by the nuclear progesterone receptors (PR-A and PR-B) in myometrial cells [158]. Progesterone has been advocated for the prevention of preterm labor [159]. Treatment with progesterone reduces the rate of spontaneous early preterm delivery in the midgestation period in women [159, 160]. CRH also has a critical role in pregnancy and labor, which is produced by the placenta during pregnancy [161–163]. CRH acts on the fetal pituitary-adrenal axis and directly on myometrial cells to facilitate labor, which determines the length of gestation and the timing of parturition and delivery. In this respect, previous studies have demonstrated that endocannabinoid signaling can modulate the activities of the hypothalamic-pituitary axis [77, 164–166] and thus is associated with normal onset and duration of labor in both mice and women [19, 167].
In mice, as described above, loss of CB1 impairs the normal oviductal embryo transport, leading to deferral of on-time embryo implantation [132]. Therefore, it was generally thought that the labor onset would accordingly be delayed. However, surprisingly, the day of birth of CB1 null mutant females is almost one day earlier than that in wild-type mice [167]. Similar premature birth can be induced in wild-type mice receiving CB1-selective antagonist SR141716, but not a CB2-selective antagonist SR144528 [120, 168]. The levels of progesterone and estrogen are largely alerted in the CB1deficient mice. An early drop of serum progesterone levels is observed on day 19 in the CB1 null mutant mice, while the estrogen level increases on days 16–18. Subsequent analysis further reveals that cytochrome P450 aromatase and 17β-HSD7, which primarily contribute to ovarian estrogen biosynthesis during gestation in mice, are upregulated in CB1 null ovaries, whereas levels of 20α-HSD, which metabolize progesterone into biologically inactive 20α-dihydroprogesterone, are substantially increased in CB1 null mutant ovaries on day 19 of pregnancy. The premature birth in mice lacking CB 1 can be restored by subcutaneous injection of progesterone on day 18. This finding suggests that endocannabinoid signaling is essential for the maintenance of normal progesterone/estrogen ratio prior to the onset of parturition. Another interesting finding is that loss of CB1 overrides cyclooxygenase- (COX-) 1 deficiency-induced delayed parturition and remarkably improves the survival rate of newborn pups. These results suggest that CB1 signaling has a unique role in regulating normal parturition that is independent of COX-1-derived prostaglandin F2α, but CB1 deficiency can correct the effects produced by COX-1 deficiency [167]. There is evidence that eCBs via CB1 can upregulate COX-2 expression and thus prostaglandin E2 production in human gestational membranes during late pregnancy [169]. Prostaglandin E and F have an important function to regulate uterine contractions in labor, and the function of prostaglandin was through prostaglandin receptor expressed in myometrial tissue [170]. It remains to be determined whether COX-1 deficiency-induced delayed parturition is associated with aberrant cannabinoid-CB1 signaling in mice. In addition, loss of CB1 induces aberrant CRH-driven endocrine activities leading to preterm labor in mice, Antalarmin hydrochloride, a selective CRH antagonist, is able to restore the normal parturition timing in CB1 deficient mice, and enhanced corticosterone activity on days 14–18 induces preterm birth with impaired fetal growth in wild-type mice. These observations show the concept that CB1 signaling is crucial for maintaining normal CRH- corticosterone activities and onset of labor in mice [167].
In women, the chronic use of marijuana is often associated with fetal abnormalities and early pregnancy termination [36, 37, 133]. Plasma AEA levels have been shown to be associated with onset of labor. Plasma AEA levels are significantly increased in laboring term than those in nonlaboring term [19, 171, 172]. Meanwhile, a significantly higher expression of CB1 has been observed in placental villous from nonlaboring compared to laboring women 173]. This finding indicates that the higher AEA level and lower placental CB1 expression are essential for the timely onset of labor.
Collectively, endocannabinoid signaling is crucial for the normal initiation of parturition. Epidemiological studies should pay a close attention to CB 1 or FAAH gene polymorphism or mutation in women with preterm labor in clinical practice.
7. Closing Remarks
In the past few years ECS has emerged as an essential player in male and female reproduction. Nowadays, eCBs together with their synthesizing and degrading enzymes, EMT, and molecular targets have been identified in reproductive cells, organs, and fluids of invertebrates, vertebrates, and mammals, highlighting the key role played by these endogenous compounds in reproduction processes along the evolutionary axis. Therefore, it comes out that the disruption of the normal physiological action of the ECS impairs the function of the male and female reproductive system and that altered AEA and/or 2-AG content is crucial during the various stages of procreation with relevant and interesting implications in the therapeutic exploitation.
Acknowledgment
Work incorporated in this paper was partially supported by Prin MIUR 2010-2011 (Rosaria Meccariello), the National Basic Research Program of China (2011CB944400), and the National Natural Science Foundation (81130009) (Haibin Wang). The authors apologize for unintended omission of any relevant references.
Conflict of interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Schuel H, Burkman LJ, Lippes J, et al. N-Acylethanolamines in human reproductive fluids. Chemistry and Physics of Lipids. 2002;121(1-2):211–227. doi: 10.1016/s0009-3084(02)00158-5. [DOI] [PubMed] [Google Scholar]
- 2.Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 3.Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochemical and Biophysical Research Communications. 1995;215(1):89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- 4.Borgen LA, Davis WM, Pace HB. Effects of synthetic Δ9-tetrahydrocannabinol on pregnancy and offspring in the rat. Toxicology and Applied Pharmacology. 1971;20(4):480–486. doi: 10.1016/0041-008x(71)90252-3. [DOI] [PubMed] [Google Scholar]
- 5.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 6.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 7.Schuel H, Goldstein E, Mechoulam R, Zimmerman AM, Zimmerman S. Anandamide (arachidonylethanolamide), a brain cannabinoid receptor agonist, reduces sperm fertilizing capacity in sea urchins by inhibiting the acrosome reaction. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(16):7678–7682. doi: 10.1073/pnas.91.16.7678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das SK, Paria BC, Chakraborty I, Dey SK. Cannabinoid ligand-receptor signaling in the mouse uterus. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(10):4332–4336. doi: 10.1073/pnas.92.10.4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paria BC, Das SK, Dey SK. The preimplantation mouse embryo is a target for cannabinoid ligand- receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(21):9460–9464. doi: 10.1073/pnas.92.21.9460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paria BC, Ma W, Andrenyak DM, et al. Effects of cannabinoids on preimplantation mouse embryo development and implantation are mediated by brain-type cannabinoid receptors. Biology of Reproduction. 1998;58(6):1490–1495. doi: 10.1095/biolreprod58.6.1490. [DOI] [PubMed] [Google Scholar]
- 11.Yang Z-M, Paria BC, Dey SK. Activation of brain-type cannabinoid receptors interferes with preimplantation mouse embryo development. Biology of Reproduction. 1996;55(4):756–761. doi: 10.1095/biolreprod55.4.756. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Matsumoto H, Guo Y, Paria BC, Roberts RL, Dey SK. Differential G protein-coupled cannabinoid receptor signaling by anandamide directs blastocyst activation for implantation. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(25):14914–14919. doi: 10.1073/pnas.2436379100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmid PC, Paria BC, Krebsbach RJ, Schmid HHO, Dey SK. Changes in anandamide levels in mouse uterus are associated with uterine receptivity for embryo implantation. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(8):4188–4192. doi: 10.1073/pnas.94.8.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karasu T, Marczylo TH, Maccarrone M, Konje JC. The role of sex steroid hormones, cytokines and the endocannabinoid system in female fertility. Human Reproduction Update. 2011;17(3):347–361. doi: 10.1093/humupd/dmq058. [DOI] [PubMed] [Google Scholar]
- 15.Paria BC, Deutsch DD, Dey SK. The uterus is a potential site for anandamide synthesis and hydrolysis: differential profiles of anandamide synthase and hydrolase activities in the mouse uterus during the periimplantation period. Molecular Reproduction and Development. 1996;45(2):183–192. doi: 10.1002/(SICI)1098-2795(199610)45:2<183::AID-MRD11>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Paria BC, Zhao X, Wang J, Das SK, Dey SK. Fatty-acid amide hydrolase is expressed in the mouse uterus and embryo during the periimplantation period. Biology of Reproduction. 1999;60(5):1151–1157. doi: 10.1095/biolreprod60.5.1151. [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Wang H, Okamoto Y, et al. N-acylphosphatidylethanolamine-hydrolyzing phospholipase D is an important determinant of uterine anandamide levels during implantation. The Journal of Biological Chemistry. 2005;280(25):23429–23432. doi: 10.1074/jbc.C500168200. [DOI] [PubMed] [Google Scholar]
- 18.Wang H, Xie H, Sun X, et al. Differential regulation of endocannabinoid synthesis and degradation in the uterus during embryo implantation. Prostaglandins and other Lipid Mediators. 2007;83(1-2):62–74. doi: 10.1016/j.prostaglandins.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habayeb OMH, Taylor AH, Evans MD, et al. Plasma levels of the endocannabinoid anandamide in women—a potential role in pregnancy maintenance and labor? The Journal of Clinical Endocrinology and Metabolism. 2004;89(11):5482–5487. doi: 10.1210/jc.2004-0681. [DOI] [PubMed] [Google Scholar]
- 20.Trabucco E, Acone G, Marenna A, et al. Endocannabinoid system in first trimester placenta: low FAAH and high CB1 expression characterize spontaneous miscarriage. Placenta. 2009;30(6):516–522. doi: 10.1016/j.placenta.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 21.Schuel H, Burkman LJ, Lippes J, et al. Evidence that anandamide-signaling regulates human sperm functions required for fertilization. Molecular Reproduction and Development. 2002;63(3):376–387. doi: 10.1002/mrd.90021. [DOI] [PubMed] [Google Scholar]
- 22.Rossato M, Popa FI, Ferigo M, Clari G, Foresta C. Human sperm express cannabinoid receptor Cb1, the activation of which inhibits motility, acrosome reaction, and mitochondrial function. The Journal of Clinical Endocrinology and Metabolism. 2005;90(2):984–991. doi: 10.1210/jc.2004-1287. [DOI] [PubMed] [Google Scholar]
- 23.Maccarrone M, Barboni B, Paradisi A, et al. Characterization of the endocannabinoid system in boar spermatozoa and implications for sperm capacitation and acrosome reaction. Journal of Cell Science. 2005;118(19):4393–4404. doi: 10.1242/jcs.02536. [DOI] [PubMed] [Google Scholar]
- 24.Gervasi MG, Osycka-Salut C, Caballero J, et al. Anandamide capacitates bull spermatozoa through CB1 and TRPV1 activation. PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0016993.e16993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aquila S, Guido C, Santoro A, et al. Rimonabant (SR141716) induces metabolism and acquisition of fertilizing ability in human sperm. British Journal of Pharmacology. 2010;159(4):831–841. doi: 10.1111/j.1476-5381.2009.00570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francavilla F, Battista N, Barbonetti A, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology. 2009;150(10):4692–4700. doi: 10.1210/en.2009-0057. [DOI] [PubMed] [Google Scholar]
- 27.Catanzaro G, Battista N, Rossi G, et al. Effect of capacitation on the endocannabinoid system of mouse sperm. Molecular and Cellular Endocrinology. 2011;343(1-2):88–92. doi: 10.1016/j.mce.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Sun X, Dey SK. Endocannabinoid signaling in female reproduction. ACS Chemical Neuroscience. 2012;3(5):349–355. doi: 10.1021/cn300014e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.M Lewis SE, Paro R, Borriello L, et al. Long-term use of HU210 adversely affects spermatogenesis in rats by modulating the endocannabinoid system. International Journal of Andrology. 2012;35(5):731–740. doi: 10.1111/j.1365-2605.2012.01259.x. [DOI] [PubMed] [Google Scholar]
- 30.Fonseca BM, Teixeira NA, Almada M, Taylor AH, Konje JC, Correia-da-Silva G. Modulation of the novel cannabinoid receptor-GPR55-during rat fetoplacental development. Placenta. 2011;32(6):462–469. doi: 10.1016/j.placenta.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 31.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends in Pharmacological Sciences. 2006;27(1):1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Grimaldi P, Orlando P, di Siena S, et al. The endocannabinoid system and pivotal role of the CB2 receptor in mouse spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(27):11131–11136. doi: 10.1073/pnas.0812789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fonseca BM, Correia-da-Silva G, Taylor AH, et al. The endocannabinoid 2-arachidonoylglycerol (2-AG) and metabolizing enzymes during rat fetoplacental development: a role in uterine remodelling. The International Journal of Biochemistry and Cell Biology. 2010;42(11):1884–1892. doi: 10.1016/j.biocel.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Cobellis G, Ricci G, Cacciola G, et al. A gradient of 2-arachidonoylglycerol regulates mouse epididymal sperm cell start-up. Biology of Reproduction. 2010;82(2):451–458. doi: 10.1095/biolreprod.109.079210. [DOI] [PubMed] [Google Scholar]
- 35.Lewis LS, Rapino C, di Tommaso M, et al. Differences in the endocannabinoid system of sperm from fertile and infertile men. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047704.e47704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A. Relation between decreased anandamide hydrolase concentrations in human lymphocytes and miscarriage. The Lancet. 2000;355(9212):1326–1329. doi: 10.1016/S0140-6736(00)02115-2. [DOI] [PubMed] [Google Scholar]
- 37.Maccarrone M, Bisogno T, Valensise H, et al. Low fatty acid amide hydrolase and high anandamide levels are associated with failure to achieve an ongoing pregnancy after IVF and embryo transfer. Molecular Human Reproduction. 2002;8(2):188–195. doi: 10.1093/molehr/8.2.188. [DOI] [PubMed] [Google Scholar]
- 38.El-Talatini MR, Taylor AH, Konje JC. Fluctuation in anandamide levels from ovulation to early pregnancy in in-vitro fertilization-embryo transfer women, and its hormonal regulation. Human Reproduction. 2009;24(8):1989–1998. doi: 10.1093/humrep/dep065. [DOI] [PubMed] [Google Scholar]
- 39.Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: Role of cytokines and implications for fertility. The Journal of Immunology. 2001;166(12):7183–7189. doi: 10.4049/jimmunol.166.12.7183. [DOI] [PubMed] [Google Scholar]
- 40.Helliwell RJA, Chamley LW, Blake-Palmer K, et al. Characterization of the endocannabinoid system in early human pregnancy. The Journal of Clinical Endocrinology and Metabolism. 2004;89(10):5168–5174. doi: 10.1210/jc.2004-0388. [DOI] [PubMed] [Google Scholar]
- 41.Rossi G, Gasperi V, Paro R, Barsacchi D, Cecconi S, Maccarrone M. Follicle-stimulating hormone activates fatty acid amide hydrolase by protein kinase A and aromatase-dependent pathways in mouse primary Sertoli cells. Endocrinology. 2007;148(3):1431–1439. doi: 10.1210/en.2006-0969. [DOI] [PubMed] [Google Scholar]
- 42.Grimaldi P, Pucci M, di Siena S, et al. The faah gene is the first direct target of estrogen in the testis: role of histone demethylase LSD1. Cellular and Molecular Life Sciences. 2012;69(24):4177–4190. doi: 10.1007/s00018-012-1074-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chioccarelli T, Cacciola G, Altucci L, et al. Cannabinoid receptor 1 influences chromatin remodeling in mouse spermatids by affecting content of transition protein 2 mRNA and histone displacement. Endocrinology. 2010;151(10):5017–5029. doi: 10.1210/en.2010-0133. [DOI] [PubMed] [Google Scholar]
- 44.Cacciola G, Chioccarelli T, Altucci L, et al. Low 17beta-estradiol levels in Cnr1 knock-out mice affect spermatid chromatin remodeling by interfering with chromatin reorganization. Biology of Reproduction. 2013;88(6):p. 152. doi: 10.1095/biolreprod.112.105726. [DOI] [PubMed] [Google Scholar]
- 45.Cacciola G, Chioccarelli T, Altucci L, et al. Nuclear size as estrogen-responsive chromatin quality parameter of mouse spermatozoa. General and Comparative Endocrinology. 2013;193:201–209. doi: 10.1016/j.ygcen.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 46.Cobellis G, Meccariello R, Fienga G, Pierantoni R, Fasano S. Cytoplasmic and nuclear Fos protein forms regulate resumption of spermatogenesis in the frog, Rana esculenta. Endocrinology. 2002;143(1):163–170. doi: 10.1210/endo.143.1.8567. [DOI] [PubMed] [Google Scholar]
- 47.Pierantoni R, Cobellis G, Meccariello R, Fasano S. Evolutionary aspects of cellular communication in the vertebrate hypothalamo-hypophysio-gonadal axis. International Review of Cytology. 2002;218:69–141. doi: 10.1016/s0074-7696(02)18012-0. [DOI] [PubMed] [Google Scholar]
- 48.Chianese R, Chioccarelli T, Cacciola G, et al. The contribution of lower vertebrate animal models in human reproduction research. General and Comparative Endocrinology. 2011;171(1):17–27. doi: 10.1016/j.ygcen.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 49.Elphick MR. The evolution and comparative neurobiology of endocannabinoid signalling. Philosophical Transactions of the Royal Society B. 2012;367(1607):3201–3215. doi: 10.1098/rstb.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fasano S, Meccariello R, Cobellis G, et al. The endocannabinoid system: an ancient signaling involved in the control of male fertility. Annals of the New York Academy of Sciences. 2009;1163:112–124. doi: 10.1111/j.1749-6632.2009.04437.x. [DOI] [PubMed] [Google Scholar]
- 51.Battista N, Meccariello R, Cobellis G, et al. The role of endocannabinoids in gonadal function and fertility along the evolutionary axis. Molecular and Cellular Endocrinology. 2012;355(1):1–14. doi: 10.1016/j.mce.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 52.Buznikov GA, Nikitina LA, Bezuglov VV, et al. A putative ’pre-nervous’ endocannabinoid system in early echinoderm development. Developmental Neuroscience. 2010;32(1):1–18. doi: 10.1159/000235758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meriaux C, Arafah K, Tasiemski A, et al. Multiple changes in peptide and lipid expression associated with regeneration in the nervous system of the medicinal leech. PLoS ONE. 2011;6(4) doi: 10.1371/journal.pone.0018359.e18359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schuel H, Burkman LJ. A tale of two cells: endocannabinoid-signaling regulates functions of neurons and sperm. Biology of Reproduction. 2005;73(6):1078–1086. doi: 10.1095/biolreprod.105.043273. [DOI] [PubMed] [Google Scholar]
- 55.Meizel S. The sperm, a neuron with a tail: ’Neuronal’ receptors in mammalian sperm. Biological Reviews of the Cambridge Philosophical Society. 2004;79(4):713–732. doi: 10.1017/s1464793103006407. [DOI] [PubMed] [Google Scholar]
- 56.Wang H, Dey SK, Maccarrone M. Jekyll and Hyde: two faces of cannabinoid signaling in male and female fertility. Endocrine Reviews. 2006;27(5):427–448. doi: 10.1210/er.2006-0006. [DOI] [PubMed] [Google Scholar]
- 57.Cottone E, Guastalla A, Mackie K, Franzoni MF. Endocannabinoids affect the reproductive functions in teleosts and amphibians. Molecular and Cellular Endocrinology. 2008;286(1-2):S41–S45. doi: 10.1016/j.mce.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 58.Cottone E, Salio C, Conrath M, Franzoni MF. Xenopus laevis CB1 cannabinoid receptor: molecular cloning and mRNA distribution in the central nervous system. The Journal of Comparative Neurology. 2003;464(4):487–496. doi: 10.1002/cne.10808. [DOI] [PubMed] [Google Scholar]
- 59.Cobellis G, Cacciola G, Scarpa D, et al. Endocannabinoid system in frog and rodent testis: type-1 cannabinoid receptor and fatty acid amide hydrolase activity in male germ cells. Biology of Reproduction. 2006;75(1):82–89. doi: 10.1095/biolreprod.106.051730. [DOI] [PubMed] [Google Scholar]
- 60.Meccariello R, Chianese R, Cacciola G, Cobellis G, Pierantoni R, Fasano S. Type-1 cannabinoid receptor expression in the frog, Rana esculenta, tissues: a possible involvement in the regulation of testicular activity. Molecular Reproduction and Development. 2006;73(5):551–558. doi: 10.1002/mrd.20434. [DOI] [PubMed] [Google Scholar]
- 61.Meccariello R, Chianese R, Cobellis G, Pierantoni R, Fasano S. Cloning of type 1 cannabinoid receptor in Rana esculenta reveals differences between genomic sequence and cDNA. FEBS Journal. 2007;274(11):2909–2920. doi: 10.1111/j.1742-4658.2007.05824.x. [DOI] [PubMed] [Google Scholar]
- 62.Ricci G, Cacciola G, Altucci L, et al. Endocannabinoid control of sperm motility: the role of epididymus. General and Comparative Endocrinology. 2007;153(1–3):320–322. doi: 10.1016/j.ygcen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 63.Gervasi MG, Rapanelli M, Ribeiro ML, et al. The endocannabinoid system in bull sperm and bovine oviductal epithelium: role of anandamide in sperm-oviduct interaction. Reproduction. 2009;137(3):403–414. doi: 10.1530/REP-08-0204. [DOI] [PubMed] [Google Scholar]
- 64.Palermo FA, Ruggeri B, Mosconi G, Virgili M, Polzonetti-Magni AM. Partial cloning of CB1 cDNA and CB1 mRNA changes in stress responses in the Solea solea . Molecular and Cellular Endocrinology. 2008;286(1-2):S52–S59. doi: 10.1016/j.mce.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 65.Cottone E, Pomatto V, Cerri F, et al. Cannabinoid receptors are widely expressed in goldfish: molecular cloning of a CB2-like receptor and evaluation of CB1 and CB2 mRNA expression profiles in different organs. Fish Physiology and Biochemistry. 2013 doi: 10.1007/s10695-013-9783-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruggeri B, Soverchia L, Mosconi G, Franzoni MF, Cottone E, Polzonetti-Magni AM. Changes of gonadal CB1 cannabinoid receptor mRNA in the gilthead seabream, Sparus aurata, during sex reversal. General and Comparative Endocrinology. 2007;150(2):263–269. doi: 10.1016/j.ygcen.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 67.Gorzalka BB, Dang SS. Minireview: Endocannabinoids and gonadal hormones: bidirectional interactions in physiology and behavior. Endocrinology. 2012;153(3):1016–1024. doi: 10.1210/en.2011-1643. [DOI] [PubMed] [Google Scholar]
- 68.Brown TT, Dobs AS. Endocrine effects of marijuana. Journal of Clinical Pharmacology. 2002;42(11):96S, 90S. doi: 10.1002/j.1552-4604.2002.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 69.López HH. Cannabinoid-hormone interactions in the regulation of motivational processes. Hormones and Behavior. 2010;58(1):100–110. doi: 10.1016/j.yhbeh.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 70.Wenger T, Ledent C, Csernus V, Gerendai I. The central cannabinoid receptor inactivation suppresses endocrine reproductive functions. Biochemical and Biophysical Research Communications. 2001;284(2):363–368. doi: 10.1006/bbrc.2001.4977. [DOI] [PubMed] [Google Scholar]
- 71.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 72.Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139(4):1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 73.Gong J-P, Onaivi ES, Ishiguro H, et al. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Research. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 74.Pierantoni R, Cobellis G, Meccariello R, et al. Testicular gonadotropin-releasing hormone activity, progression of spermatogenesis, and sperm transport in vertebrates. Annals of the New York Academy of Sciences. 2009;1163:279–291. doi: 10.1111/j.1749-6632.2008.03617.x. [DOI] [PubMed] [Google Scholar]
- 75.Murphy LL, Muñoz RM, Adrian BA, Villanúa MA. Function of cannabinoid receptors in the neuroendocrine regulation of hormone secretion. Neurobiology of Disease. 1998;5(6):432–446. doi: 10.1006/nbdi.1998.0224. [DOI] [PubMed] [Google Scholar]
- 76.Gammon CM, Freeman GM, Jr., Xie W, Petersen SL, Wetsel WC. Regulation of gonadotropin-releasing hormone secretion by cannabinoids. Endocrinology. 2005;146(10):4491–4499. doi: 10.1210/en.2004-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Scorticati C, Fernández-Solari J, de Laurentiis A, et al. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(32):11891–11896. doi: 10.1073/pnas.0404366101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oláh M, Milloh H, Wenger T. The role of endocannabinoids in the regulation of luteinizing hormone and prolactin release. Differences between the effects of AEA and 2AG. Molecular and Cellular Endocrinology. 2008;286(1-2):S36–S40. doi: 10.1016/j.mce.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 79.Nakane R, Oka Y. Excitatory action of GABA in the terminal nerve gonadotropin-releasing hormone neurons. Journal of Neurophysiology. 2010;103(3):1375–1384. doi: 10.1152/jn.00910.2009. [DOI] [PubMed] [Google Scholar]
- 80.Farkas I, Kalló I, Deli L, et al. Retrograde endocannabinoid signaling reduces GABAergic synaptic transmission to gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(12):5818–5829. doi: 10.1210/en.2010-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. The Journal of Neuroscience. 2005;25(24):5740–5749. doi: 10.1523/JNEUROSCI.0913-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. The Journal of Neuroscience. 2009;29(31):9809–9818. doi: 10.1523/JNEUROSCI.2509-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21(11):1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- 84.Shivachar AC. Cannabinoids inhibit sodium-dependent, high-affinity excitatory amino acid transport in cultured rat cortical astrocytes. Biochemical Pharmacology. 2007;73(12):2004–2011. doi: 10.1016/j.bcp.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 85.Glanowska KM, Moenter SM. Endocannabinoids and prostaglandins both contribute to GnRH neuron-GABAergic afferent local feedback circuits. Journal of Neurophysiology. 2011;106(6):3073–3081. doi: 10.1152/jn.00046.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chianese R, Cobellis G, Pierantoni R, Fasano S, Meccariello R. Non-mammalian vertebrate models and the endocannabinoid system: Relationships with gonadotropin-releasing hormone. Molecular and Cellular Endocrinology. 2008;286(1-2):S46–S51. doi: 10.1016/j.mce.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Chianese R, Ciaramella V, Fasano S, Pierantoni R, Meccariello R. Anandamide modulates the expression of GnRH-II and GnRHRs in frog, Rana esculenta, diencephalon. General and Comparative Endocrinology. 2011;173(3):389–395. doi: 10.1016/j.ygcen.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 88.Meccariello R, Franzoni MF, Chianese R, et al. Interplay between the endocannabinoid system and GnRH-I in the forebrain of the anuran amphibian Rana esculenta. Endocrinology. 2008;149(5):2149–2158. doi: 10.1210/en.2007-1357. [DOI] [PubMed] [Google Scholar]
- 89.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocrine Reviews. 2009;30(6):713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clarkson J, Herbison AE. Oestrogen, kisspeptin, GPR54 and the pre-ovulatory luteinising hormone surge. Journal of Neuroendocrinology. 2009;21(4):305–311. doi: 10.1111/j.1365-2826.2009.01835.x. [DOI] [PubMed] [Google Scholar]
- 91.de Tassigny XD, Colledge WH. The role of Kisspeptin signaling in reproduction. Physiology. 2010;25(4):207–217. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- 92.Meccariello R, Chianese R, Fasano S, Pierantoni R. Endocannabinoids and kisspeptins: two modulators in fight for the regulation of GnRH activity. In: Vizcarra J, editor. Gonadotropins. Rijeka, Croatia: InTech; 2013. pp. 57–88. [Google Scholar]
- 93.González S, Mauriello-Romanazzi G, Berrendero F, Ramos JA, Fosca Franzoni M, Fernández-Ruiz J. Decreased cannabinoid CB1 receptor mRNA levels and immunoreactivity in pituitary hyperplasia induced by prolonged exposure to estrogens. Pituitary. 2000;3(4):221–226. doi: 10.1023/a:1012874029689. [DOI] [PubMed] [Google Scholar]
- 94.González S, Bisogno T, Wenger T, et al. Sex steroid influence on cannabinoid CB1 receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochemical and Biophysical Research Communications. 2000;270(1):260–266. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- 95.Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2006;291(2):R349–R358. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- 96.Waleh NS, Cravatt BF, Apte-Deshpande A, Terao A, Kilduff TS. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene. 2002;291(1-2):203–210. doi: 10.1016/s0378-1119(02)00598-x. [DOI] [PubMed] [Google Scholar]
- 97.Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology. 2007;32(4):350–357. doi: 10.1016/j.psyneuen.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 98.Habayeb OMH, Bell SC, Konje JC. Endogenous cannabinoids: metabolism and their role in reproduction. Life Sciences. 2002;70(17):1963–1977. doi: 10.1016/s0024-3205(01)01539-9. [DOI] [PubMed] [Google Scholar]
- 99.Maccarrone M, Gasperi V, Fezza F, Finazzi-Agrò A, Rossi A. Differential regulation of fatty acid amide hydrolase promoter in human immune cells and neuronal cells by leptin and progesterone. European Journal of Biochemistry. 2004;271(23-24):4666–4676. doi: 10.1111/j.1432-1033.2004.04427.x. [DOI] [PubMed] [Google Scholar]
- 100.Mani SK, Mitchell A, O’Malley BW. Progesterone receptor and dopamine receptors are required in Δ9-tetrahydrocannabinol modulation of sexual receptivity in female rats. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(3):1249–1254. doi: 10.1073/pnas.031563998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gordon JH, Bromley BL, Gorski RA, Zimmermann E. Δ9-Tetrahydrocannabinol enhancement of lordosis behavior in estrogen treated female rats. Pharmacology Biochemistry and Behavior. 1978;8(5):603–608. [PubMed] [Google Scholar]
- 102.Craft RM, Leitl MD. Gonadal hormone modulation of the behavioral effects of Δ9-tetrahydrocannabinol in male and female rats. European Journal of Pharmacology. 2008;578(1):37–42. doi: 10.1016/j.ejphar.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. British Journal of Pharmacology. 2007;152(5):795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daniel JM, Winsauer PJ, Brauner IN, Moerschbaecher JM. Estrogen improves response accuracy and attenuates the disruptive effects of Δ9-THC in ovariectomized rats responding under a multiple schedule of repeated acquisition and performance. Behavioral Neuroscience. 2002;116(6):989–998. [PubMed] [Google Scholar]
- 105.Pierantoni R, Cobellis G, Meccariello R, et al. CB1 activity in male reproduction: mammalian and nonmammalian animal models. Vitamins and Hormones. 2009;81(C):367–387. doi: 10.1016/S0083-6729(09)81014-5. [DOI] [PubMed] [Google Scholar]
- 106.Cacciola G, Chianese R, Chioccarelli T, et al. Cannabinoids and reproduction: a lasting and intriguing history. Pharmaceuticals. 2010;3(10):3275–3323. [Google Scholar]
- 107.Marczylo TH, Lam PMW, Amoako AA, Konje JC. Anandamide levels in human female reproductive tissues: solid-phase extraction and measurement by ultraperformance liquid chromatography tandem mass spectrometry. Analytical Biochemistry. 2010;400(2):155–162. doi: 10.1016/j.ab.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 108.Gervasi MG, Marczylo TH, Lam PM, et al. Anandamide levels fluctuate in the bovine oviduct during the oestrous cycle. PLoS ONE. 2013;8(8) doi: 10.1371/journal.pone.0072521.e72521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gye MC, Kang HH, Kang HJ. Expression of cannabinoid receptor 1 in mouse testes. Archives of Andrology. 2005;51(3):247–255. doi: 10.1080/014850190898845. [DOI] [PubMed] [Google Scholar]
- 110.Cacciola G, Chioccarelli T, Mackie K, et al. Expression of type-1 cannabinoid receptor during rat postnatal testicular development: possible involvement in adult leydig cell differentiation. Biology of Reproduction. 2008;79(4):758–765. doi: 10.1095/biolreprod.108.070128. [DOI] [PubMed] [Google Scholar]
- 111.Chianese R, Ciaramella V, Scarpa D, Fasano S, Pierantoni R, Meccariello R. Anandamide regulates the expression of GnRH1, GnRH2, and GnRH-Rs in frog testis. American Journal of Physiology: Endocrinology and Metabolism. 2012;303(4):E475–E487. doi: 10.1152/ajpendo.00086.2012. [DOI] [PubMed] [Google Scholar]
- 112.Mizrak SC, van Dissel-Emiliani FMF. Transient receptor potential vanilloid receptor-1 confers heat resistance to male germ cells. Fertility and Sterility. 2008;90(4):1290–1293. doi: 10.1016/j.fertnstert.2007.10.081. [DOI] [PubMed] [Google Scholar]
- 113.White RB, Eisen JA, Kasten TL, Fernald RD. Second gene for gonadotropin-releasing hormone in humans. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(1):305–309. doi: 10.1073/pnas.95.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.van Biljon W, Wykes S, Scherer S, Krawetz SA, Hapgood J. Type II gonadotropin-releasing hormone receptor transcripts in human sperm. Biology of Reproduction. 2002;67(6):1741–1749. doi: 10.1095/biolreprod.101.002808. [DOI] [PubMed] [Google Scholar]
- 115.Lin YM, Poon SL, Choi JH, Lin JSN, Leung PCK, Huang BM. Transcripts of testicular gonadotropin-releasing hormone, steroidogenic enzymes, and intratesticular testosterone levels in infertile men. Fertility and Sterility. 2008;90(5):1761–1768. doi: 10.1016/j.fertnstert.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 116.Cobellis G, Meccariello R, Minucci S, Palmiero C, Pierantoni R, Fasano S. Cytoplasmic versus nuclear localization of fos-related proteins in the frog, Rana esculenta, testis: In vivo and direct in vitro effect of a gonadotropin-releasing hormone agonist. Biology of Reproduction. 2003;68(3):954–960. doi: 10.1095/biolreprod.102.008938. [DOI] [PubMed] [Google Scholar]
- 117.Treen N, Itoh N, Miura H, et al. Mollusc gonadotropin-releasing hormone directly regulates gonadal functions: a primitive endocrine system controlling reproduction. General and Comparative Endocrinology. 2012;176(2):167–172. doi: 10.1016/j.ygcen.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 118.Morales P, Pasten C, Pizarro E. Inhibition of in vivo and in vitro fertilization in rodents by gonadotropin-releasing hormone antagonists. Biology of Reproduction. 2002;67(4):1360–1365. doi: 10.1095/biolreprod67.4.1360. [DOI] [PubMed] [Google Scholar]
- 119.Chianese R, Ciaramella V, Scarpa D, Fasano S, Pierantoni R, Meccariello R. Endocannabinoids and endovanilloids: a possible balance in the regulation of the testicular GnRH signaling. International Journal of Endocrinology. 2013;2013:9 pages. doi: 10.1155/2013/904748.904748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rinaldi-Carmona M, Barth F, Heaulme M, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Letters. 1994;350(2-3):240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 121.Cacciola G, Chioccarelli T, Ricci G, et al. The endocannabinoid system in vertebrate male reproduction: a comparative overview. Molecular and Cellular Endocrinology. 2008;286(1-2):S24–S30. doi: 10.1016/j.mce.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 122.Sun X, Wang H, Okabe M, et al. Genetic loss of Faah compromises male fertility in mice. Biology of Reproduction. 2009;80(2):235–242. doi: 10.1095/biolreprod.108.072736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amoako AA, Marczylo TH, Marczylo EL, et al. Anandamide modulates human sperm motility: implications for men with asthenozoospermia and oligoasthenoteratozoospermia. Human Reproduction. 2013;28(8):2058–2066. doi: 10.1093/humrep/det232. [DOI] [PubMed] [Google Scholar]
- 124.Sun X, Dey SK. Cannabinoid/Endocannabinoid signaling impact on early pregnancy events. Current Topics in Behavioral Neurosciences. 2009;1:255–273. doi: 10.1007/978-3-540-88955-7_10. [DOI] [PubMed] [Google Scholar]
- 125.El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertility and Sterility. 2010;93(6):1989–1996. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 126.Lazzarin N, Valensise H, Bari M, et al. Fluctuations of fatty acid amide hydrolase and anandamide levels during the human ovulatory cycle. Gynecological Endocrinology. 2004;18(4):212–218. doi: 10.1080/09513590410001692492. [DOI] [PubMed] [Google Scholar]
- 127.Rossant J, Tam PPL. Emerging asymmetry and embryonic patterning in early mouse development. Developmental Cell. 2004;7(2):155–164. doi: 10.1016/j.devcel.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 128.Zernicka-Goetz M. Cleavage pattern and emerging asymmetry of the mouse embryo. Nature Reviews Molecular Cell Biology. 2005;6(12):919–928. doi: 10.1038/nrm1782. [DOI] [PubMed] [Google Scholar]
- 129.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nature Reviews Genetics. 2006;7(3):185–199. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 130.Chan HW, McKirdy NC, Peiris HN, Rice GE, Mitchell MD. The role of endocannabinoids in pregnancy. Reproduction. 2013;146(3):R101–R109. doi: 10.1530/REP-12-0508. [DOI] [PubMed] [Google Scholar]
- 131.Paria BC, Song H, Wang X, et al. Dysregulated cannabinoid signaling disrupts uterine receptivity for embryo implantation. The Journal of Biological Chemistry. 2001;276(23):20523–20528. doi: 10.1074/jbc.M100679200. [DOI] [PubMed] [Google Scholar]
- 132.Wang H, Guo Y, Wang D, et al. Aberrant cannabinoid signaling impairs oviductal transport of embryos. Nature Medicine. 2004;10(10):1074–1080. doi: 10.1038/nm1104. [DOI] [PubMed] [Google Scholar]
- 133.Wang H, Xie H, Guo Y, et al. Fatty acid amide hydrolase deficiency limits early pregnancy events. Journal of Clinical Investigation. 2006;116(8):2122–2131. doi: 10.1172/JCI28621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Horne AW, Phillips JA, III, Kane N, et al. CB1 expression is attenuated in Fallopian tube and decidua of women with ectopic pregnancy. PLoS ONE. 2008;3(12) doi: 10.1371/journal.pone.0003969.e3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gebeh AK, Willets JM, Marczylo EL, Taylor AH, Konje JC. Ectopic pregnancy is associated with high anandamide levels and aberrant expression of FAAH and CB1 in fallopian tubes. The The Journal of Clinical Endocrinology and Metabolism. 2012;97(8):2827–2835. doi: 10.1210/jc.2012-1780. [DOI] [PubMed] [Google Scholar]
- 136.Ribeiro ML, Vercelli CA, Sordelli MS, et al. 17β-oestradiol and progesterone regulate anandamide synthesis in the rat uterus. Reproductive BioMedicine Online. 2009;18(2):209–218. doi: 10.1016/s1472-6483(10)60258-1. [DOI] [PubMed] [Google Scholar]
- 137.Sordelli MS, Beltrame JS, Cella M, et al. Interaction between lysophosphatidic acid, prostaglandins and the endocannabinoid system during the window of implantation in the rat uterus. PLoS ONE. 2012;7(9) doi: 10.1371/journal.pone.0046059.e46059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Habayeb OMH, Taylor AH, Bell SC, Taylor DJ, Konje JC. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology. 2008;149(10):5052–5060. doi: 10.1210/en.2007-1799. [DOI] [PubMed] [Google Scholar]
- 139.Varmuza S, Prideaux V, Kothary R, Rossant J. Polytene chromosomes in mouse trophoblast giant cells. Development. 1988;102(1):127–134. doi: 10.1242/dev.102.1.127. [DOI] [PubMed] [Google Scholar]
- 140.Rossant J, Cross JC. Placental development: lessons from mouse mutants. Nature Reviews Genetics. 2001;2(7):538–548. doi: 10.1038/35080570. [DOI] [PubMed] [Google Scholar]
- 141.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology. 2005;20(3):180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 142.Sun X, Xie H, Yang J, Wang H, Bradshaw HB, Dey SK. Endocannabinoid signaling directs differentiation of trophoblast cell lineages and placentation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(39):16887–16892. doi: 10.1073/pnas.1010892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xie H, Sun X, Piao Y, et al. Silencing or amplification of endocannabinoid signaling in blastocysts via CB1 compromises trophoblast cell migration. The Journal of Biological Chemistry. 2012;287(38):32288–32297. doi: 10.1074/jbc.M112.381145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kenney SP, Kekuda R, Prasad PD, Leibach FH, Devoe LD, Ganapathy V. Cannabinoid receptors and their role in the regulation of the serotonin transporter in human placenta. American Journal of Obstetrics and Gynecology. 1999;181(2):491–497. doi: 10.1016/s0002-9378(99)70583-1. [DOI] [PubMed] [Google Scholar]
- 145.Park B, Gibbons HM, Mitchell MD, Glass M. Identification of the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) in the human placenta. Placenta. 2003;24(10):990–995. doi: 10.1016/s0143-4004(03)00165-6. [DOI] [PubMed] [Google Scholar]
- 146.Chamley LW, Bhalla A, Stone PR, et al. Nuclear localisation of the endocannabinoid metabolizing enzyme fatty acid amide hydrolase (FAAH) in invasive trophoblasts and an association with recurrent miscarriage. Placenta. 2008;29(11):970–975. doi: 10.1016/j.placenta.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 147.Taylor AH, Finney M, Lam PMW, Konje JC. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reproductive Biology and Endocrinology. 2011;9, article 152 doi: 10.1186/1477-7827-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Aban C, Leguizamon GF, Cella M, Damiano A, Franchi AM, Farina MG. Differential expression of endocannabinoid system in normal and preeclamptic placentas: effects on nitric oxide synthesis. Placenta. 2013;34(1):67–74. doi: 10.1016/j.placenta.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 149.Khare M, Taylor AH, Konje JC, Bell SC. Δ9-Tetrahydrocannabinol inhibits cytotrophoblast cell proliferation and modulates gene transcription. Molecular Human Reproduction. 2006;12(5):321–333. doi: 10.1093/molehr/gal036. [DOI] [PubMed] [Google Scholar]
- 150.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. The Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 151.Chang HH, Larson J, Blencowe H, et al. Preventing preterm births: analysis of trends and potential reductions with interventions in 39 countries with very high human development index. The Lancet. 2013;381(9862):223–234. doi: 10.1016/S0140-6736(12)61856-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gravett MG, Rubens CE. A framework for strategic investments in research to reduce the global burden of preterm birth. American Journal of Obstetrics and Gynecology. 2012;207(5):368–373. doi: 10.1016/j.ajog.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 153.Dolan SM, Hollegaard MV, Merialdi M, et al. Synopsis of preterm birth genetic association studies: the preterm birth genetics knowledge base (PTBGene) Public Health Genomics. 2010;13(7-8):514–523. doi: 10.1159/000294202. [DOI] [PubMed] [Google Scholar]
- 154.Ding T, McConaha M, Boyd KL, Osteen KG, Bruner-Tran KL. Developmental dioxin exposure of either parent is associated with an increased risk of preterm birth in adult mice. Reproductive Toxicology. 2011;31(3):351–358. doi: 10.1016/j.reprotox.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Horvath B, Grasselly M, Bodecs T, Boncz I, Bodis J. Screening pregnant women for group B streptococcus infection between 30 and 32 weeks of pregnancy in a population at high risk for premature birth. International Journal of Gynecology & Obstetrics. 2013;122(1):9–12. doi: 10.1016/j.ijgo.2013.01.027. [DOI] [PubMed] [Google Scholar]
- 156.Mogami H, Kishore AH, Shi H, Keller PW, Akgul Y, Word RA. Fetal fibronectin signaling induces matrix metalloproteases and cyclooxygenase-2 (COX-2) in amnion cells and preterm birth in mice. The Journal of Biological Chemistry. 2013;288(3):1953–1966. doi: 10.1074/jbc.M112.424366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sonne SR, Bhalla VK, Barman SA, et al. Hyperhomocysteinemia is detrimental to pregnancy in mice and is associated with preterm birth. Biochimica et Biophysica Acta. 2013;1832(8):1149–1158. doi: 10.1016/j.bbadis.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 158.Tan H, Yi L, Rote NS, Hurd WW, Mesiano S. Progesterone receptor-A and -B have opposite effects on proinflammatory gene expression in human myometrial cells: implications for progesterone actions in human pregnancy and parturition'. The Journal of Clinical Endocrinology and Metabolism. 2012;97(5):E719–E730. doi: 10.1210/jc.2011-3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Dodd JM, Flenady V, Cincotta R, Crowther CA. Prenatal administration of progesterone for preventing preterm birth. Cochrane Database of Systematic Reviews. 2006;(1) doi: 10.1002/14651858.CD004947.pub2.CD004947 [DOI] [PubMed] [Google Scholar]
- 160.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. The New England Journal of Medicine. 2007;357(5):462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 161.Grammatopoulos DK, Hillhouse EW. Role of corticotropin-releasing hormone in onset of labour. The Lancet. 1999;354(9189):1546–1549. doi: 10.1016/S0140-6736(99)03418-2. [DOI] [PubMed] [Google Scholar]
- 162.Grammatopoulos DK. The role of CRH receptors and their agonists in myometrial contractility and quiescence during pregnancy and labour. Frontiers in Bioscience. 2007;12(2):561–571. doi: 10.2741/2082. [DOI] [PubMed] [Google Scholar]
- 163.Vrachnis N, Malamas FM, Sifakis S, Tsikouras P, Iliodromiti Z. Immune aspects and myometrial actions of progesterone and CRH in labor. Clinical and Developmental Immunology. 2012;2012 doi: 10.1155/2012/937618.937618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Steger RW, Silverman AY, Johns A, Asch RH. Interactions of cocaine and Δ9-tetrahydrocannabinol with the hypothalamic-hypophysial axis of the female rat. Fertility and Sterility. 1981;35(5):567–572. [PubMed] [Google Scholar]
- 165.Field E, Tyrey L. Tolerance to the luteinizing hormone and prolactin suppressive effects of delta-9-tetrahydrocannabinol develops during chronic prepubertal treatment of female rats. Journal of Pharmacology and Experimental Therapeutics. 1986;238(3):1034–1038. [PubMed] [Google Scholar]
- 166.Fernández-Ruiz JJ, Muñoz; RM, Romero J, Villanua MA, Makriyannis A, Ramos JA. Time course of the effects of different cannabimimetics on prolactin and gonadotrophin secretion: Evidence for the presence of CB1 receptors in hypothalamic structures and their involvement in the effects of cannabimimetics. Biochemical Pharmacology. 1997;53(12):1919–1927. doi: 10.1016/s0006-2952(97)00168-8. [DOI] [PubMed] [Google Scholar]
- 167.Wang H, Xie H, Dey SK. Loss of cannabinoid receptor CB1 induces preterm birth. PLoS ONE. 2008;3(10) doi: 10.1371/journal.pone.0003320.e3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rinaldi-Carmona M, Barth F, Millan J, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. Journal of Pharmacology and Experimental Therapeutics. 1998;284(2):644–650. [PubMed] [Google Scholar]
- 169.Mitchell MD, Sato TA, Wang A, Keelan JA, Ponnampalam AP, Glass M. Cannabinoids stimulate prostaglandin production by human gestational tissues through a tissue- and CB1-receptor-specific mechanism. American Journal of Physiology: Endocrinology and Metabolism. 2008;294(2):E352–E356. doi: 10.1152/ajpendo.00495.2007. [DOI] [PubMed] [Google Scholar]
- 170.O’Brien WF. The role of prostaglandins in labor and delivery. Clinics in Perinatology. 1995;22(4):973–984. [PubMed] [Google Scholar]
- 171.Lam PMW, Marczylo TH, El-Talatini M, et al. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Analytical Biochemistry. 2008;380(2):195–201. doi: 10.1016/j.ab.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 172.Nallendran V, Lam P, Marczylo T, et al. The plasma levels of the endocannabinoid, anandamide, increase with the induction of labour. BJOG. 2010;117(7):863–869. doi: 10.1111/j.1471-0528.2010.02555.x. [DOI] [PubMed] [Google Scholar]


