Abstract
Aging is a complex process regulated by multiple cellular pathways, including the proteostasis network. The proteostasis network consists of molecular chaperones, stress-response transcription factors, and protein degradation machines that sense and respond to proteotoxic stress and protein misfolding to ensure cell viability. A loss of proteostasis is associated with aging and age-related disorders in diverse model systems, moreover, genetic or pharmacological enhancement of the proteostasis network has been shown to extend lifespan and suppress age-related disease. However, our understanding of the relationship between aging, proteostasis, and the proteostasis network remains unclear. Here, we propose, from studies in Caenorhabditis elegans, that proteostasis collapse is not gradual but rather a sudden and early life event that triggers proteome mismanagement, thereby affecting a multitude of downstream processes. Furthermore, we propose that this phenomenon is not stochastic but is instead a programmed re-modeling of the proteostasis network that may be conserved in other species. As such, we postulate that changes in the proteostasis network may be one of the earliest events dictating healthy aging in metazoans.
Aging, proteome integrity, and the proteostasis network
Aging is inextricably linked to the world around us and regularly pervades everyday life. Despite this, why organisms age remains a complex mystery that if understood could have profound implications for the quality of human health. While the physiological decline associated with old age is easily recognizable, the mechanisms that determine aging are poorly understood; however, wide-scale loss of protein homeostasis (proteostasis) is proposed to be one of the “primary hallmarks of aging” [1].
Protein aggregation is associated with many age-related disorders, and increased protein oxidation, mislocalization, and aggregation are observed in aged organisms [2-7]. Intuitively, these findings can be explained by a gradual decline in protein biosynthetic and quality control pathways and a progressive accumulation of protein damage. However, recent findings in Caenorhabditis elegans challenge this view, suggesting that a decline in proteome integrity may be the result of early programmed events rather than the consequence of a random and gradual accrual of molecular damage.
Proteostasis is maintained by the proteostasis network (PN), a system of molecular chaperones, protein degradation machines, and stress response pathways that act alone or together in various subnetworks to sense and respond to protein misfolding in all cellular compartments [8]. This amalgamation of constitutive and inducible quality control mechanisms is central to the identity and health of the proteome [8]. Given the importance of the proteome to cell function, it is salient to ask, what is the relationship between proteostasis and aging? Attempts to address this in C. elegans have yielded a surprising and consistent result; a pronounced, widespread decline in proteostasis is a sudden event in early adulthood. Below we discuss the temporal relationship between aging, the PN, and proteostasis in C. elegans and highlight relevant observations in flies and rodents that suggest that early remodeling of the PN may be conserved.
Age-related loss of proteostasis: random chance or programmed event?
Initial efforts to precisely examine proteostasis during aging employed C. elegans temperature-sensitive (ts) mutants as protein folding sensors [9]. These worms express endogenous metastable proteins with a propensity to misfold and aggregate when animals are shifted to higher ambient temperatures (25°C). The misfolding of ts proteins is suppressed when animals are grown at lower temperatures (15°C) during development. This changes aggregation propensity from a temperature-dependent to age-dependent phenomenon as correct protein folding becomes more dependent on the action of molecular chaperones [9]. Remarkably, aggregation and subcellular mislocalization of metastable ts proteins are observed in neurons and body wall muscle cells at an early stage of adulthood (day three), with aggregation increased substantially by day seven of adulthood [9]. These observations extend to multiple unrelated ts proteins expressed in different tissues, suggesting that a widespread loss of proteostasis occurs well before the age-related appearance of motility defects (~ day eleven), cessation of reproduction (~ day seven), and median lifespan (~ day 19) in worms [9-11].
A subsequent proteomics study of C. elegans aging examined the detergent-insoluble fraction of aggregated proteins and demonstrated that a subset of the proteome formed aggregates by day three of adulthood, extending the observations predicted from the use of ts mutants. Aggregation increased with age and occurred in both the soma and germline [3]. The composition of the age-aggregated proteome was strikingly reproducible (~64% overlap), containing proteins with sequence and structural similarities (high β-sheet content), suggesting that protein aggregation in early adulthood is a non-random event dictated, in part, by protein sequence [3]. To monitor aggregation in living animals, fluorescently tagged proteins were expressed and visualized throughout C. elegans life. While all animals exhibited age-dependent protein misfolding, there was substantial heterogeneity in the onset of aggregation, indicating variance in the rate of proteostasis collapse within a population [3].
These findings suggest that protein aggregation is an early, non-random event and that the transition into early adulthood may be the critical threshold for proteostasis maintenance or collapse. This leads to questions, such as how do changes in the composition and activity of the PN contribute to the decline of proteostasis in early adulthood and how do these changes impact on aging? Below, we discuss the components of the PN that have been found to change in conjunction with, or preceding, proteostasis collapse in C. elegans.
Protein synthesis and aging: doing more with less?
The quantity and quality of newly synthesized proteins are primary modulators of proteostasis. Both a paucity or excess of nascent polypeptides could cause cellular dysfunction; therefore, translation rates must be finely tuned to match cellular requirements. In addition, chaperone machines, including the 70-kilodalton heat shock protein (HSP70) and the nascent polypeptide chain-associated complex (NAC), must be present and active at the ribosome to maintain faithful folding and ensure the fidelity of nascent polypeptides [12]. A simple explanation for proteostasis collapse could be that a high rate of protein synthesis is necessary to accommodate rapid cellular differentiation early in life but becomes excessive as cells enter senescence, thereby placing a load on the PN that cannot be sustained during aging. Alternatively, proteostasis collapse could arise from a loss of chaperone activity and concomitant reduction in the capability of the PN to maintain the necessary quality of nascent polypeptides.
The use of polysome profiling has revealed that global translation rates progressively decline between day two and day five of adulthood in C. elegans [13], indicating that an overload of the PN due to increased protein synthesis is not the likely basis for proteostasis collapse. Ribosomal subunits and components of the translation machinery are consistently found in detergent-insoluble protein fractions, suggesting that early misfolding of these proteins could contribute to, if not catalyze, proteostasis collapse [3,13]. Similarly, soluble levels of the ribosomal chaperone NAC also decline by day three of adulthood due to sequestration by protein aggregates [13]. Multiple molecular chaperones, including HSP70 and HSP90, are also associated with detergent insoluble aggregates early in adulthood; therefore, it is possible that proteostasis collapse exacerbates misfolding and aging through imbalance and sequestration of major components of the quality control machinery [3,13]. This theory is further supported by observations that RNA interference (RNAi) knockdown of molecular chaperones decreases lifespan in worms [14].
Further support for the importance of protein synthesis dynamics in aging comes from observations that reduced translation correlates with lifespan extension and enhanced stress resistance through genetic or pharmacological inhibition of the target of rapamycin complex (TORC1) in worms and flies [15]. Therefore, reduction of protein synthesis early in life could represent a beneficial remodeling of the proteome that conserves metabolic energy and minimizes the load on the PN. Alternatively, reduced translation may affect some proteins more than others. If this is the case, it is possible that highly chaperone-dependent or aggregation-prone proteins become over-represented in the proteome early in life, thereby initializing proteostasis collapse. Given that the proteome differs substantially between cell and tissue types [16], it is possible that the mechanism of proteostasis collapse could vary between tissues if different proteins become over-represented during proteome remodeling.
The ubiquitin-proteasome system in aging
Another way that cells can re-shape proteome identity and functionality is through protein degradation pathways; however, alterations to this balance can also cause proteotoxicity and reduce lifespan [17-19]. As such, it is feasible that a reduction in the protein degradation capacity of cells could contribute to proteostasis collapse and aging.
The ubiquitin-proteasome system (UPS) is a key component of protein quality control and cellular function [17]. Old and/or damaged substrates are recognized by molecular chaperone complexes and targeted for degradation through E3-ligase-mediated ubiquitylation [17]. Ubiquitylation can take many forms; however, substrates tagged with lysine residue 48 (K48)-linked polyubiquitin chains are predominantly directed to the proteasome, a multi-subunit machine consisting of a 19S regulatory particle and a 20S proteolytic core, which act in tandem to clear potentially harmful proteins. In addition to its central role in proteostasis, the UPS has roles in transcription, DNA repair, apoptosis, and signal transduction; therefore, changes in UPS activity could have far-reaching consequences [17].
Studies investigating proteasome activity in C. elegans have typically relied upon in vitro assays that measure the turnover of synthetic substrates and in vivo reporter systems monitoring the turnover of chimeric fluorescent proteins fused to a non-cleavable ubiquitin moiety (UbG76V). C. elegans UPS reporters have revealed that spatial and temporal changes in proteasome activity occur throughout life [20-22]. Initial studies with a photoconvertible reporter found that early in adulthood (day two adults), fluorescence intensity after photoconversion declines strongly throughout the worm, corresponding to the efficient degradation of the reporter [20]. However, by day five of adulthood, the fluorescence signal declined at a much slower rate following photoconversion, suggesting that proteasome activity is significantly reduced between day two and day five of adulthood. Intriguingly, this was found to be the case in dorsorectal neurons but not in body wall muscle cells, suggesting that age-related changes in the UPS may be tissue specific [20]. While these observations suggest that changes in the UPS are not associated with age-related sarcopenia in C. elegans, UPS decline may contribute to neuronal dysfunction and loss of motor activity early in life [23,24].
A second study that monitored proteasome activity earlier in adulthood (between day one and day two) found that UbG76V-green fluorescent protein (GFP) fluorescence declined sharply (by 80%) in all somatic tissues within the first 2 days of adulthood, independent of changes in GFP stability or reporter expression [21]. This increase in proteasome activity is most prominent between 24 and 36 hours into adulthood (continuing 48 hours into adulthood) [21]. In addition, global levels of K48-linked polyubiquitylated proteins increase in whole-worm lysates, indicating that the increased activity of the UPS as a whole occurs in early adulthood as opposed to a specific increase in the proteolytic properties of the proteasome [21]. Together, these findings suggest that the overall activity of the UPS increases substantially in the soma as animals reach reproductive maturity and declines thereafter in a tissue-specific manner. Reduced proteasome activity has also been reported in early adulthood in Drosophila melanogaster heads (between day one and day five of adulthood) and in rat spinal cord (between 3 and 12 months of age) [25,26], suggesting that an early change in UPS activity is a conserved feature of aging.
A critical question is whether changes in UPS activity are beneficial, detrimental, or neutral to normal aging? Enhanced proteasomal activity is linked to increased lifespan in yeast, worms, rodents, and humans; furthermore, the lifespan-extending effects of dietary restriction (DR) have been attributed to increased proteasome activity [27-31]. This suggests that the burst of proteasome activity early in life is protective and that the decline thereafter is detrimental. Given that increased proteasome activity coincides with decreased protein synthesis, these observations could represent a wide-scale protective remodeling of the proteome as animals enter the reproductive period. If this is the case, why does this event correlate with proteostasis collapse? One possibility is that without this event, proteostasis collapse would be even more extreme, leading to accelerated aging. Alternatively, the global changes in protein synthesis and degradation rates might preferentially affect specific protein subsets. Theoretically, this could lead to a reduction in proteome stability that initiates proteostasis collapse; however, a more detailed analysis of the proteomic changes that occur early in adulthood is required to ascertain whether this is the case. Investigating the activity of other protein degradation pathways early in life will be essential for a complete understanding of the relationship between the PN and aging. In particular, autophagy is the primary method by which cells can remove large protein aggregates or damaged organelles and has been linked to increased lifespan and disease prevention [32,33]. Autophagy can take one of three forms, chaperone-mediated autophagy (CMA), microautophagy, and macroautophagy, each of which differ in the mechanism by which targets are recognized and directed to the lysosome for degradation [34]. Defects in any of the three arms of autophagy could contribute to proteostasis collapse, while activation of autophagy in early adulthood could act to protect some or all tissues from proteostasis collapse. If changes in protein synthesis and degradation alone do not explain proteostasis collapse, what does? Other lines of evidence suggest that a decline in stress-inducible pathways could be the answer.
Early changes in stress response pathways: the initiating switch for aging?
Stress response pathways safeguard the proteome against increased reactive oxygen species, exposures to elevated temperatures, and errors in protein biogenesis [2]. These pathways are regulated by the heat shock response (HSR), the unfolded protein response (UPR) of the endoplasmic reticulum (ER), the mitochondrial UPR (UPRmt), and the oxidative stress response (OxSR). Gene induction through these pathways is coordinated by the transcription factors HSF-1 (HSR), XBP-1 and ATF-6 (UPRER), ATFS-1 (UPRmt), SKN-1/NRF-2 (OxSR), and Daf-16/FOXO3A (HSR and OxSR) [35-40]. We propose that these stress pathways represent an integrated stress network that provides robust protection against a wide range of acute and chronic stress conditions and therefore are essential modifiers of aging and disease [14,41-44]. The inducibility of the HSR and OxSR is reduced in old flies, aged rat tissues, and senescent human cells [45-47], possibly leading to less efficient detection and removal of misfolded or damaged proteins. Recent observations indicate that stress response pathways in C. elegans decline abruptly as animals reach reproductive maturity.
When challenged with toxic insults, such as HS or the ER stress-inducer tunicamycin, C. elegans rapidly induces the expression of cytoprotective genes. Remarkably, the induction of HSR and UPR regulated genes is reduced in somatic tissues by ~80% by day two/three of adulthood [9,43,48]. This rapid decline occurs within a 24-hour period and correlates with a pronounced reduction in stress resistance, indicating that a collapse of the HSR and UPR has functional consequences that may precede proteostasis collapse [43,48]. Perhaps related to this are observations that a similarly timed collapse of the HSR occurs in the aging rat adrenal cortex (in response to restraint stress) and in aged flies subjected to hyperthermia [49,50], supporting the early transcriptional dysregulation of stress responses as a conserved event in metazoans.
The collapse of the HSR and UPR could reflect a change in the requirements of the proteome with age, which is perhaps in part due to the changes in protein synthesis and degradation described previously or perhaps due to increased basal expression of chaperone genes and low levels of endogenous peroxide and protein oxidation [4,51,52]. As such, changes in the potency of stress responses may be neutral or beneficial. However, overexpression of HSF-1 or Daf-16/FOXO3A suppresses proteostasis collapse and enhances longevity, while mutations that extend lifespan also result in the activation of multiple stress response pathways and increased stress resistance, suggesting that the ability to maintain optimal stress response pathways is beneficial to lifespan [53].
If stress responses are beneficial, why do they decline in early adulthood? The collapse of stress responses could represent an uncontrolled, passive process due to dysregulation of signaling events or pathways. This would seem unlikely given the rapidity and uniformity of the collapse, both within individuals and throughout the population. Alternatively, if this is an active process, what are the organismal benefits of such an event? Perhaps other lifespan-modulating stress, responses, such as the OxSR and mitochondrial UPR (UPRmt), compensate and the resources redirected from tuning down the HSR and UPR are used for progeny production. This seems unlikely in the case of the UPRmt as the beneficial effects of this pathway have also been proposed to be most relevant early in life [54]. Furthermore, the likely importance of the UPR to yolk protein secretion suggests that a dampening of this pathway would negatively impact reproduction in C. elegans [55]. Finally, dysregulation of the HSR and UPR could reflect global changes in gene expression and may be proxies for other lifespan-regulating changes in transcription. Future experiments to determine the molecular basis of these events as well as which other stress response pathways also decline early in life will greatly enhance our understanding of aging.
Is the remodeling of the proteostasis network an active or passive event?
If the coordinated changes in the PN are an active rather than passive process, what are the signals that instigate PN remodeling, where might these signals originate, and why might such a system have evolved? One possibility is that neurons and the intestine in young adults coordinately sense nutrients and determine the availability of resources to the organism. In favorable conditions (suitable temperature, abundant food and ample space), signals are relayed to the gonad, promoting egg-laying, which in turn results in germline-dependent signaling to promote somatic remodeling of the PN, as resources are redirected from the soma to the germline. This would provide energy for the production of progeny at the expense of protecting the soma, a model consistent with the disposable soma theory of aging [56,57].
In support of this, long-lived C. elegans mutants, in which germline stem cell (GSC) proliferation is suppressed (glp-1) and reproduction is prevented, exhibit a pronounced delay in the collapse of proteostasis and the HSR [48] as well as enhanced proteasome activity through the daf-16-mediated upregulation of the proteasomal subunit rpn-6, which stabilizes the interaction between the 19S cap and the 20S proteolytic core of the proteasome. glp-1 mutants also show increased autophagy, enhanced resistance to oxidative stress, and reduced susceptibility to pathogenic bacteria, suggesting that GSCs can modulate a range of protective pathways [58-60]. While these observations support a link between somatic proteostasis, aging, and reproduction, the signaling pathways involved are likely to be complex as the removal of the entire gonad does not result in the lifespan extension or maintenance of proteostasis [48,61].
At the organismal level in C. elegans, neurons and the intestine are known to regulate proteostasis and lifespan in a cell non-autonomous fashion [43,62,63]; therefore, a better understanding of how these tissues cooperate to influence proteostasis collapse will be essential. Finally, it will be interesting to understand whether this model could explain age-related changes in non-hermaphroditic metazoans. At present, this seems possible (if difficult to put into the context of a complex mammalian system) as both the importance of the PN to aging and disease and early changes in the PN have been described in other systems.
Summary
Aging is a complex and multi-faceted process that has prompted many new theories in regard to its process and origin. Many studies of aging have focused on molecular changes across the lifetime of an organism with the reasonable assumption that a series of progressive events collectively contribute to the aging process. Here, we propose that a battery of sudden, early changes in key aspects of the PN could be among the earliest events that set lifespan according to resources, metabolic rates, and protein biogenesis (Figure 1). These events may be modulated, at least in part, by the germline and fit well with the disposable soma theory of aging. At present, the molecular mechanism(s) responsible for PN remodeling and the upstream signals that promote these changes are unknown. However, further insight into the full repertoire of PN changes that occur early in adulthood as well as the signaling pathways responsible could have profound implications on our understanding of the aging process.
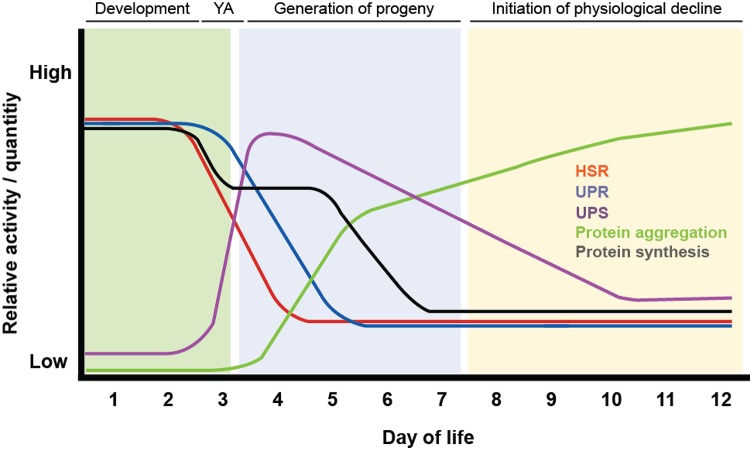
Figure 1. Temporal relationship between Caenorhabditis elegans reproduction, aging, and changes in proteostasis.
The C. elegans lifespan can be broken into three distinct stages: development (pale green region) through four larval stages (L1-L4) to become young adults (YAs), maturation into reproductively active adults that generate progeny (pale blue region), and post-reproductive adults that show progressive changes in physiology and behavior (pale red region). These life stages correlate with pronounced changes in the heat shock response (HSR) (red line), unfolded protein response (UPR) (blue line), ubiquitin-proteasome system (UPS) (purple line), protein synthesis (black line), and onset of protein aggregation (green line) early in life (colored lines). Relative activity/quantity is an arbitrary scale intended to reflect published data [3,9-11,13,21,43,48]. Days of life are representative for animals grown at 20°C; however, C. elegans life stages are shifted by growth at lower (15°C) or higher (25°C) temperatures. For example, C. elegans reach YA approximately 3, 2, or 1.5 days after hatching when grown at 15, 20, or 25°C, respectively.
Abbreviations
- C. elegans
Caenorhabditis elegans
- ER
endoplasmic reticulum
- GFP
green fluorescent protein
- HSP
heat shock protein
- HSR
heat shock response
- NAC
nascent polypeptide chain-associated complex
- OxSR
oxidative stress response
- PN
proteostasis network
- ts
temperature-sensitive
- UbG76V
non-cleavable ubiquitin moiety
- UPR
unfolded protein response
- UPS
ubiquitin proteasome system
Disclosures
The authors declare that they have no disclosures.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/b/6/7
References
- 1.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morimoto RI. Proteotoxic stress and inducible chaperone networks in neurodegenerative disease and aging. Genes Dev. 2008;22:1427–38. doi: 10.1101/gad.1657108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Della David C, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/4877956
- 4.Knoefler D, Thamsen M, Koniczek M, Niemuth NJ, Diederich A, Jakob U. Quantitative in vivo redox sensors uncover oxidative stress as an early event in life. Mol Cell. 2012;47:767–76. doi: 10.1016/j.molcel.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242999
- 5.Rana A, Rera M, Walker DW. Parkin overexpression during aging reduces proteotoxicity, alters mitochondrial dynamics, and extends lifespan. Proc Natl Acad Sci USA. 2013;110:8638–43. doi: 10.1073/pnas.1216197110. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718132852
- 6.Demontis F, Perrimon N. FOXO/4E-BP signaling in Drosophila muscles regulates organism-wide proteostasis during aging. Cell. 2010;143:813–25. doi: 10.1016/j.cell.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/8687959
- 7.Reis-Rodrigues P, Czerwieniec G, Peters TW, Evani US, Alavez S, Gaman EA, Vantipalli M, Mooney SD, Gibson BW, Lithgow GJ, Hughes RE. Proteomic analysis of age-dependent changes in protein solubility identifies genes that modulate lifespan. Aging Cell. 2012;11:120–7. doi: 10.1111/j.1474-9726.2011.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–9. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1104930
- 9.Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc Natl Acad Sci USA. 2009;106:14914–9. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1164752
- 10.Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004;101:8084–9. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes SE, Evason K, Xiong C, Kornfeld K. Genetic and pharmacological factors that influence reproductive aging in nematodes. PLoS Genet. 2007;3:e25. doi: 10.1371/journal.pgen.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preissler S, Deuerling E. Ribosome-associated chaperones as key players in proteostasis. Trends Biochem Sci. 2012;37:274–83. doi: 10.1016/j.tibs.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Kirstein-Miles J, Scior A, Deuerling E, Morimoto RI. The nascent polypeptide-associated complex is a key regulator of proteostasis. EMBO J. 2013;32:1451–68. doi: 10.1038/emboj.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu A, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300:1142–5. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1013615
- 15.Bjedov I, Partridge L. A longer and healthier life with TOR down-regulation: genetics and drugs. Biochem Soc Trans. 2011;39:460–5. doi: 10.1042/BST0390460. [DOI] [PubMed] [Google Scholar]
- 16.Geiger T, Velic A, Macek B, Lundberg E, Kampf C, Nagaraj N, Uhlen M, Cox J, Mann M. Initial quantitative proteomic map of 28 mouse tissues using the SILAC mouse. Mol Cell Proteomics. 2013;12:1709–22. doi: 10.1074/mcp.M112.024919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsakiri EN, Sykiotis GP, Papassideri IS, Terpos E, Dimopoulos MA, Gorgoulis VG, Bohmann D, Trougakos IP. Proteasome dysfunction in Drosophila signals to an Nrf2-dependent regulatory circuit aiming to restore proteostasis and prevent premature aging. Aging Cell. 2013;12:802–13. doi: 10.1111/acel.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Zhao D, Wei H, Yao L, Dang Y, Amjad A, Xu J, Liu J, Guo L, Li D, Li Z, Di Zuo, Zhang Y, Liu J, Huang S, Jia C, Wang L, Wang Y, Xie Y, Luo J, Zhang B, Luo H, Donehower LA, Moses RE, Xiao J, O'Malley BW, Li X. REGγ deficiency promotes premature aging via the casein kinase 1 pathway. Proc Natl Acad Sci USA. 2013;110:11005–10. doi: 10.1073/pnas.1308497110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamer G, Matilainen O, Holmberg CI. A photoconvertible reporter of the ubiquitin-proteasome system in vivo. Nat Methods. 2010;7:473–8. doi: 10.1038/nmeth.1460. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242981
- 21.Liu G, Rogers J, Murphy CT, Rongo C. EGF signalling activates the ubiquitin proteasome system to modulate C. elegans lifespan. EMBO J. 2011;30:2990–3003. doi: 10.1038/emboj.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]; f1000.com/prime/718242988
- 22.Segref A, Torres S, Hoppe T. A screenable in vivo assay to study proteostasis networks in Caenorhabditis elegans. Genetics. 2011;187:1235–40. doi: 10.1534/genetics.111.126797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, Paupard MC, Hall DH, Driscoll M. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419:808–14. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1009702
- 24.Liu J, Zhang B, Lei H, Feng Z, Liu J, Hsu A, Xu XZS. Functional aging in the nervous system contributes to age-dependent motor activity decline in C. elegans. Cell Metab. 2013;18:392–402. doi: 10.1016/j.cmet.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tonoki A, Kuranaga E, Tomioka T, Hamazaki J, Murata S, Tanaka K, Miura M. Genetic evidence linking age-dependent attenuation of the 26S proteasome with the aging process. Mol Cell Biol. 2009;29:1095–106. doi: 10.1128/MCB.01227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242991
- 26.Keller JN, Huang FF, Markesbery WR. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–56. doi: 10.1016/S0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242992
- 27.Carrano AC, Liu Z, Dillin A, Hunter T. A conserved ubiquitination pathway determines longevity in response to diet restriction. Nature. 2009;460:396–9. doi: 10.1038/nature08130. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1162407
- 28.Chondrogianni N, Petropoulos I, Franceschi C, Friguet B, Gonos ES. Fibroblast cultures from healthy centenarians have an active proteasome. Exp Gerontol. 2000;35:721–8. doi: 10.1016/S0531-5565(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 29.Kruegel U, Robison B, Dange T, Kahlert G, Delaney JR, Kotireddy S, Tsuchiya M, Tsuchiyama S, Murakami CJ, Schleit J, Sutphin G, Carr D, Tar K, Dittmar G, Kaeberlein M, Kennedy BK, Schmidt M. Elevated proteasome capacity extends replicative lifespan in Saccharomyces cerevisiae. PLoS Genet. 2011;7:e1002253. doi: 10.1371/journal.pgen.1002253. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13781958
- 30.Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, Ward W, Richardson A, Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106:3059–64. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/3266975
- 31.Vilchez D, Morantte I, Liu Z, Douglas PM, Merkwirth C, Rodrigues APC, Manning G, Dillin A. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–8. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/717957667
- 32.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–95. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 33.Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–6. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- 34.Singh R, Cuervo AM. Autophagy in the cellular energetic balance. Cell Metab. 2011;13:495–504. doi: 10.1016/j.cmet.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat Rev Mol Cell Biol. 2010;11:545–55. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 37.Sykiotis GP, Bohmann D. Stress-activated cap'n'collar transcription factors in aging and human disease. Sci Signal. 2010;3:re3. doi: 10.1126/scisignal.3112re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–12. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 39.Riedel CG, Dowen RH, Lourenco GF, Kirienko NV, Heimbucher T, West JA, Bowman SK, Kingston RE, Dillin A, Asara JM, Ruvkun G. DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat Cell Biol. 2013;15:491–501. doi: 10.1038/ncb2720. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718008425
- 40.Haynes CM, Fiorese CJ, Lin Y. Evaluating and responding to mitochondrial dysfunction: the mitochondrial unfolded-protein response and beyond. Trends Cell Biol. 2013;23:311–8. doi: 10.1016/j.tcb.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henis-Korenblit S, Zhang P, Hansen M, McCormick M, Lee S, Cary M, Kenyon C. Insulin/IGF-1 signaling mutants reprogram ER stress response regulators to promote longevity. Proc Natl Acad Sci USA. 2010;107:9730–5. doi: 10.1073/pnas.1002575107. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/4958956
- 42.Morley JF, Morimoto RI. Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Mol Biol Cell. 2004;15:657–64. doi: 10.1091/mbc.E03-07-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor RC, Dillin A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 2013;153:1435–47. doi: 10.1016/j.cell.2013.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718022157
- 44.Tullet JMA, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–38. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1104851
- 45.Rahman MM, Sykiotis GP, Nishimura M, Bodmer R, Bohmann D. Declining signal dependence of Nrf2-MafS-regulated gene expression correlates with aging phenotypes. Aging Cell. 2013;12:554–62. doi: 10.1111/acel.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242995
- 46.Westerheide SD, Anckar J, Stevens SM, Sistonen L, Morimoto RI. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science. 2009;323:1063–6. doi: 10.1126/science.1165946. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1157911
- 47.Blake MJ, Fargnoli J, Gershon D, Holbrook NJ. Concomitant decline in heat-induced hyperthermia and HSP70 mRNA expression in aged rats. Am J Physiol. 1991;260:R663–7. doi: 10.1152/ajpregu.1991.260.4.R663. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718242996
- 48.Shemesh N, Shai N, Ben-Zvi A. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell. 2013;12:814–22. doi: 10.1111/acel.12110. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718029849
- 49.Blake MJ, Udelsman R, Feulner GJ, Norton DD, Holbrook NJ. Stress-induced heat shock protein 70 expression in adrenal cortex: an adrenocorticotropic hormone-sensitive, age-dependent response. Proc Natl Acad Sci USA. 1991;88:9873–7. doi: 10.1073/pnas.88.21.9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pappas C, Hyde D, Bowler K, Loeschcke V, Sørensen JG. Post-eclosion decline in ‘knock-down’ thermal resistance and reduced effect of heat hardening in Drosophila melanogaster. Comp Biochem Physiol., Part A Mol Integr Physiol. 2007;146:355–9. doi: 10.1016/j.cbpa.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 51.Golden TR, Hubbard A, Melov S. Microarray analysis of variation in individual aging C. elegans: approaches and challenges. Exp Gerontol. 2006;41:1040–5. doi: 10.1016/j.exger.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 52.Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE. Transcriptional profile of aging in C. elegans. Curr Biol. 2002;12:1566–73. doi: 10.1016/S0960-9822(02)01146-6. [DOI] [PubMed] [Google Scholar]
- 53.Shore DE, Carr CE, Ruvkun G. Induction of cytoprotective pathways is central to the extension of lifespan conferred by multiple longevity pathways. PLoS Genet. 2012;8:e1002792. doi: 10.1371/journal.pgen.1002792. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242997
- 54.Durieux J, Wolff S, Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/8487957
- 55.Moore KA, Hollien J. The unfolded protein response in secretory cell function. Annu Rev Genet. 2012;46:165–83. doi: 10.1146/annurev-genet-110711-155644. [DOI] [PubMed] [Google Scholar]
- 56.Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–4. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 57.Gems D, Partridge L. Genetics of longevity in model organisms: debates and paradigm shifts. Annu Rev Physiol. 2013;75:621–44. doi: 10.1146/annurev-physiol-030212-183712. [DOI] [PubMed] [Google Scholar]
- 58.Lapierre LR, Gelino S, Meléndez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21:1507–14. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13324008
- 59.Alper S, McElwee MK, Apfeld J, Lackford B, Freedman JH, Schwartz DA. The Caenorhabditis elegans germ line regulates distinct signaling pathways to control lifespan and innate immunity. J Biol Chem. 2010;285:1822–8. doi: 10.1074/jbc.M109.057323. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718242998
- 60.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–5. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1003695
- 61.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–6. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P, Judy M, Lee S, Kenyon C. Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab. 2013;17:85–100. doi: 10.1016/j.cmet.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Prahlad V, Morimoto RI. Neuronal circuitry regulates the response of Caenorhabditis elegans to misfolded proteins. Proc Natl Acad Sci USA. 2011;108:14204–9. doi: 10.1073/pnas.1106557108. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13360104