Progesterone has a neuroprotective effect in preclinical models of stroke and traumatic brain injury, but the therapeutic window remains uncertain. Wali et al. examine infarct size, and sensory, motor and cognitive measures, and report a neuroprotective effect of progesterone when administered as late as 6 hours after experimental stroke onset.
Keywords: dose response, functional recovery, progesterone, stroke, therapeutic time window
Abstract
Currently, the only approved treatment for ischaemic stroke is tissue plasminogen activator, a clot-buster. This treatment can have dangerous consequences if not given within the first 4 h after stroke. Our group and others have shown progesterone to be beneficial in preclinical studies of stroke, but a progesterone dose-response and time-window study is lacking. We tested male Sprague-Dawley rats (12 months old) with permanent middle cerebral artery occlusion or sham operations on multiple measures of sensory, motor and cognitive performance. For the dose-response study, animals received intraperitoneal injections of progesterone (8, 16 or 32 mg/kg) at 1 h post-occlusion, and subcutaneous injections at 6 h and then once every 24 h for 7 days. For the time-window study, the optimal dose of progesterone was given starting at 3, 6 or 24 h post-stroke. Behavioural recovery was evaluated at repeated intervals. Rats were killed at 22 days post-stroke and brains extracted for evaluation of infarct volume. Both 8 and 16 mg/kg doses of progesterone produced attenuation of infarct volume compared with the placebo, and improved functional outcomes up to 3 weeks after stroke on locomotor activity, grip strength, sensory neglect, gait impairment, motor coordination and spatial navigation tests. In the time-window study, the progesterone group exhibited substantial neuroprotection as late as 6 h after stroke onset. Compared with placebo, progesterone showed a significant reduction in infarct size with 3- and 6-h delays. Moderate doses (8 and 16 mg/kg) of progesterone reduced infarct size and improved functional deficits in our clinically relevant model of stroke. The 8 mg/kg dose was optimal in improving motor, sensory and memory function, and this effect was observed over a large therapeutic time window. Progesterone shows promise as a potential therapeutic agent and should be examined for safety and efficacy in a clinical trial for ischaemic stroke.
Introduction
In the USA stroke is now downgraded from the third to fourth leading cause of death, partly because of better acute stroke prevention and management among the elderly (Roger et al., 2012; Go et al., 2013), but at the same time there has been a significant increase of stroke cases in middle-aged, often obese, people (Kissela et al., 2012). Survivors of stroke absorb 6% of the total US healthcare budget (Durukan and Tatlisumak, 2007). Ischaemic stroke, the most common form, accounts for up to 80% of all strokes and if not resolved early, leads to an infarcted brain region that may not be therapeutically salvaged (Murray and Lopez, 1997; Hankey, 1999; Muntner et al., 2006). Despite tremendous efforts, only one therapy, tissue plasminogen activator (tPA), has been proven effective (Muntner et al., 2006), and only ∼2–3% of stroke patients are treated with tPA because of its high risk-to-benefit ratio and short treatment window. Thus, finding an effective therapeutic strategy for stroke remains a high priority.
A large body of evidence now suggests that the steroid hormone progesterone has neuroprotective effects in several experimental CNS injury models including stroke and trauma (Roof et al., 1994; Jiang et al., 1996; Asbury et al., 1998; Chen et al., 1999; Roof and Stein, 1999; Thomas et al., 1999; Alkayed et al., 2000; Kumon et al., 2000; Shear et al., 2002; Sayeed and Stein, 2009; Hua et al., 2012; Liu et al., 2012; Stein, 2013). The neuroprotective efficacy of progesterone in acute functional and morphological recovery in young adult rats has been shown in both permanent and transient stroke models by our recent studies (Sayeed et al., 2006, 2007; Ishrat et al., 2009, 2010, 2012) and previous studies of others (Betz and Coester, 1990; Jiang et al., 1996; Chen et al., 1999; Murphy et al., 2002; Gibson and Murphy, 2004; Gibson et al., 2005). Recent systematic reviews and a meta-analysis reported progesterone to be neuroprotective after brain injuries (Gibson et al., 2008; Wong et al., 2013a). After two positive phase II clinical trials of progesterone in traumatic brain injury (Wright et al., 2007; Xiao et al., 2008), a phase III NIH-sponsored, 31-centre clinical trial and a pharmaceutical company trial are nearing completion (http://clinicaltrials.gov/ct2/show/record/NCT00822900 and http://www.synapse-trial.com/). In light of the evidence for progesterone’s safety and efficacy in different brain injury models, and despite the fact that stroke and traumatic brain injury have different aetiologies, progesterone may prove to be an attractive therapeutic candidate for stroke. However, before it can be tested in clinical trial, more parametric data are needed to support an Investigational New Drug (IND) application. At present, a systematic preclinical progesterone dose-response and time-window study in clinically relevant ischaemic stroke models is lacking.
The variability of preclinical evaluation and numerous clinical trial failures culminated in the Stroke Therapy Academic Industrial Roundtable (STAIR) guidelines for the preclinical evaluation of candidate drugs (Fisher, 2003; Fisher et al., 2005, 2009; Savitz et al., 2011). One STAIR recommendation calls for evaluating dose-response and treatment time-window studies using focal permanent middle cerebral artery occlusion (MCAO) in older animals to better simulate the typical human stroke injury without reperfusion. STAIR also calls for assessing histological and functional outcome measures over an extended period to ensure that early treatment effects are not transient. The present study sought to address these recommendations and determine progesterone’s dose- and time-response effects on infarct size and behavioural performance on a variety of clinically relevant tests given after permanent MCAO.
Materials and methods
One hundred and twenty-six 12-month-old virgin male Sprague-Dawley rats (Harlan) underwent permanent MCAO by electrocoagulation or sham operation. We used virgin male rats instead of retired breeders because, in our preliminary study, training older retired breeders on behavioural measures was challenging, with wide variation in behavioural performance. We calculated the starting sample sizes to be at least eight animals/group to reject the null hypothesis (of no differences among the treatment groups relative to untreated controls) at a power of 0.8 with a P-value of 0.05. The animals were housed in individual cages with free access to pellet chow and water, quarantined for 1 week, and handled at least five times before starting training. Room temperature was maintained at 21–25°C, and humidity at 45–50%. Rats were maintained under a 12:12-h reverse light–dark cycle (0900–2100h) so that behavioural testing would occur during their active phase. All experimental animal procedures were approved by the Emory University Institutional Animal Care and Use Committee (IACUC), Protocol # 2001517.
Treatment
For the dose-response experiments, 51 animals underwent and 46 survived permanent MCAO by electrocoagulation or sham operation and were randomly assigned to one of five groups: sham-vehicle (n = 10), permanent MCAO-vehicle (n = 9), progesterone 8 mg/kg (P8) (n = 9), progesterone 16 mg/kg (P16) (n = 9), or progesterone 32 mg/kg (P32) (n = 9). Both the permanent MCAO and sham control groups received 22.5% 2-hydroxypropyl-β-cyclodextrin (HBC). Each dose of progesterone (P-0130; Sigma-Aldrich) was administered in equal volumes relative to body weight (a volume of 2 ml/kg body weight was necessary to dissolve all doses of progesterone). One hour post-permanent MCAO, the animals were given an intraperitoneal injection of one of the three doses (8, 16 or 32 mg/kg) of progesterone, followed by subcutaneous injections at 6 h post-injury and then every 24 h for the next 7 days. The dose was tapered over the final two treatments. Permanent MCAO-vehicle and sham control groups received equal volumes of HBC relative to body weight. One rat each in the vehicle and P32 groups died at 21 and 9 days post-permanent MCAO, respectively.
In the time-window experiments, 75 animals underwent and 72 animals survived permanent MCAO or sham operation and were randomly assigned to one of five groups: progesterone treatment begun at 3 h (n = 8), 6 h (n = 8), or 24 h (n = 8), permanent MCAO-vehicle [n = 24, combined from 3 h (n = 8), 6 h (n = 8), and 24 h (n = 8) delay experiments], or sham-vehicle [n = 24, combined from 3 h (n = 8), 6 h (n = 8), and 24 h (n = 8) delay experiments]. The first dose of progesterone (8 mg/kg) was given intraperitoneally to ensure more rapid absorption followed by subcutaneous injections 5 h later and then every 24 h for the next 7 days. The last two doses were tapered. For the purposes of blinding, separate vehicle-treated permanent MCAO and vehicle-treated sham groups with delays in post-stroke injections of 3, 6 and 24 h were used in the time-window study. Animals were euthanized at 23 days post-injury and their brains removed and cryoprotected for infarct size measurements. Behavioural training for Rotarod and gait scan tests was started 1 week before surgery. Baseline testing for all behavioural parameters was performed a day before surgery. One rat each in the vehicle- and progesterone-treated groups died at 8 and 10 days post-permanent MCAO, respectively.
Induction of permanent focal cerebral ischaemia
Permanent MCAO was induced as previously described (Tamura et al., 1981; Yamamoto et al., 1988) with minor modifications to produce consistency in the ischaemic injury. All rats were anaesthetized using 5% isoflurane and maintained at 1.5–2% (2:1 nitrous oxide and oxygen) during surgery. The incision area was shaved and sterilized with Betadine® antiseptic and 70% isopropanol. A ventral midline incision was made for exposure of both common carotid arteries. The contralateral right common carotid artery was permanently ligated using a 3/0 silk suture, and the left ipsilateral common carotid artery was temporarily occluded for 90 min using a Mayfield micro-aneurysm clip. A vertical incision was made midway between the left orbit and the left external auditory canal. The left temporalis muscles were separated and retracted interiorly downward to expose the zygomatic and squamosal bone and then the pterygoid muscles and mandibular nerve were retracted to expose the ventral surface of the skull. Under an operating microscope, the bone around the foramen ovale was burnished (2–3 mm) to expose the middle cerebral artery, and the craniotomy was extended dorsally up to the first major branch of the middle cerebral artery. Care was taken to avoid thermal and physical injury to the cortex during preparation for exposing the middle cerebral artery. The dura was opened with a bent 26-gauge needle, the arachnoid membrane was gently removed and the middle cerebral artery cauterized and cut permanently to prevent recanalization with a bipolar electrocauterizer without damaging the brain surface. The site of the occlusion is midway between the inferior cerebral vein and olfactory tract. Sutures were used to close the incision after bleeding stopped. The rats were allowed to recover from anaesthesia on the heating pad and then returned to their home cages. After 90 min of temporary occlusion, the micro-aneurysm clip was removed from the left ipsilateral side and rats were again permitted to recover from anaesthesia on the heating pad. Sham-operated rats were subjected only to exposure of the middle cerebral artery without coagulation. Physiological parameters were monitored with pulse oximetry (SurgiVet™ model V3304): heart rate was maintained around 350 beats/min and blood oxygen level (SpO2) was kept >95%. Core body temperature (37 ± 2° C) was maintained with a homoeothermic heating blanket system (Harvard Apparatus). Sham animals were anaesthetized and a ventral midline incision was made for exposure of both common carotid arteries. After this, a vertical incision was made midway between the left orbit and the left external auditory canal. Then the incision was sutured closed.
Assessment of functional outcome
Behavioural recovery was evaluated at repeated time intervals on locomotor activity, grip strength, ability to remain on an accelerating Rotarod, sensory neglect, gait impairment, spatial navigation, learning and memory. All functional assessments were done before giving daily hormone treatments to avoid any possibility of progesterone ‘intoxication’. Throughout the injections, behavioural testing, and histological analysis, experimenters were blinded to the experimental conditions of the animals.
Grip strength
To evaluate the rats’ forelimb grip strength, we used an electronic digital force gauge grip-strength meter (Columbus Instruments) that measured the peak force exerted by the animal while gripping the sensor bar as previously described (Ishrat et al., 2009). A digital reading (in Newtons (N)) of three successive trials was obtained for each rat, and the best (highest) score was used for data analysis. Baseline was determined 1 day before ischaemia and at post-ischaemia Days 2, 6, 9 and 22 for the dose-response study and Days 3, 9 and 21 for the time-window study. The best (highest) score was used for data analysis.
Motor impairment
Motor impairment was assessed with an accelerating Rotarod (Columbus Instruments). Rats were given five training sessions 5 min apart before establishing baseline as described previously (Ishrat et al., 2009). Two trials were given to each animal and the best (longest) latency values were used for analysis. Latency to fall off the Rotarod was determined before ischaemia and at post-surgery Days 3, 7, 10, and 21 days for the dose-response study, and 4, 10, and 22 days for the time-window study. Animals were given equal rest time between the two trials.
Somatosensory neglect
An adhesive removal test was performed as previously described (Yousuf et al., 2013) to evaluate somatosensory function. Habituation and testing occurred under red light. Adhesive (0.5-in round) labels were placed on the ventral surface of the contralateral forepaw. The experimenter recorded the latency for each rat to remove the adhesive label with its mouth up to a maximum latency of 2 min. Testing was done 1 day before surgery and after surgery at Days 3, 7, 10 and 21 for the dose-response and 4, 10 and 22 days for the time-window study.
Locomotor activity
Rats were tested for open field activity under red light in a quiet environment. For each trial, two animals were tested simultaneously in individual boxes using the Opto-Varimex© activity monitoring system (Columbus Instruments). The rats were tested 1 day before injury, and at post-surgery Days 2, 6, 9 and 22 for the dose-response and Days 3, 9 and 21 for the time-window study.
Spatial navigation
To evaluate learning, memory and spatial navigation after permanent MCAO, Morris water maze tests were conducted as previously described (Wali et al., 2011). Testing began at Day 13 after surgery for both dose-response and time-window studies. A probe trial was conducted at Day 21 post-injury for both dose-response and time-window studies. The hidden platform was removed and rats were placed in the pool once for 60 s starting from the same location as in the previous 5 days of testing. The time spent swimming in the quadrant where the platform was located was recorded and used for final analysis.
Gait analysis
The CatWalk (Noldus Information Technology), a quantitative gait analysis system, was used for assessment of gait-related anomalies after stroke (Wang et al., 2008; Lubjuhn et al., 2009). A fluorescent light is reflected internally in the glass floor of the walkway when the animal crosses the walkway. The video images with paw prints were classified by assigning labels (e.g. right and left, fore and hind paw) automatically. The experiment was performed in red light. Before surgery all animals were trained to traverse a glass walkway toward their home cages from one end to another without interruptions, so that all the animals started from more or less the same baseline. On subsequent training days, three complete runs across the walkway were recorded. If an animal failed to complete a run, walked backwards, or reared during the run, it was given an additional run. Baseline was taken 1 day before surgery and at post-surgery Days 2, 6 and 21 for the dose-response and Days 3, 9 and 21 for the time-window study.
Assessment of infarct volume
Cerebral infarct size was assessed using previously applied methods (Ishrat et al., 2010). The infarct areas, defined as areas showing reduced Nissl staining under light microscopy, were traced and quantified by digital image-analysis system (Image Pro System, Media Cybernatics), and expressed as a percentage of the contralateral side ± standard error of the mean (SEM).
Data analysis
As noted, based on a delta-value of 1.5 we calculated the sample sizes and power needed to reject the null hypothesis to achieve >80% power to detect a 50% difference. The number of rats per group at these criteria was determined to be at least eight. All results were expressed as mean ± SEM and calculations were obtained using SPSS 11.0 software. All behavioural data were analysed by repeated-measures ANOVA, followed by a Tukey post hoc test. Other results were analysed with one-way ANOVA followed by least significant difference and Tukey honestly significant difference post-test for multiple comparisons. The criterion for statistical significance was set at P < 0.05.
Results
Dose-response effects of progesterone in attenuating functional deficits after permanent middle cerebral artery occlusion
Grip strength
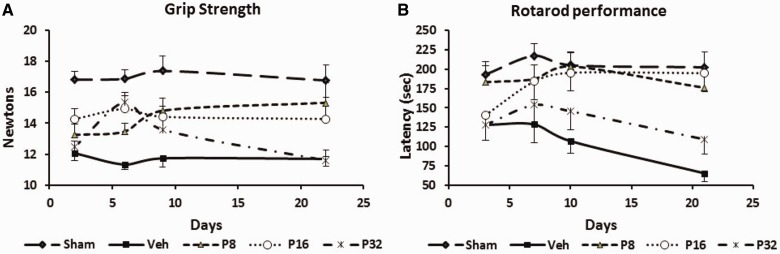
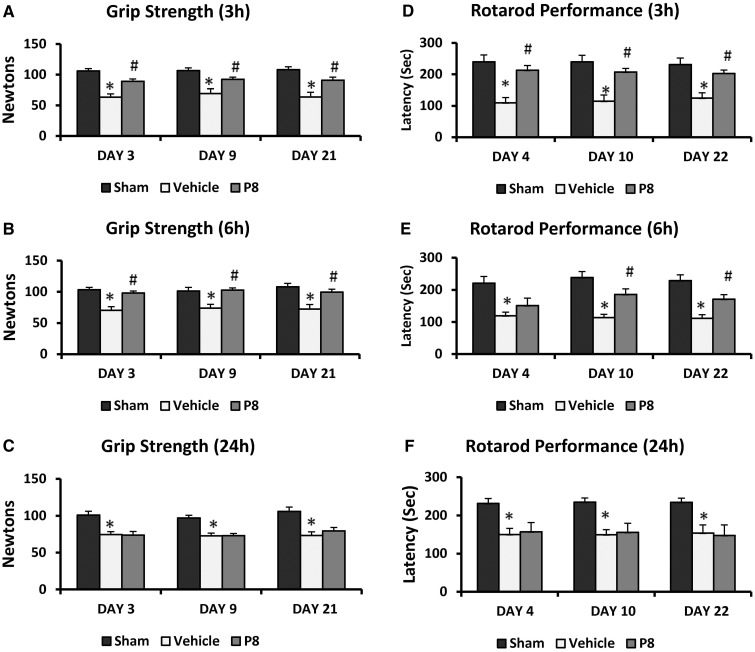
The maximum digital reading (in N) of three successive trials obtained for each rat was used as the dependent variable. Progesterone improved grip strength after permanent MCAO (Fig. 1A). There were significant group [F(4,41) = 17.15, P < 0.001] effects. Grip strength decreased significantly (P < 0.05) in rats subjected to permanent MCAO (12.07 ± 0.49 N, 11.33 ± 0.34 N, 11.74 ± 0.58 N, 11.70 ± 0.47 N at 2, 6, 9 and 22 days post-occlusion, respectively) at all time points compared with intact rats (16.82 ± 0.54 N, 16.88 ± 0.58 N, 17.38 ± 0.95 N, 16.77 ± 0.98 N at 2, 6, 9 and 22 days post-surgery, respectively). Post hoc analyses showed that repeated treatments with 8 mg/kg progesterone after permanent MCAO significantly (P < 0.05) improved grip strength at 6, 9 and 22 days.
Figure 1.
Dose-response effect of progesterone on permanent MCAO-induced grip strength and motor deficits. (A) Grip strength: the rats subjected to permanent MCAO + vehicle showed significantly (P < 0.05) lower grip-strength scores at all time points compared with sham + vehicle rats. Progesterone-treated rats showed significant improvement in average scores compared to permanent MCAO + vehicle-treated animals. (B) Motor impairment as assessed using the Rotarod after permanent MCAO: rotometric performance significantly (P < 0.05) decreased in rats subjected to permanent MCAO + vehicle compared with sham-operated + vehicle rats. Progesterone-treated rats were significantly less impaired than vehicle-treated rats at all time points. Values are expressed as mean ± SE.
Rotarod performance
Measures of latency to remain on the Rotarod (in seconds (s)) showed significant group [F(4,40) = 6.43, P < 0.05] and time [F(4,160) = 2.90, P < 0.05] effects. There were significant (P < 0.05) deficits (Fig. 1B) in motor performance (time spent on the Rotarod) in permanent MCAO-vehicle-treated rats (127.89 ± 19.59 s, 128.67 ± 23.88 s, 106.89 ± 15.77 s, 65.33 ± 10.98 s at 3, 7, 10 and 21 days post-occlusion, respectively) at all time points compared with shams (193.10 ± 16.59 s, 217.40 ± 15.37 s, 205.10 ± 17.34 s, 202.40 ± 20.05 s at 3, 7, 10 and 21 days post-occlusion, respectively). Post hoc analyses showed that repeated treatments with 8 mg/kg progesterone after permanent MCAO significantly (P < 0.05) improved ability to remain on the Rotarod (183.25 ± 21.00 s, 187.25 ± 18.09 s, 203.62 ± 17.69 s, 175.87 ± 26.17 s) at 3, 7, 10 and 21 days.
Somatosensory neglect
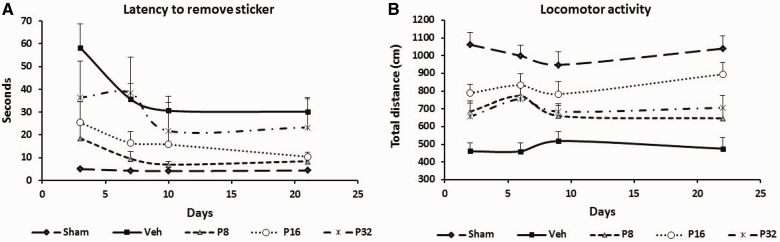
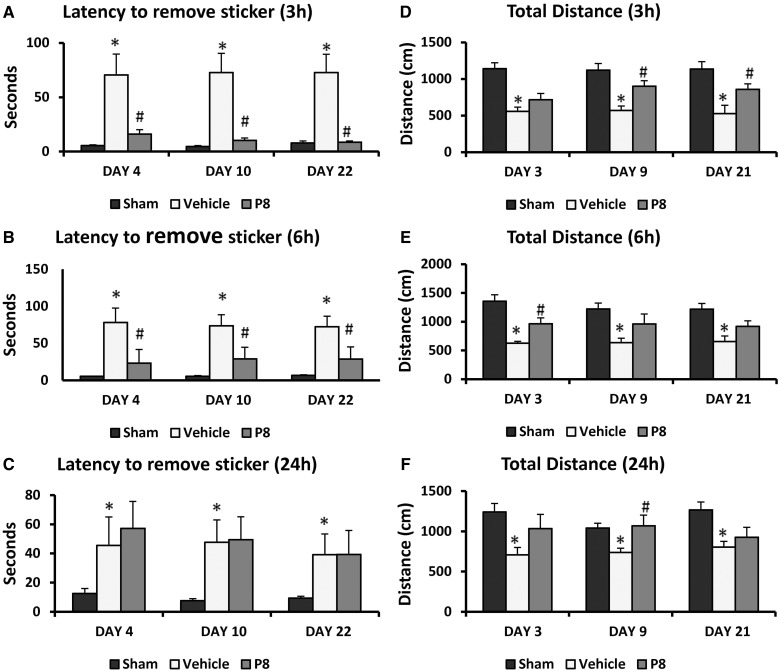
Latency to remove sticky tape from the contralateral forepaw showed significant group [F(4,41) = 4.42, P = 0.005] effects. There were significant (P < 0.05) deficits (Fig. 2A) in latency to remove the sticky tape in permanent MCAO-vehicle-treated rats (58.33 ± 10.47 s, 35.67 ± 6.70 s, 30.67 ± 6.29 s, 25.78 ± 6.02 s at 3, 7, 10 and 21 days post-occlusion, respectively) at all time points compared to shams (5.00 ± 0.69 s, 4.30 ± 0.47 s, 4.10 ± 0.38 s, 4.40 ± 0.49 s at 3, 7, 10 and 21 days post-occlusion, respectively). Repeated treatments with progesterone (8 mg) after permanent MCAO significantly (P < 0.05) decreased the latency to remove the sticker (18.56 ± 7.73 s, 9.56 ± 3.1 s, 7.00 ± 1.24 s, 8.44 ± 1.76 s) at all time points (3, 7, 10 and 21 days). Post hoc analyses showed that treatment with 16 and 32 mg were not significantly effective on this measure of performance.
Figure 2.
Dose-response effect of progesterone on permanent MCAO-induced sensory neglect and locomotor activity. (A) Sensory neglect: mean latencies to remove stickers from contralateral forepaws (sensory neglect task) after permanent MCAO: progesterone reduces sensory impairment after permanent MCAO. (B) Locomotor activity: total distance travelled after permanent MCAO. Mean post-stroke locomotor activity at Days 2, 6, 9 and 22 showed increased total distance in progesterone-treated animals. Values are expressed as mean ± SE.
Locomotor activity
Open field activity was measured after permanent MCAO. Total distance travelled showed significant group [F(4,41) = 14.63, P < 0.0001] effects. There was a significant (P < 0.05) decrease (Fig. 2B) in total distance travelled by the vehicle-treated rats (461.44 ± 46.41 cm, 461.00 ± 46.18 cm, 519.11 ± 54.35 cm, 476.33 ± 60.64 cm at 2, 6, 9 and 22 days post-injury, respectively) compared with intact controls (1062.60 ± 66.51 cm, 999.60 ± 59.33 cm, 948.60 ± 72.16 cm, 1039.90 ± 71.04 cm at 2, 6, 9 and 22 days, respectively). Post hoc analyses showed that treatment with 16 mg progesterone significantly (P < 0.05) increased the total distance traversed on all days compared with vehicle. The increase in the progesterone-treated group at 32 mg did not show any significant effect except on Day 6.
Spatial memory
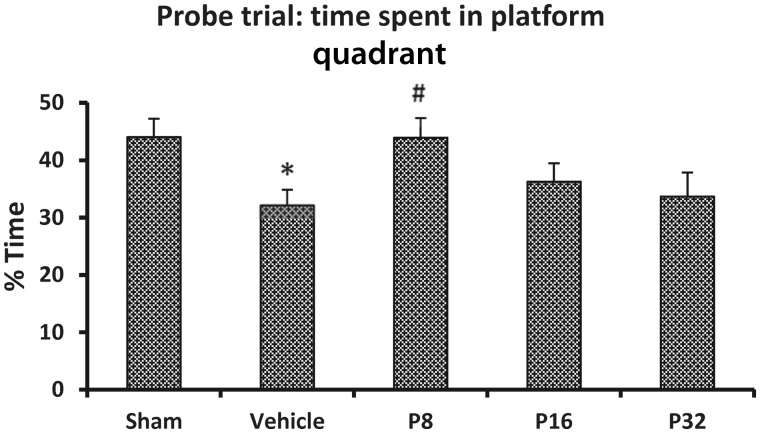
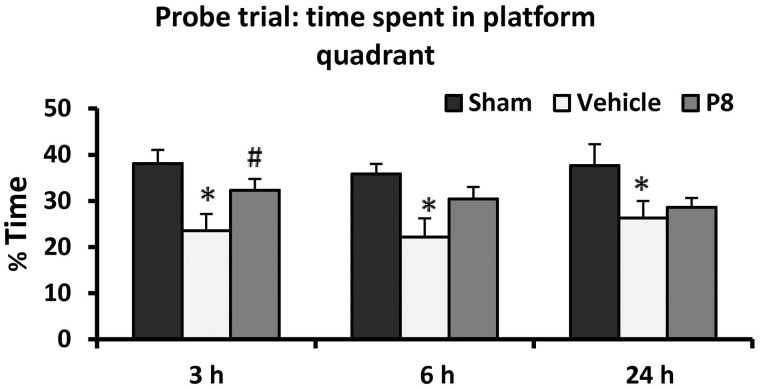
There was a significant difference among the treatment groups [F(4,40) = 2.7, P < 0.05]. Post hoc analyses showed that subjects in the stroke + vehicle-treated group spent significantly less time in the platform quadrant (32.12 ± 2.7%) compared to the sham group (43.98 ± 3.21%; P < 0.05). Although all progesterone-treated groups showed a trend towards more time spent in the platform quadrant (43.88 ± 3.42%, 36.24 ± 3.24%, 33.69 ± 4.20%, respectively) compared with vehicle-treated rats, post hoc analysis showed that the rats given 8 mg/kg were significantly better than the permanent MCAO-vehicle group (P < 0.05) (Fig. 3).
Figure 3.
Dose-response effect of progesterone on a memory task as assessed by per cent time spent in the platform quadrant during Morris water maze probe trial. Values are expressed as mean ± SE. *Permanent MCAO + vehicle versus sham + vehicle; #Permanent MCAO + vehicle versus permanent MCAO + progesterone.
Gait impairment
Gait changes are considered reliable indices of stroke severity and efficacy of rehabilitative therapies in humans. We assessed changes in gait using an automated computer-assisted gait analysis system and observed significant differences on the following gait parameters:
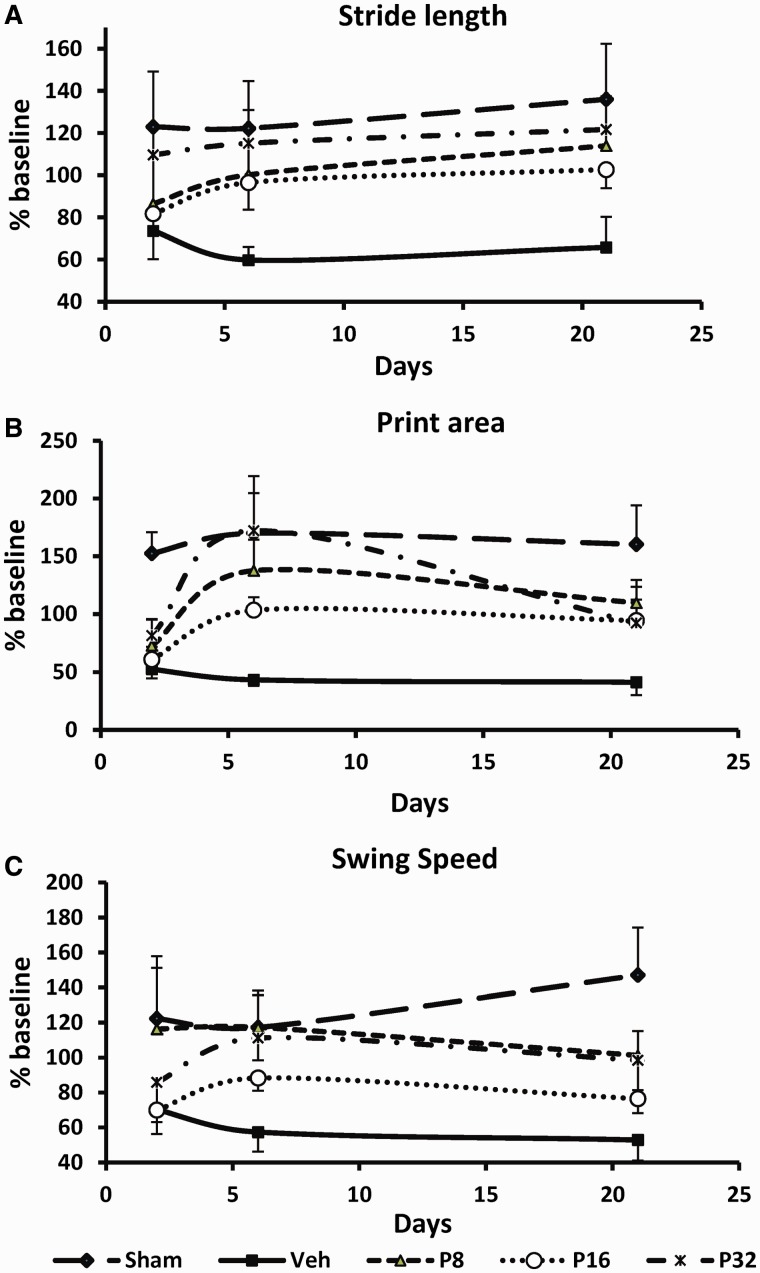
Stride length is the distance between successive placements of the same paw and is a measure of normal performance. After permanent MCAO, stride length significantly decreased in the affected forepaw (Fig. 4A). Progesterone treatment improved forepaw stride length of the affected (contralateral) limb. The measures of stride length (% baseline) of the contralateral forepaw showed significant group [F(4,40) = 2.75, P = 0.05] effects. All progesterone groups showed greater stride length with the affected contralateral hind limb compared with stroke + vehicle-treated rats but the observed differences were significant at 6 and 21 days post-permanent MCAO.
Figure 4.
Dose-response effect of progesterone on gait impairment after permanent MCAO. (A) Stride length; (B) print area; (C) swing speed as assessed at 2, 6 and 21 days after permanent MCAO. Values are expressed as mean ± SE.
Paw print area is a measure of spasticity. Permanent MCAO led to a persistent reduction of maximal paw contact area. The print area at maximal contact of contralateral forepaws (right paw) of rats during floor contact was significantly smaller than that of sham rats at 2, 6 and 21 days after ischaemia (Fig. 4B). Measures of paw print area covered (% baseline) of the contralateral forepaw showed significant group [F(4,40) = 4.7, P = 0.05] effects. All progesterone-treated rats showed greater contact area with the contralateral forelimb.
Swing speed is the speed (distance unit/s) of the paw during swing and is a measure of normal gait. Swing speed significantly decreased in the affected forepaw at all the measured time points after permanent MCAO (Fig. 4C). Measures of swing speed (% baseline) of the contralateral forepaw showed significant group [F(4,40) = 2.65, P < 0.05] effects. The rats treated with 8 mg/kg progesterone showed significant improvement compared to the permanent MCAO-vehicle group (P < 0.05).
Progesterone attenuates infarct volume after permanent middle cerebral artery occlusion
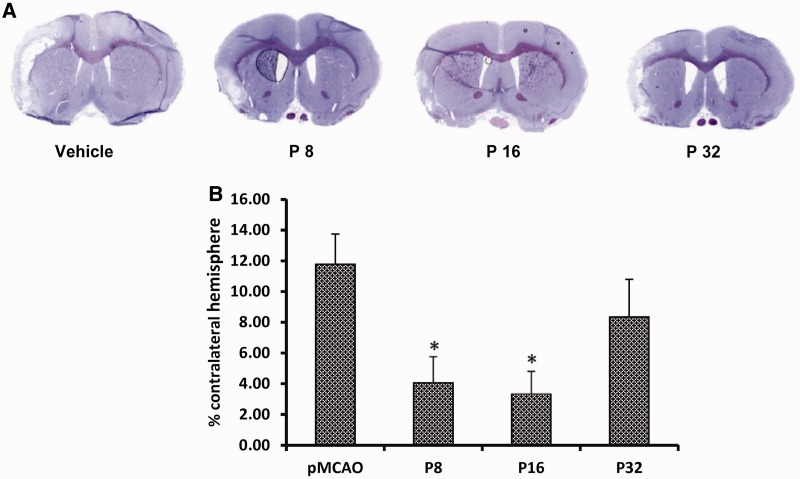
Figure 5A shows representative brain slices stained with cresyl violet 23 days after permanent MCAO in vehicle- and progesterone-treated rats. ANOVA showed a difference among the treatment groups [F(3,24) = 4.14, P < 0.05]. Post hoc analysis showed that 8 mg/kg progesterone (P8) and 16 mg/kg progesterone (P16) produced significant attenuation in infarct volume compared to the stroke + vehicle only group (4.05 ± 1.71 and 3.34 ± 1.47 versus 11.78 ± 1.97, respectively; Fig. 5B). The decrease in the 32 mg/kg (P32) group (8.34 ± 2.91) was not significant.
Figure 5.
Dose-response effect of progesterone on infarct volume. (A) Cresyl violet-stained coronal sections from representative animals given either vehicle or progesterone, with brains harvested at 23 days post-occlusion. Infarcts are shown as pale (unstained) regions involving the cortex. The infarct area in progesterone-treated animals is substantially reduced. (B) Infarct volumes after 23 days of occlusion. Compared to vehicle alone, P8 and P16 significantly reduced infarct volumes (% of contralateral hemisphere). The data are represented as mean ± SE; *P < 0.05 = significant difference compared with permanent MCAO + vehicle.
Therapeutic time window for progesterone in attenuating functional deficits after permanent middle cerebral artery occlusion
Grip strength
Three hour delay of treatment
Progesterone improved grip strength after permanent MCAO (Fig. 6A). There was a significant group [F(2,21) = 19.43, P < 0.001] effect. Grip strength decreased significantly (P < 0.05) in stroked rats (63.34 ± 5.53 N, 69.46 ± 7.70 N, 63.74 ± 7.97 N at 3, 9 and 21 days post-occlusion, respectively) at all time points compared to intact rats (105.89 ± 3.95 N, 106.67 ± 4.31 N, 108.06 ± 4.84 N at 3, 9 and 21 days post-surgery, respectively). Post hoc analyses showed 3-h delayed and repeated treatments with progesterone given after permanent MCAO significantly (P < 0.001) improved grip strength at all days after injury (89.05 ± 4.01 N, 92.28 ± 4.31 N, 90.78 ± 5.26 N at 3, 9 and 21 days post-surgery, respectively).
Figure 6.
Effect of delayed progesterone on permanent MCAO-induced grip strength and motor deficit. (A) 3-h; (B) 6-h; and (C) 24-h delay of progesterone treatment. Values are expressed as mean ± SE. Motor impairment as assessed using the Rotarod. (D) 3-h; (E) 6-h; and (F) 24-h delayed progesterone treatment. Time spent on the Rotarod is expressed as per cent of pre-surgery value (mean ± SE). *Permanent MCAO + vehicle versus sham + vehicle; #Permanent MCAO + vehicle versus permanent MCAO + progesterone.
Six hour delay
Our analysis revealed a significant group [F(2,21) = 13.67, P < 0.001] effect. Grip strength decreased significantly (P < 0.05) in permanent MCAO rats (70.41 ± 5.80 N, 73.70 ± 6.21 N, 72.30 ± 7.37 N at 3, 9 and 21 days post-occlusion, respectively) at all time points compared to intact rats (103.32 ± 3.74 N, 101.33 ± 5.89 N, 107.97 ± 5.72 N at 3, 9 and 21 days post-surgery, respectively) (Fig. 6B). Post hoc analyses showed 6-h delayed and repeated treatments with 8 mg/kg progesterone after permanent MCAO significantly (P < 0.001) improved grip strength (98.00 ± 3.27 N, 102.77 ± 3.61 N, 99.48 ± 4.72 N at 3, 9 and 21 days post-surgery, respectively) at all days after injury.
Twenty-four hour delay
There was a significant group [F(2,21) = 20.46, P < 0.001] effect (Fig. 6C). Post hoc analyses showed 24-h delayed repeated treatments with progesterone after permanent MCAO led to no significant improvement in grip strength at any time tested.
Performance on the Rotarod
Three hour delay of treatment
The measures of latency to remain on the Rotarod showed significant group differences [F(2,21) = 15.39, P < 0.001]. There were significant (P < 0.001) deficits (Fig. 6D) in motor performance (time spent on the Rotarod) in permanent MCAO-vehicle-treated rats (109.75 ± 16.57 s, 114.62 ± 20.02 s, 124.50 ± 17.26 s at 4, 10 and 22 days post-occlusion, respectively) at all time points compared with shams (240.00 ± 21.59 s, 239.62 ± 20.76 s, 231.12 ± 20.84 s at 4, 10 and 22 days post-occlusion, respectively). Post hoc analyses found that repeated treatments with progesterone after permanent MCAO significantly (P < 0.001) improved ability to remain on the Rotarod (212.87 ± 15.34 s, 207.00 ± 12.09 s, 202.87 ± 10.63 s) at all time points (4, 10 and 22 days, respectively). The most substantial deficit (46.61% compared with sham) in motor strength was observed in the vehicle-treated group compared to intact rats at 10 days post-surgery.
Six hour delay
Measures of latency to remain on the Rotarod showed significant group [F(2,21) = 16.06, P < 0.001] effects. There were significant (P < 0.05) deficits (Fig. 6E) in time spent on the Rotarod in permanent MCAO-vehicle-treated rats (119.25 ± 11.43 s, 113.81 ± 10.21 s, 111.56 ± 11.11 s at 4, 10 and 22 days post-occlusion, respectively) at all time points compared with shams (221.00 ± 20.85 s, 238.25 ± 18.98 s, 228.75 ± 18.28 s at 4, 10 and 22 days post-occlusion, respectively). Post hoc analyses showed that repeated treatments with progesterone after permanent MCAO significantly (P < 0.05) improved ability to remain on the Rotarod (151.00 ± 23.13 s, 185.37 ± 17.83 s, 170.75 ± 14.16 s at 4, 10 and 22 days, respectively) at 10 and 22 days post-surgery.
Twenty-four hour delay
ANOVA on latency to remain on the Rotarod showed significant group [F(2,19) = 19.46] effects. There were significant (P < 0.05) deficits (Fig. 6F) in time spent on the Rotarod in permanent MCAO-vehicle-treated rats (150.00 ± 16.00 s, 149.43 ± 13.40 s, 153.86 ± 21.42 s at 4, 10 and 22 days post-occlusion, respectively) at all time points compared with shams (231.62 ± 12.86 s, 234.62 ± 11.13 s, 234.50 ± 10.77 s at 4, 10 and 22 days post-occlusion, respectively). The 24-h delayed progesterone treatment group showed no significant improvement in motor performance on the Rotarod after permanent MCAO (157.00 ± 24.23 s, 155.43 ± 23.84 s, 147.43 ± 27.86 s at 4, 10 and 22 days, respectively) on any testing days.
Sensory neglect
Three hour delay of treatment
Measures of latency to remove sticky tape from the contralateral forepaw showed significant group [F(2,21) = 15.73] effects. There was a significant (P < 0.05) increase in latency to remove the stickers in permanent MCAO-vehicle-treated rats (70.50 ± 19.24 s, 72.75 ± 17.70 s, 72.75 ± 16.88 s at 4, 10 and 22 days post-occlusion, respectively) compared to shams (5.50 ± 0.76 s, 4.75 ± 0.818 s, 7.87 ± 1.83 s at 4, 10 and 22 days post-occlusion, respectively) (Fig. 7A). A 3-h delay of progesterone treatment after permanent MCAO significantly (P < 0.05) decreased latency to remove the sticker (16.12 ± 4.015 s, 10.25 ± 2.24 s, 8.50 ± 1.25 s) at all time points.
Figure 7.
Effect of delayed progesterone treatment on permanent MCAO-induced sensory neglect and locomotor deficit. Mean latencies to remove stickers from contralateral forepaws (sensory neglect task): (A) 3-h; (B) 6-h; (C) 24-h delay of progesterone treatment. Total distance travelled after permanent MCAO: (D) 3-h; (E) (6-h); (F) 24-h delayed progesterone treatment. Values are expressed as mean ± SE. *Permanent MCAO + vehicle versus sham + vehicle; #Permanent MCAO + vehicle versus permanent MCAO + progesterone.
Six hour delay
ANOVA on latency to remove sticky tape from the contralateral forepaw showed significant group [F(2,21) = 9.71] effects. There was a significant (P < 0.05) increase in latency to remove the stickers in permanent MCAO-vehicle-treated rats (78.25 ± 20.37 s, 73.75 ± 18.95 s, 72.50 ± 16.74 s at 4, 10 and 22 days post-occlusion, respectively) compared with shams (5.50 ± 0.38 s, 5.62 ± 0.80 s, 6.87 ± 0.77 s at 4, 10 and 22 days post-occlusion, respectively). With the 6-h delay, repeated progesterone treatments after permanent MCAO significantly (P < 0.05) decreased the time taken to remove the sticker (23.37 ± 9.63 s, 29.00 ± 11.34 s, 28.75 ± 13.45 s) at all time points (Fig. 7B).
Twenty-four hour delay
Our analyses showed that 24-h delayed and repeated treatments with progesterone did not result in significant improvement in ability to remove sticky tape from the contralateral forepaw on any of the days tested (Fig. 7C).
Locomotor activity
Three hour delay of treatment
Analysis of total distance travelled showed significant group [F(2,22) = 21.49, P < 0.05] effects. There was a significant (P < 0.05) decrease in total distance travelled by the vehicle-treated rats (557.62 ± 56.73 cm, 569.12 ± 61.65 cm, 527.12 ± 114.45 cm at 3, 9 and 21 days post-injury, respectively) compared with intact controls (1141.12 ± 79.90 cm, 1121.75 ± 89.39 cm, 1135.12 ± 101.13 cm at 3, 9 and 21 days, respectively; Fig. 7D). Post hoc analyses showed that 3-h delayed treatments with progesterone after permanent MCAO significantly (P < 0.05) improved spontaneous activity (716.78 ± 85.31 cm, 901.55 ± 75.20 cm, 858.44 ± 75.39 cm) at all time points.
Six hour delay
ANOVA on total distance travelled showed significant group [F(2,22) = 15.27, P < 0.05] effects. There was a significant (P < 0.05) decrease in total distance travelled by the vehicle-treated rats (626.50 ± 32.19 cm, 635.87 ± 78.14 cm, 654.12 ± 96.53 cm at 3, 9 and 21 days post-injury, respectively) compared with intact controls (1355.37 ± 114.51 cm, 1219.62 ± 105.35 cm, 1219.12 ± 99.54 cm at 3, 9 and 21 days, respectively). Post hoc analyses showed that 6-h delayed treatments with progesterone after permanent MCAO significantly (P < 0.05) improved spontaneous activity (716.78 ± 85.31 cm, 901.55 ± 75.20 cm, 858.44 ± 75.39 cm) at all time points (Fig. 7E).
Twenty-four hour delay
We observed a significant group [F(2,20) = 8.14, P < 0.05] effect on total distance travelled. There was a significant (P < 0.05) decrease in total distance travelled by the vehicle-treated rats (707.50 ± 91.00 cm, 737.37 ± 53.43 cm, 803.25 ± 71.14 cm at 3, 9 and 21 days post-injury, respectively) compared with intact controls (1240.00 ± 105.32 cm, 1039.87 ± 60.89 cm, 1265.87 ± 97.85 cm at 3, 9 and 21 days, respectively). Improvement in spontaneous activity with 24-h delayed treatments with progesterone after permanent MCAO was 716.78 ± 85.31 cm, 901.55 ± 75.20 cm, 858.44 ± 75.39 cm at 3, 9 and 21 days, respectively (Fig. 7F).
Spatial memory
Three hour delay of treatment
There was a significant difference among the treatment groups [F(2,20) = 5.8, P < 0.01]. Post hoc analyses showed that subjects in the stroke + vehicle-treated group spent significantly less time (23.53 ± 3.61%) in the platform quadrant compared with the sham-vehicle-treated group (38.06 ± 2.94%, P < 0.05). Post hoc analysis showed that the 3-h delayed progesterone-treatment group (32.33 ± 2.41%) was significantly different from the permanent MCAO-vehicle group (P < 0.05) (Fig. 8).
Figure 8.
Delayed progesterone treatment effects on a memory task as assessed by per cent time spent in the platform quadrant during Morris water maze probe trial. Values are expressed as mean ± SE. *Permanent MCAO + vehicle versus sham + vehicle; #Permanent MCAO + vehicle versus permanent MCAO + progesterone.
Six hour delay
There was a significant difference among the treatment groups [F(2,20) = 5.22, P < 0.01]. Post hoc analyses showed that subjects in the stroke + vehicle-treated group spent significantly less time (22.19 ± 4.01%) in the platform quadrant compared to the sham group (35.82 ± 2.22%; P < 0.05). Post hoc analysis showed that the 6-h delayed progesterone-treatment group (30.39 ± 2.62%) was significantly different from the permanent MCAO-vehicle group (P < 0.05; Fig. 8).
Twenty-four hour delay
There was a significant difference among the treatment groups [F(2,20) = 2.66, P = 0.05]. Subjects in the stroke + vehicle-treated group spent less time (26.32 ± 3.7%) in the platform quadrant compared with the sham-vehicle-treated group (37.65 ± 4.60%). The 24-h delayed progesterone-treatment group (28.62 ± 1.99%) was not significantly different from the permanent MCAO-vehicle group (Fig. 8).
Gait impairment
Stride length
Three hour delay of treatment
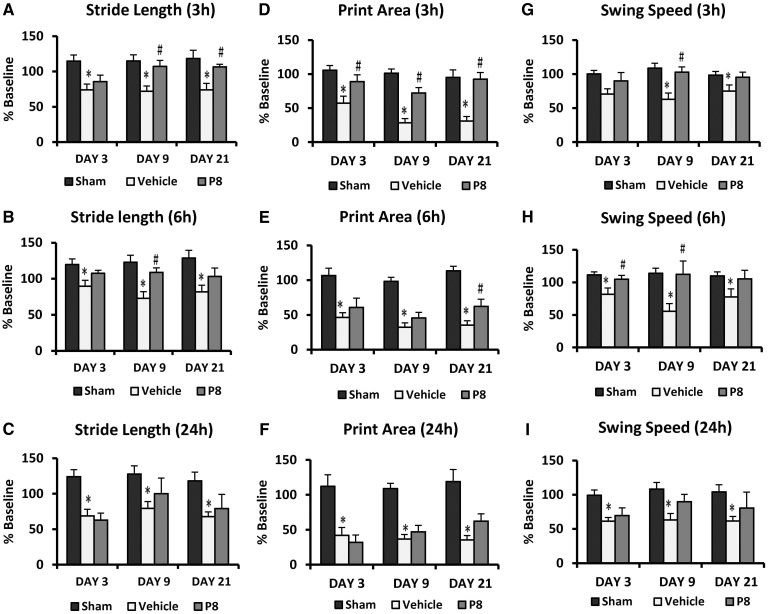
The measures of stride length (% baseline) of the contralateral forepaw showed significant group [F(2,21) = 9.65, P < 0.05] effects (Fig. 9A). At all time points, stride length in the affected hind limb decreased significantly (P < 0.05) in rats subjected to permanent MCAO (74.07 ± 8.21%, 71.93 ± 7.63%, 73.93 ± 9.38% at Day 3, 9 and 21 post-injury, respectively) compared with intact rats (114.64 ± 8.80%, 114.97 ± 8.75%, 118.28 ± 11.81% at Days 3, 9 and 21 post-injury, respectively). Progesterone treatment improved (85.60 ± 9.3%, 107.33 ± 8.35%, 106.36 ± 3.85% at Days 3, 9 and 21 post-injury, respectively) stride length of the affected limb (Fig. 9A). All progesterone groups showed significantly greater stride length with the affected contralateral fore limb compared to stroke + vehicle-treated rats at all testing days.
Figure 9.
Delayed progesterone treatment effect on permanent MCAO-induced gait impairments. (A–C) Stride length; (D–F) print area; (G–I) swing speed following 3-, 6-, and 24- h delayed progesterone treatment. Values are expressed as mean ± SE. *Permanent MCAO + vehicle versus sham + vehicle; #Permanent MCAO + vehicle versus permanent MCAO + progesterone.
Six hour delay
The measures of stride length (% baseline) of the contralateral forepaw showed significant group [F(2,21) = 10.43, P < 0.05] effects (Fig. 9B). At all time points, stride length decreased significantly (P < 0.05) in rats subjected to permanent MCAO (89.74 ±8.14%, 72.95 ± 9.15%, 81.87 ± 9.26% at Day 3, 9 and 21 post-injury, respectively) compared with intact rats (119.79 ± 7.76%, 122.83 ± 9.86%, 128.64 ± 10.95% at Days 2, 6, and 21 post-injury, respectively) in the affected hind limb. Progesterone treatment improved (85.60 ± 9.3%, 107.33 ± 8.35%, 106.36 ±3.85% at Days 3, 9 and 21 post-injury, respectively) stride length of the affected limb (Fig. 9B). All progesterone groups showed significantly greater stride length with the affected contralateral hind limb compared to stroke + vehicle-treated rats.
Twenty-four hour delay
The measures of stride length showed significant group [F(2,20) = 9.13, P < 0.05] effects (Fig. 9C). Stride length decreased significantly (P < 0.05) in rats subjected to permanent MCAO compared with intact rats in the affected forepaw, but progesterone treatment did not significantly improve stride length of the affected forelimb at any time points compared to vehicle.
Paw print area
Three hour delay of treatment
Repeated measures ANOVA on print area (% baseline) of contralateral forepaw showed a difference among the treatment groups [F(2,22) = 22.68, P < 0.05]. At all time points print area decreased (Fig. 9D) significantly (P < 0.05) in rats subjected to permanent MCAO (57.35 ± 10.19%, 28.42 ± 6.3%, 31.07 ± 6.55% at Days 3, 9 and 21 post-injury, respectively) compared with intact rats (105.69 ± 7.0%, 101.24 ± 6.36%, 94.99 ± 11.28% at Days 3, 9 and 21 post-injury, respectively) in the affected hind limb. Progesterone treatment improved (88.89 ± 10.16%, 72.21 ± 8.02%, 92.54 ± 9.89% at Days 3, 9 and 21 post-injury, respectively) print area of the affected limb. All progesterone groups showed significantly greater print area with the affected contralateral hind limb compared to stroke + vehicle-treated rats.
Six hour delay
Repeated measures ANOVA on print area (% baseline) of the contralateral forepaw showed a difference among the treatment groups [F(2,21) = 38.21, P < 0.05]. At all time points print area decreased (Fig. 9E) significantly (P < 0.05) in rats subjected to permanent MCAO (46.32 ± 6.89%, 32.14 ± 6.37%, 35.35 ± 6.26% at Days 3, 9 and 21 post-injury, respectively) compared with intact rats (106.66 ± 10.61%, 98.16 ± 6.02%, 113.47 ± 6.57% at Day 3, 9 and 21 post-injury, respectively) in the affected hind limb. Progesterone treatment improved (60.59 ± 13.38%, 45.58 ± 8.25%, 62.14 ± 10.46% at Days 3, 9 and 21 post-injury, respectively) print area of the affected limb. All progesterone groups showed significantly greater print area with the affected contralateral hind limb compared with stroke + vehicle-treated rats.
Twenty-four hour delay
Repeated measures ANOVA on print area (% baseline) of contralateral forepaw showed a difference among the treatment groups [F(2,21) = 32.25, P < 0.05]. At all time points print area decreased (Fig. 9F) significantly (P < 0.05) in rats subjected to permanent MCAO (41.90 ± 11.21%, 36.58 ± 6.54%, 37.87 ± 7.83% at Days 3, 9 and 21 post-injury, respectively) compared with intact rats (112.10 ± 16.47%, 108.87 ± 7.49%, 118.78 ± 17.24% at Days 3, 9 and 21 post-injury, respectively) in the affected hind limb. All progesterone groups showed a trend toward greater print area compared with the stroke + vehicle-treated group but the difference was not significant.
Swing speed
Three hour delay of treatment
Analysis of swing speed (% baseline) of the contralateral forepaw showed a difference among the treatment groups [F(2,22) = 6.55, P < 0.05]. At all time points, swing speed decreased (Fig. 9G) significantly (P < 0.05) in rats subjected to permanent MCAO (70.63 ± 7.64%, 62.92 ± 9.30%, 74.91 ± 9.16% at Days 3, 9 and 21 post-injury, respectively) compared with intact rats (100.31 ± 5.0%, 108.81 ± 7.08%, 98.38 ± 5.47% at Days 3, 9 and 21 post-injury) in the affected hind limb. Progesterone treatment improved (89.96 ± 12.37%, 102.72 ± 7.79%, 95.28 ±7.65% at Days 3, 9 and 21 post-injury, respectively) swing speed of the affected limb. All progesterone groups showed significantly greater swing speed compared with stroke + vehicle-treated rats.
Six hour delay
Analysis of swing speed (% baseline) of the contralateral forepaw showed a difference among the treatment groups [F(2,21) = 7.34, P < 0.05]. At all time points swing speed decreased (Fig. 9H) significantly (P < 0.05) in rats subjected to permanent MCAO (81.55 ± 9.61%, 55.59 ± 11.84%, 77.50 ± 12.51% at Days 3, 9 and 21 post-injury) compared to intact rats (111.36 ± 4.77%, 113.88 ± 7.81%, 109.83 ± 6.28% at Days 3, 9 and 21 post-injury, respectively) in the affected limb. Progesterone treatment improved (104.78 ± 5.90%, 112.32 ± 20.44%, 105.39 ± 13.08% at Days 3, 9 and 21 post-injury, respectively) swing speed of the affected limb. All progesterone groups showed significantly greater swing speed compared with stroke + vehicle-treated rats.
Twenty-four hour delay
Analysis of swing speed (% baseline) of the contralateral forepaw showed a difference among the treatment groups [F(2,20) = 7.98, P < 0.05]. Post hoc tests showed no significant difference in improvement with progesterone treatment on any days of testing (Fig. 9I).
Infarct volume
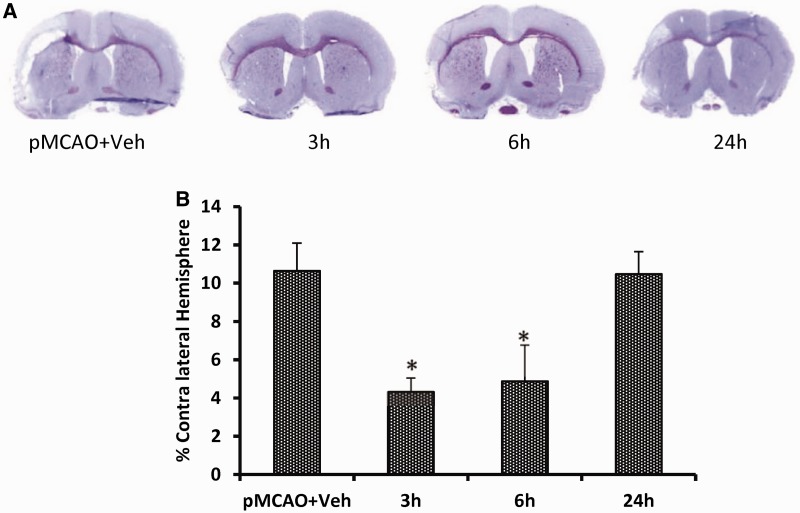
ANOVA showed a difference among the treatment groups [F(3,36) = 4.78, P < 0.05]. Post hoc analysis showed that 3- and 6-h delayed progesterone treatment produced significant attenuation in infarct volume (percent contralateral hemisphere) compared with the stroke + vehicle-only group (4.33 ± 0.72 and 4.87 ± 1.91 versus 10.65 ± 1.46, respectively). The decrease in the 24-h delay group (10.47 ± 1.17) was not significant (Fig. 10). Maximum decrease in infarct volume was observed for the 3-h delay group (59.29 %) compared with vehicle.
Figure 10.
Delayed progesterone treatment effects on infarct volume at 23 days post-occlusion. (A) Cresyl violet-stained coronal sections from representative animals given either vehicle or progesterone, with brains harvested at 22 days post-occlusion. Infarcts are shown as pale (unstained) regions involving the cortex. (B) Delayed progesterone treatment reduces infarct volume at 3 h and 6 h at 23 d post-permanent MCAO. Compared to vehicle alone, progesterone significantly reduced infarct volumes (% of contralateral hemisphere). Progesterone treatment begun at 24 h did not reduce the infarct volume. The data are represented as mean ± SE; *P < 0.01 = significant difference compared to permanent MCAO + vehicle.
Discussion
The most common type of focal stroke in humans is the permanent or thromboembolic occlusion of the cerebral arteries, especially MCAO (Delcker et al., 1993; Hacke et al., 1996). To simulate typical human stroke without reperfusion (Fisher et al., 2005, 2009), STAIR endorses the testing of agents in permanent models of ischaemia. Accordingly, we used permanent MCAO for our comprehensive dose-response and therapeutic time-window study.
Although progesterone has been shown to be neuroprotective in senescent rats with traumatic brain injury (Cutler et al., 2007; Wali et al., 2011), a recent review (Wong et al., 2013b), highlighted the need for experimental studies in aged animals, as most preclinical studies reporting progesterone efficacy in stroke models have used young, healthy animals. A recent American Heart Association report shows sharp increases in midlife stroke occurrence (Roger et al., 2012). By using middle-aged rats we took a more novel and clinically relevant approach compared with previous studies in younger animals, including our own, and assessed neuroprotection over a longer period of time.
Most studies report progesterone to be effective on acute reduction in lesion size and early behavioural impairments, but less is known about its long-term neuroprotective effects after stroke. To address this problem, we examined progesterone’s effects on a panel of behavioural tests sensitive to unilateral ischaemic insult beginning 3 weeks after the injury. We observed that the rats showed severe behavioural impairments and diminished functional recovery over the 3 weeks of post-stroke testing. We found that compared with intact rats, older rats with permanent MCAO showed functional deficits up to 3 weeks post-injury on a wide array of outcome measures. We observed sustained sensory, motor, and gait impairments and cognitive deficits compared to the sham group. At 3 weeks post-injury we observed sustained deficits (Table 1) in grip strength (30.24%), ability to stay on an accelerating Rotarod (56.13%), sensory neglect (>10-fold), locomotor activity (54.19%) and spatial memory (26.97%) compared with sham-operated rats. Here we suggest that the use of older rats tested over a longer duration is a more appropriate permanent stroke model for evaluating neuroprotection in middle age than evaluation of acute infarct and short-term functional measures in very young animals.
Table 1.
Per cent deficit and improvement by different doses of progesterone on test parameters
| Test day (D) | % Deficit compared with sham control | % Improvement compared with deficit |
|||
|---|---|---|---|---|---|
| P8 | P16 | P32 | |||
| Grip strength | D2 | 28.26* | 24.94 | 46.40# | 8.67 |
| D6 | 32.86* | 38.53 | 64.99# | 72.95# | |
| D9 | 32.48* | 54.56# | 47.67# | 32.79 | |
| D22 | 30.24* | 71.56# | 50.86 | −2.07 | |
| Rotarod | D3 | 33.77* | 84.89# | 19.59 | 0.17 |
| D7 | 40.82* | 66.02# | 62.36 | 28.30 | |
| D10 | 47.88* | 98.49# | 90.06# | 39.37 | |
| D21 | 67.72* | 80.64# | 94.52# | 32.02 | |
| Sensory neglect | D3 | 10.66* folds (1066%) | 74.58# | 61.67# | 41.25 |
| D7 | 7.29* folds (729%) | 83.24# | 61.28# | −8.86 | |
| D10 | 6.48* folds (648%) | 89.08# | 56.04 | 33.46 | |
| D21 | 5.87* | 84.34# | 76.59# | 26.68 | |
| Locomotor activity | D2 | 56.57* | 37.26# | 54.45# | 32.46# |
| D6 | 53.88* | 57.95# | 69.19# | 54.67# | |
| D9 | 45.28* | 33.09# | 61.52# | 38.75 | |
| D22 | 54.19* | 30.40 | 74.35# | 41.01 | |
| Memory (Morris water maze) | D22 | 26.97* | 99.16# | 34.74 | 13.15 |
| Stride length | D2 | 40.09 | 25.91 | 16.43 | 72.98 |
| D6 | 51.10* | 64.66# | 58.76 | 88.60# | |
| D21 | 51.62* | 68.60# | 52.52 | 79.62# | |
| Print area | D2 | 65.56* | 20.09 | 8.19 | 28.87 |
| D6 | 74.56* | 38.24# | 47.43 | 101.72# | |
| D21 | 74.31* | 26.83 | 44.55 | 42.80 | |
| Swing speed | D2 | 42.44 | 88.63 | −0.83 | 29.81 |
| D6 | 51.11* | 100.10# | 51.79 | 90.02# | |
| D21 | 64.04* | 51.39# | 25.02 | 48.43 | |
| Infarct volume | D23 | − | 65.60# | 71.63# | 29.21 |
*Permanent MCAO + vehicle versus Sham + vehicle.
#Permanent MCAO + vehicle versus permanent MCAO + progesterone.
P = progesterone.
A key objective of the present study was to determine a dose–response curve for progesterone treatment at repeated time points over 3 weeks after permanent MCAO on histological and functional improvement. Using doses of 8, 16 and 32 mg/kg, we hypothesized that an inverted U-shaped dose–response curve would emerge for most of the behavioural tasks, as previously observed in a traumatic brain injury model in young adult rats (Goss et al., 2003). On behavioural testing days we administered progesterone after, rather than before, testing because of its known anxiolytic and anaesthetic effects (Bixo and Backstrom, 1990; Bitran et al., 1993, 1995; Bitran and Dowd, 1996; Reddy and Kulkarni, 1997; Djebaili et al., 2004).
We found that 8 and 16 mg/kg doses of progesterone substantially reduce stroke infarct volume and improve functional recovery. Although both doses were more or less equally effective on sensory and motor function tests, 8 mg/kg produced better performance on the spatial memory Morris water maze task. We therefore suggest that 8 mg/kg is the optimal effective dose for systemic administration of progesterone for ischaemic stroke in older rats. These observations are consistent with previous reports, including our own, demonstrating the neuroprotective efficacy of an 8 mg/kg dose of progesterone after ischaemic stroke (Jiang et al., 1996; Chen et al., 1999; Kumon et al., 2000; Goss et al., 2003; Djebaili et al., 2004). As we would have predicted from our earlier studies (Goss et al., 2003), we found that the highest dose (32 mg/kg) was less effective, or ineffective, in improving outcomes over time on measures of performance such as grip strength, forepaw neglect and spatial memory. Although the weight loss in the 32 mg/kg group was observed to be significantly lower compared to the vehicle-treated sham group (Supplementary Fig. 1), in no case did we observe any of the three doses to be harmful on functional tests, a finding which is potentially important for selecting optimal doses for clinical use. Consistent with our finding, Wang et al. (2010) using permanent MCAO with the intraluminal filament technique in aged rats, showed that progesterone (8 mg/kg) improved Rotarod performance and reduced neurological deficit scores compared with vehicle-treated rats. Although the Wang et al. (2010) study used aged subjects, the outcome measures were limited to infarct assessment and Rotarod testing only at the 72-h survival time point. The model of permanent MCAO with intraluminal thread technique shows variability in infarct volumes and higher mortality rates (Kitagawa et al., 1998), thus limiting long-term survival studies. The present study in a model of permanent MCAO by direct ‘ligation’ (cauterization) of the proximal middle cerebral artery allowed assessment of neuroprotection over a longer period of time. However, this model has limitations in approximating all aspects of the stroke injury, producing only cortical infarct and not being a pertinent model of subcortical ischaemic stroke. Our previous studies have shown progesterone to attenuate subcortical (caudate putamen) infarct in both transient and permanent intraluminal suture models of stroke (Sayeed et al., 2006, 2007), but a more detailed examination of dose- and time-response relationships is needed in these models that, in fact, result in subcortical damage.
Our recent results showed that progesterone at 8 and 16 mg not only attenuates the functional deficits induced by transient MCAO, but also exerts neuroprotection after lipopolysaccharide-induced exacerbated ischaemic brain injury (Yousuf et al., 2013). Consistent with our current approach, a study by Wang et al. (2010), using permanent MCAO with the intraluminal filament technique in older rats, showed that progesterone (8 mg/kg) improved Rotarod performance and reduced neurological deficit scores compared with vehicle-treated rats.
Our previous animal studies have shown that neurosteroids given after stroke onset can reduce infarct size and improve functional outcome measures (Sayeed et al., 2007; Ishrat et al., 2009; Yousuf et al., 2013). Unfortunately, in most experiments initiation of drug administration has not been sufficiently delayed to mimic what can happen in a clinical setting. After investigating the dose–response relationship, we investigated treatment with the optimal dose of progesterone (8 mg/kg) on morphological and functional outcomes when treatment is delayed for 3, 6 or 24 h after ischaemic stroke. To the best of our knowledge this is the first study reporting the delayed treatment effects of progesterone in a stroke model. We note that the neuroprotective efficacy of progesterone can be seen even when administered at 6 h post-occlusion (Table 2). Perhaps more importantly, we observed slight salutary effects even when the treatment was delayed for 24 h after permanent MCAO. In light of the fact that in most of the completed stroke clinical trials, the enrolment time was beyond 6 h, it will be important to investigate an intermediate time point between 6 and 24 h post-occlusion.
Table 2.
Percent improvement on test parameters after delayed progesterone treatment
| Test day (D) post-injury | 3-h delay | 6-h delay | 24-h delay | |
|---|---|---|---|---|
| Grip strength | D3 | 60.43 | 83.85 | −2.28 |
| D9 | 61.32 | 105.22 | 0.06 | |
| D21 | 61.01 | 76.21 | 19.10 | |
| Rotarod | D4 | 79.17 | 31.20 | 8.58 |
| D10 | 73.90 | 57.51 | 7.04 | |
| D22 | 73.50 | 50.51 | −7.97 | |
| Sensory neglect | D4 | 83.65 | 75.43 | 99.04 |
| D10 | 91.91 | 65.69 | 66.67 | |
| D22 | 99.04 | −4.28 | −0.48 | |
| Locomotor activity | D3 | 27.28 | 46.49 | 61.13 |
| D9 | 60.15 | 55.89 | – | |
| D21 | 54.49 | 46.72 | 26.47 | |
| Memory (Morris water maze) | D22 | 60.52 | 60.18 | 20.35 |
| Stride length | D3 | 28.42 | 59.73 | −10.60 |
| D9 | 82.25 | 71.87 | 42.84 | |
| D21 | 73.11 | 45.56 | 22.60 | |
| Print area | D3 | 65.24 | 23.66 | −14.20 |
| D9 | 60.13 | 20.36 | 14.30 | |
| D21 | 96.15 | 34.29 | 32.11 | |
| Swing speed | D3 | 65.14 | 77.94 | 21.95 |
| D9 | 86.72 | 97.32 | 59.11 | |
| D21 | 86.82 | 86.27 | 44.32 | |
| Infarct volume | D23 | 59.29 | 54.24 | 1.62 |
Previous studies in different stroke models have shown a varying extent and duration of gait impairments in younger rodents (Wang et al., 2008; Encarnacion et al., 2011; Hetze et al., 2012; Parkkinen et al., 2013). In the present study, we used an automated gait analysis system to evaluate the effect of progesterone on gait function in older rats with permanent MCAO. We repeatedly measured stride length, print area and swing speed of the contralateral paw. Vehicle-treated animals showed significant post-stroke gait impairments, whereas progesterone treatment at 1, 3 and 6 h reduced these impairments. To the best of our knowledge our study is the first to report the beneficial effects of progesterone on stroke-induced gait impairments—clinically relevant indicators of motor function status.
Although it will be necessary to examine the mechanisms involved in the age-related response of the brain to stroke treatment, the purpose of this study was to measure progesterone’s efficacy on the reduction of stroke infarct size and the enhancement of functional recovery in middle-aged subjects. We have previously shown that progesterone exerts its effects through a variety of intranuclear and membrane-bound molecular mechanisms and pathways (Djebaili et al., 2004; Pettus et al., 2005; Guo et al., 2006; Vanlandingham et al., 2006, 2007, 2008; Ishrat et al., 2010, 2012; Atif et al., 2013), making it likely that progesterone’s interacting pleiotropic actions on the reduction of many complex molecular and genomic cascades of injury are responsible for the improved functional outcomes. The multiple effects of injury, inflammation and repair become manifest at different times—sometimes days, weeks or months—after injury. There are reports showing that progesterone consistently inhibits inflammatory reactions following traumatic brain injury and acute stroke (Gibson et al., 2005; Hua et al., 2011). Our earlier studies in aged rats showed that progesterone mitigates cellular damage caused by the inflammation cascade (Cutler et al., 2007; Cekic et al., 2009, 2011). Our laboratory has also shown (VanLandingham et al., 2007) that progesterone increases the expression of CD-55, a cell surface protein which reduces complement factors that can trigger the debilitating inflammatory cascade. Progesterone can also decrease apoptosis after stroke through the PI3K/Akt pathway (Ishrat et al., 2012) and suppress glial activation leading to scar formation (Labombarda et al., 2011). Progesterone can also repair the blood–brain barrier after permanent MCAO by reducing metalloproteinases, and this in turn helps to prevent degradation of tight junction proteins such as occludin 1 and claudin 5 (Ishrat et al., 2010). More recently, we showed that progesterone inhibits the tPA-induced increase in matrix metalloproteinase 9 and vascular endothelial growth factor. This observation may explain some of the mechanisms underlying progesterone’s vascular protection and attenuation of cerebral haemorrhage after ischaemic stroke and delayed tPA treatment (Won et al., 2013). Progesterone confers many neuroprotective benefits because it acts on many different pathways in the injury/repair cascade. Other mechanisms for the hormone’s pleiotropic actions in enhancing neuronal sparing and repair after various kinds of brain injury have been reviewed elsewhere (Schumacher et al., 2007; Stein et al., 2008; Liu et al., 2012; Go et al., 2013).
For the current study only males were used as subjects. Most recently, Wong et al. (2013a) conducted individual animal meta-analyses from limited, published preclinical studies of progesterone in females with experimental stroke and concluded that there was an increase in the incidence of stroke-related death in adult, ovariectomized females, highlighting the fact that further investigations are needed to evaluate hormonal status of the females at time of injury as well as dose- and time-response relationships for progesterone. Recently Gibson et al. (2011) demonstrated that progesterone treatment is beneficial after transient MCAO in aged and ovariectomized female mice. Although the numbers were small, recently completed phase II clinical trials with progesterone for traumatic brain injury in both male and female patients showed salutary effects of the hormone (NCT00562016; ACTRN12607000545460) (Wright et al., 2007; Xiao et al., 2008). Two independent phase III trials are now nearing completion and there will apparently be enough patients to permit some stratification based on gender to determine whether there are sex differences in outcomes following progesterone treatment for traumatic brain injury (NCT00822900; NCT01143064).
Given that there are now >240 articles demonstrating the beneficial effects of progesterone in a wide variety of neural injury models, we can conclude that both laboratory and clinical evidence indicates that progesterone may be a promising neuroprotective agent and a candidate for future clinical studies in ischaemic stroke.
Supplementary Material
Acknowledgements
The authors thank Leslie McCann for her editorial assistance.
Glossary
Abbreviations
- MCAO
middle cerebral artery occlusion
- tPA
tissue plasminogen activator
Funding
This work was supported by National Institutes of Health grants UO1 NS062676 to D.G.S. and American Heart Association grant 11SDG5430002 to I.S.
Conflict of interest
D.G.S. is entitled to royalty from products of BHR Pharma related to the research described in this paper, and may receive research funding from BHR, which is developing products related to this research. The terms of this arrangement have been reviewed and approved by Emory University, which receives the largest share of any benefits in accordance with its business practices and conflict of interest policies.
Supplementary material
Supplementary material is available at Brain online.
References
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31:161–8. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Asbury ET, Fritts ME, Horton JE, Isaac WL. Progesterone facilitates the acquisition of avoidance learning and protects against subcortical neuronal death following prefrontal cortex ablation in the rat. Behav Brain Res. 1998;97:99–106. doi: 10.1016/s0166-4328(98)00031-x. [DOI] [PubMed] [Google Scholar]
- Atif F, Yousuf S, Sayeed I, Ishrat T, Hua F, Stein DG. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology. 2013;67:78–87. doi: 10.1016/j.neuropharm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz AL, Coester HC. Effect of steroids on edema and sodium uptake of the brain during focal ischemia in rats. Stroke. 1990;21:1199–204. doi: 10.1161/01.str.21.8.1199. [DOI] [PubMed] [Google Scholar]
- Bitran D, Dowd JA. Ovarian steroids modify the behavioral and neurochemical responses of the central benzodiazepine receptor. Psychopharmacology (Berl) 1996;125:65–73. doi: 10.1007/BF02247394. [DOI] [PubMed] [Google Scholar]
- Bitran D, Purdy RH, Kellogg CK. Anxiolytic effect of progesterone is associated with increases in cortical allopregnanolone and GABAA receptor function. Pharmacol Biochem Behav. 1993;45:423–8. doi: 10.1016/0091-3057(93)90260-z. [DOI] [PubMed] [Google Scholar]
- Bitran D, Shiekh M, McLeod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABAA receptors. J Neuroendocrinol. 1995;7:171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- Bixo M, Backstrom T. Regional distribution of progesterone and 5 alpha-pregnane-3,20-dione in rat brain during progesterone-induced “anesthesia”. Psychoneuroendocrinology. 1990;15:159–62. doi: 10.1016/0306-4530(90)90025-5. [DOI] [PubMed] [Google Scholar]
- Cekic M, Cutler SM, VanLandingham JW, Stein DG. Vitamin D deficiency reduces the benefits of progesterone treatment after brain injury in aged rats. Neurobiol Aging. 2011;32:864–74. doi: 10.1016/j.neurobiolaging.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, Sayeed I, Stein DG. Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease. Front Neuroendocrinol. 2009;30:158–72. doi: 10.1016/j.yfrne.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chopp M, Li Y. Neuroprotective effects of progesterone after transient middle cerebral artery occlusion in rat. J Neurol Sci. 1999;171:24–30. doi: 10.1016/s0022-510x(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Cutler SM, Cekic M, Miller DM, Wali B, VanLandingham JW, Stein DG. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J Neurotrauma. 2007;24:1475–86. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- Delcker A, Diener HC, Timmann D, Faustmann P. The role of vertebral and internal carotid artery disease in the pathogenesis of vertebrobasilar transient ischemic attacks. Eur Arch Psychiatry Clin Neurosci. 1993;242:179–83. doi: 10.1007/BF02189960. [DOI] [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat pre-frontal cortex. Neuroscience. 2004;123:349–59. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87:179–97. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Encarnacion A, Horie N, Keren-Gill H, Bliss TM, Steinberg GK, Shamloo M. Long-term behavioral assessment of function in an experimental model for ischemic stroke. J Neurosci Methods. 2011;196:247–57. doi: 10.1016/j.jneumeth.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. Recommendations for advancing development of acute stroke therapies: Stroke Therapy Academic Industry Roundtable 3. Stroke. 2003;34:1539–46. doi: 10.1161/01.STR.0000072983.64326.53. [DOI] [PubMed] [Google Scholar]
- Fisher M, Albers GW, Donnan GA, Furlan AJ, Grotta JC, Kidwell CS, et al. Enhancing the development and approval of acute stroke therapies: Stroke Therapy Academic Industry roundtable. Stroke. 2005;36:1808–13. doi: 10.1161/01.STR.0000173403.60553.27. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–50. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193:522–30. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Coomber B, Murphy SP. Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain. 2011;134(Pt 7):2125–33. doi: 10.1093/brain/awr132. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Gray LJ, Bath PM, Murphy SP. Progesterone for the treatment of experimental brain injury; a systematic review. Brain. 2008;131(Pt 2):318–28. doi: 10.1093/brain/awm183. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–13. doi: 10.1097/01.WCB.0000125365.83980.00. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss CW, Hoffman SW, Stein DG. Behavioral effects and anatomic correlates after brain injury: a progesterone dose-response study. Pharmacol Biochem Behav. 2003;76:231–42. doi: 10.1016/j.pbb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Guo Q, Sayeed I, Baronne LM, Hoffman SW, Guennoun R, Stein DG. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp Neurol. 2006;198:469–78. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–15. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- Hankey GJ. Stroke: how large a public health problem, and how can the neurologist help? Arch Neurol. 1999;56:748–54. doi: 10.1001/archneur.56.6.748. [DOI] [PubMed] [Google Scholar]
- Hetze S, Romer C, Teufelhart C, Meisel A, Engel O. Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. J Neurosci Methods. 2012;206:7–14. doi: 10.1016/j.jneumeth.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Hua F, Reiss JI, Tang H, Wang J, Fowler X, Sayeed I, et al. Progesterone and low-dose vitamin D hormone treatment enhances sparing of memory following traumatic brain injury. Horm Behav. 2012;61:642–51. doi: 10.1016/j.yhbeh.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua F, Wang J, Ishrat T, Wei W, Atif F, Sayeed I, et al. Genomic profile of Toll-like receptor pathways in traumatically brain-injured mice: effect of exogenous progesterone. J Neuroinflammation. 2011;8:42. doi: 10.1186/1742-2094-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol. 2010;226:183–90. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Hua F, Stein DG. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience. 2012;210:442–50. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishrat T, Sayeed I, Atif F, Stein DG. Effects of progesterone administration on infarct volume and functional deficits following permanent focal cerebral ischemia in rats. Brain Res. 2009;1257:94–101. doi: 10.1016/j.brainres.2008.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–7. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Kissela BM, Khoury JC, Alwell K, Moomaw CJ, Woo D, Adeoye O, et al. Age at stroke: temporal trends in stroke incidence in a large, biracial population. Neurology. 2012;79:1781–7. doi: 10.1212/WNL.0b013e318270401d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa H, Hayashi T, Mitsumoto Y, Koga N, Itoyama Y, Abe K. Reduction of ischemic brain injury by topical application of glial cell line-derived neurotrophic factor after permanent middle cerebral artery occlusion in rats. Stroke. 1998;29:1417–22. doi: 10.1161/01.str.29.7.1417. [DOI] [PubMed] [Google Scholar]
- Kumon Y, Kim SC, Tompkins P, Stevens A, Sakaki S, Loftus CM. Neuroprotective effect of postischemic administration of progesterone in spontaneously hypertensive rats with focal cerebral ischemia. J Neurosurg. 2000;92:848–52. doi: 10.3171/jns.2000.92.5.0848. [DOI] [PubMed] [Google Scholar]
- Labombarda F, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, et al. Progesterone attenuates astro- and microgliosis and enhances oligodendrocyte differentiation following spinal cord injury. Exp Neurology. 2011;231:135–46. doi: 10.1016/j.expneurol.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Liu A, Margaill I, Zhang S, Labombarda F, Coqueran B, Delespierre B, et al. Progesterone receptors: a key for neuroprotection in experimental stroke. Endocrinology. 2012;153:3747–57. doi: 10.1210/en.2012-1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubjuhn J, Gastens A, von Wilpert G, Bargiotas P, Herrmann O, Murikinati S, et al. Functional testing in a mouse stroke model induced by occlusion of the distal middle cerebral artery. J Neurosci Methods. 2009;184:95–103. doi: 10.1016/j.jneumeth.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Muntner P, DeSalvo KB, Wildman RP, Raggi P, He J, Whelton PK. Trends in the prevalence, awareness, treatment, and control of cardiovascular disease risk factors among noninstitutionalized patients with a history of myocardial infarction and stroke. Am J Epidemiol. 2006;163:913–20. doi: 10.1093/aje/kwj124. [DOI] [PubMed] [Google Scholar]
- Murphy SJ, Littleton-Kearney MT, Hurn PD. Progesterone administration during reperfusion, but not preischemia alone, reduces injury in ovariectomized rats. J Cereb Blood Flow Metab. 2002;22:1181–8. doi: 10.1097/01.WCB.0000037990.07114.07. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Mortality by cause for eight regions of the world: Global Burden of Disease Study. Lancet. 1997;349:1269–76. doi: 10.1016/S0140-6736(96)07493-4. [DOI] [PubMed] [Google Scholar]
- Parkkinen S, Ortega FJ, Kuptsova K, Huttunen J, Tarkka I, Jolkkonen J. Gait impairment in a rat model of focal cerebral ischemia. Stroke Res Treat. 2013;2013:410972. doi: 10.1155/2013/410972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain Res. 2005;1049:112–9. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–9. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Roof RL, Stein DG. Gender differences in Morris water maze performance depend on task parameters. Physiol Behav. 1999;68:81–6. doi: 10.1016/s0031-9384(99)00162-6. [DOI] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael ST, Phinney D, Wechsler L. Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke. 2011;42:825–9. doi: 10.1161/STROKEAHA.110.601914. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Guo Q, Hoffman SW, Stein DG. Allopregnanolone, a progesterone metabolite, is more effective than progesterone in reducing cortical infarct volume after transient middle cerebral artery occlusion. Ann Emerg Med. 2006;47:381–9. doi: 10.1016/j.annemergmed.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Stein DG. progesterone as a neuroprotective factor in traumatic and ischemic brain injury. In: Verhaagen J, Hol EM, Huitinga I, Wijnholds W, Bergen AB, Boer GJ, et al., editors. Neurotherapy. New York: Elsevier; 2009. pp. 219–37. [DOI] [PubMed] [Google Scholar]
- Sayeed I, Wali B, Stein DG. Progesterone inhibits ischemic brain injury in a rat model of permanent middle cerebral artery occlusion. Restor Neurol Neurosci. 2007;25:151–9. [PubMed] [Google Scholar]
- Schumacher M, Guennoun R, Stein DG, De Nicola AF. Progesterone: therapeutic opportunities for neuroprotection and myelin repair. Pharmacol Ther. 2007;116:77–106. doi: 10.1016/j.pharmthera.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Shear DA, Galani R, Hoffman SW, Stein DG. Progesterone protects against necrotic damage and behavioral abnormalities caused by traumatic brain injury. Exp Neurol. 2002;178:59–67. doi: 10.1006/exnr.2002.8020. [DOI] [PubMed] [Google Scholar]
- Stein DG. A clinical/translational perspective: can a developmental hormone play a role in the treatment of traumatic brain injury? Horm Behav. 2013;63:291–300. doi: 10.1016/j.yhbeh.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Stein DG, Wright DW, Kellermann AL. Does progesterone have neuroprotective properties? Ann Emerg Med. 2008;51:164–72. doi: 10.1016/j.annemergmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine. 1999;24:2134–8. doi: 10.1097/00007632-199910150-00013. [DOI] [PubMed] [Google Scholar]
- VanLandingham JW, Cekic M, Cutler S, Hoffman SW, Stein DG. Neurosteroids reduce inflammation after TBI through CD55 induction. Neurosci Lett. 2007;425:94–8. doi: 10.1016/j.neulet.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanLandingham JW, Cekic M, Cutler SM, Hoffman SW, Washington ER, Johnson SJ, et al. Progesterone and its metabolite allopregnanolone differentially regulate hemostatic proteins after traumatic brain injury. J Cereb Blood Flow Metab. 2008;28:1786–94. doi: 10.1038/jcbfm.2008.73. [DOI] [PubMed] [Google Scholar]
- Vanlandingham JW, Cutler SM, Virmani S, Hoffman SW, Covey DF, Krishnan K, et al. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51:1078–85. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Wali B, Sayeed I, Stein DG. Improved behavioral outcomes after progesterone administration in aged male rats with traumatic brain injury. Restor Neurol Neurosci. 2011;29:61–71. doi: 10.3233/RNN-2011-0579. [DOI] [PubMed] [Google Scholar]
- Wang J, Jiang C, Liu C, Li X, Chen N, Hao Y. Neuroprotective effects of progesterone following stroke in aged rats. Behav Brain Res. 2010;209:119–22. doi: 10.1016/j.bbr.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, et al. A comprehensive analysis of gait impairment after experimental stroke and the therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow Metab. 2008;28:1936–50. doi: 10.1038/jcbfm.2008.82. [DOI] [PubMed] [Google Scholar]
- Won S, Wali B, Sayeed I, Stein DG. Progesterone prevents hemorrhagic transformation after tPAa treatment in experimental stroke. International Stroke Conference: February 2013; Honolulu, HI. Stroke 2013; 44: ATMP33. [Google Scholar]
- Wong R, Renton C, Gibson CL, Murphy SJ, Kendall DA, Bath PM. Progesterone treatment for experimental stroke: an individual animal meta-analysis. J Cereb Blood Flow Metab. 2013a;33:1362–72. doi: 10.1038/jcbfm.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R, Bath PM, Kendall D, Gibson CL. Progesterone and cerebral ischaemia: the relevance of ageing. J Neuroendocrinol. 2013b doi: 10.1111/jne.12042. in press. [DOI] [PubMed] [Google Scholar]
- Wright DW, Kellermann AL, Hertzberg VS, Clark PL, Frankel M, Goldstein FC, et al. ProTECT: a randomized clinical trial of progesterone for acute traumatic brain injury. Ann Emerg Med. 2007;49:391–402. doi: 10.1016/j.annemergmed.2006.07.932. [DOI] [PubMed] [Google Scholar]
- Xiao G, Wei J, Yan W, Wang W, Lu Z. Improved outcomes from the administration of progesterone for patients with acute severe traumatic brain injury: a randomized controlled trial. Crit Care. 2008;12:R61. doi: 10.1186/cc6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Tamura A, Kirino T, Shimizu M, Sano K. Behavioral changes after focal cerebral ischemia by left middle cerebral artery occlusion in rats. Brain Res. 1988;452:323–8. doi: 10.1016/0006-8993(88)90036-4. [DOI] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I, Wang J, Stein DG. Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: immunomodulation neuroprotection by progesterone. Neuroscience. 2013;239:92–102. doi: 10.1016/j.neuroscience.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.