Abstract
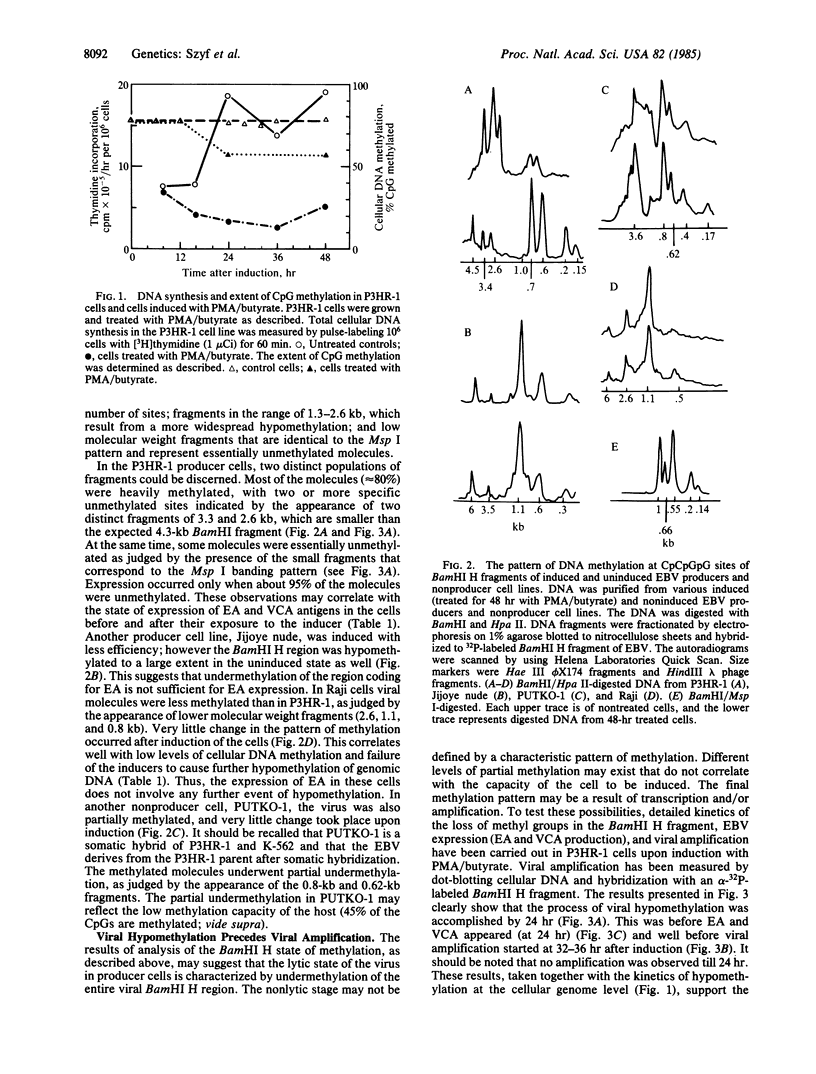
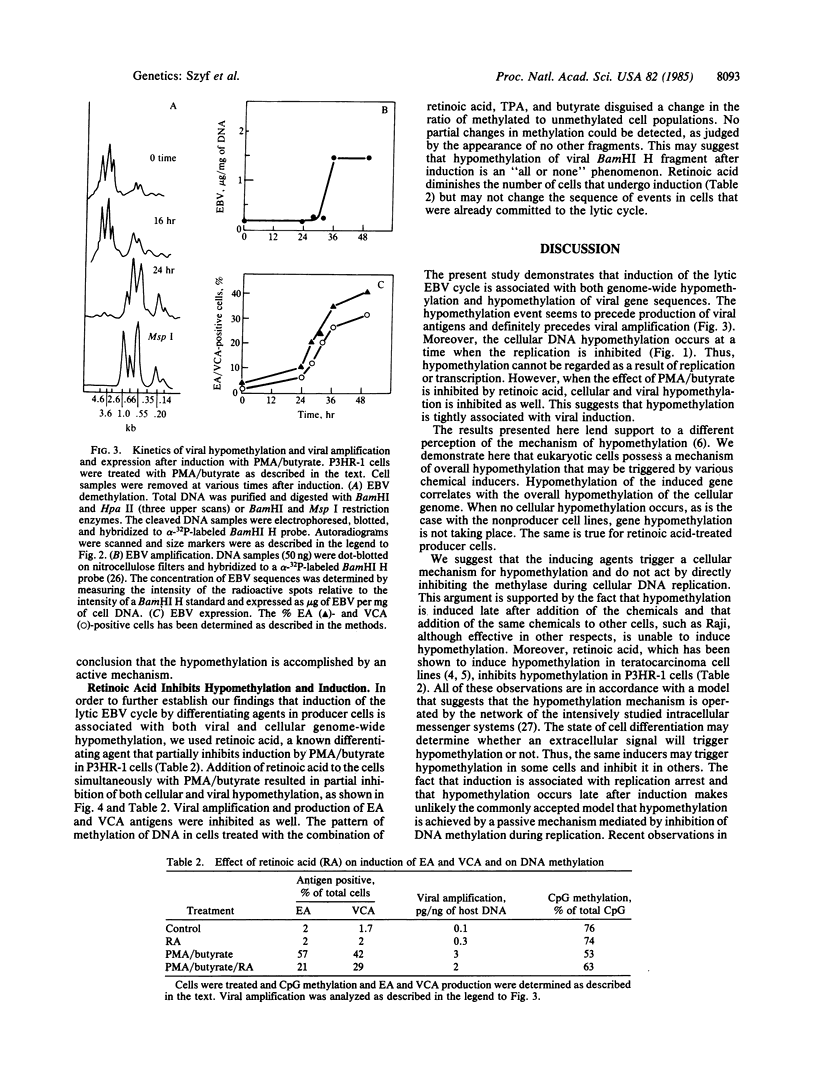
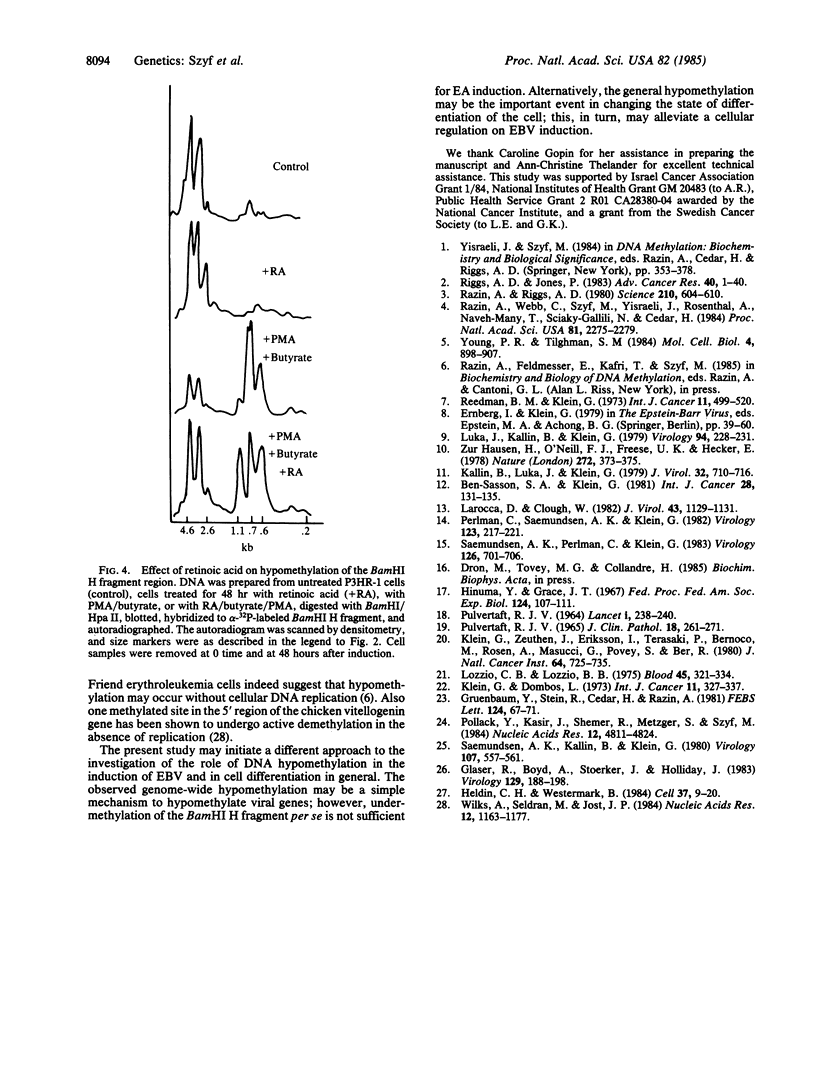
Epstein-Barr virus (EBV) producer and nonproducer cell lines have been treated with a combination of phorbol 12-myristate 13-acetate and n-butyrate (sodium salt). These inducers caused a massive hypomethylation of the EBV producer line P3HR-1 DNA (about 30%) at the time when DNA replication was inhibited. The viral DNA in these cells is heavily methylated as judged by digestion with Hpa II and probing with the Bam HI H fragment of EBV. However, upon induction with phorbol 12-myristate 13-acetate and n-butyrate, total hypomethylation of this viral DNA region was observed within 24 hr. This hypomethylation preceded EBV amplification, which became apparent only 32-36 hr after induction. When induction was carried out in the presence of retinoic acid, hypomethylation of cellular and viral DNA, viral DNA amplification, and production of the viral early antigen and viral capsid antigen were substantially inhibited. EBV DNA in another producer line (Jijoye nude) and in the nonproducer line Raji was hypomethylated and did not undergo further hypomethylation in response to induction. The observed hypomethylation of P3HR-1 and EBV DNA in the absence of DNA replication suggests that it is achieved by an active demethylation mechanism. This changes our perception of the DNA methylation phenomenon, since it has been generally accepted that hypomethylation of DNA takes place by a passive mechanism that involves DNA replication in the absence of methylation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Sasson S. A., Klein G. Activation of the Epstein-Barr virus genome by 5-aza-cytidine in latently infected human lymphoid lines. Int J Cancer. 1981 Aug 15;28(2):131–135. doi: 10.1002/ijc.2910280204. [DOI] [PubMed] [Google Scholar]
- Glaser R., Boyd A., Stoerker J., Holliday J. Functional mapping of the Epstein-Barr virus genome: identification of sites coding for the restricted early antigen, the diffuse early antigen, and the nuclear antigen. Virology. 1983 Aug;129(1):188–198. doi: 10.1016/0042-6822(83)90405-1. [DOI] [PubMed] [Google Scholar]
- Gruenbaum Y., Stein R., Cedar H., Razin A. Methylation of CpG sequences in eukaryotic DNA. FEBS Lett. 1981 Feb 9;124(1):67–71. doi: 10.1016/0014-5793(81)80055-5. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Kallin B., Luka J., Klein G. Immunochemical characterization of Epstein-Barr virus-associated early and late antigens in n-butyrate-treated P3HR-1 cells. J Virol. 1979 Dec;32(3):710–716. doi: 10.1128/jvi.32.3.710-716.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Dombos L. Relationship between the sensitivity of EBV-carrying lymphoblastoid lines to superinfection and the inducibility of the resident viral genome. Int J Cancer. 1973 Mar 15;11(2):327–337. doi: 10.1002/ijc.2910110210. [DOI] [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Eriksson I., Terasaki P., Bernoco M., Rosén A., Masucci G., Povey S., Ber R. Hybridization of a myeloid leukemia-derived human cell line (K562) with a human Burkitt's lymphoma line (P3HR-1). J Natl Cancer Inst. 1980 Apr;64(4):725–738. [PubMed] [Google Scholar]
- Larocca D., Clough W. Hypomethylation of Epstein-Barr virus DNA in the nonproducer B-cell line EBR. J Virol. 1982 Sep;43(3):1129–1131. doi: 10.1128/jvi.43.3.1129-1131.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozzio C. B., Lozzio B. B. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975 Mar;45(3):321–334. [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PULVERTAFT J. V. CYTOLOGY OF BURKITT'S TUMOUR (AFRICAN LYMPHOMA). Lancet. 1964 Feb 1;1(7327):238–240. doi: 10.1016/s0140-6736(64)92345-1. [DOI] [PubMed] [Google Scholar]
- Perlmann C., Saemundsen A. K., Klein G. A fraction of Epstein--Barr virus virion DNA is methylated in and around the EcoRI-J fragment. Virology. 1982 Nov;123(1):217–221. doi: 10.1016/0042-6822(82)90308-7. [DOI] [PubMed] [Google Scholar]
- Pollack Y., Kasir J., Shemer R., Metzger S., Szyf M. Methylation pattern of mouse mitochondrial DNA. Nucleic Acids Res. 1984 Jun 25;12(12):4811–4824. doi: 10.1093/nar/12.12.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Razin A., Webb C., Szyf M., Yisraeli J., Rosenthal A., Naveh-Many T., Sciaky-Gallili N., Cedar H. Variations in DNA methylation during mouse cell differentiation in vivo and in vitro. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2275–2279. doi: 10.1073/pnas.81.8.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Saemundsen A. K., Kallin B., Klein G. Effect of n-butyrate on cellular and viral DNA synthesis in cells latently infected with Epstein-Barr virus. Virology. 1980 Dec;107(2):557–561. doi: 10.1016/0042-6822(80)90326-8. [DOI] [PubMed] [Google Scholar]
- Saemundsen A. K., Perlmann C., Klein G. Intracellular Epstein-Barr virus DNA is methylated in and around the EcoRI-J fragment in both producer and nonproducer cell lines. Virology. 1983 Apr 30;126(2):701–706. doi: 10.1016/s0042-6822(83)80026-9. [DOI] [PubMed] [Google Scholar]
- Wilks A., Seldran M., Jost J. P. An estrogen-dependent demethylation at the 5' end of the chicken vitellogenin gene is independent of DNA synthesis. Nucleic Acids Res. 1984 Jan 25;12(2):1163–1177. doi: 10.1093/nar/12.2.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P. R., Tilghman S. M. Induction of alpha-fetoprotein synthesis in differentiating F9 teratocarcinoma cells is accompanied by a genome-wide loss of DNA methylation. Mol Cell Biol. 1984 May;4(5):898–907. doi: 10.1128/mcb.4.5.898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H., O'Neill F. J., Freese U. K., Hecker E. Persisting oncogenic herpesvirus induced by the tumour promotor TPA. Nature. 1978 Mar 23;272(5651):373–375. doi: 10.1038/272373a0. [DOI] [PubMed] [Google Scholar]


