Abstract
More than 300 years after Antonie van Leeuwenhoek gave the first description of microbes that colonize human body surfaces, the re-discovery of this multifaceted microbial world within our bodies has challenged our principal view on microbes. Novel sequencing techniques provide a plethora of (meta)genomic data, which elucidate the unique properties of mircobiota in different subjects. Moreover, the variety of metabolic and immunologic interactions between the mircobiota and the host's epithelial surfaces has challenged the paradigm of a unidirectional interplay between a given pathogen and the host's immune defense. The newly discovered mechanisms that underlie the symbiosis between the host, specific colonizers, and the mircobiota as a whole indicate that this colonization is more than a friendly coexistence. In fact, it represents a complex ecosystem with implications for the human metabolic homeostasis and immune tolerance. The resilience of the mircobiota and the capability to maintain a well-established equilibrium between symbionts and potential pathogens seem to be determining factors in shaping health or disease.
Introduction
The microbial world is a fascinating microcosm with many hazardous impacts and benefits for humans. Namely at the mucosal interface of epithelial barriers, host microbe interactions contribute to either the establishment of immune tolerance against beneficial microbes or the recognition and defense of jeopardous pathogens [1]. Since Robert Koch's identification of pathogenic microbes as principles of infection, we so far considered host-microbe interactions as a “duel between two players”. The development of next-generation sequencing techniques, accompanied by sophisticated biostatistical approaches, has widened our microbial horizon and led to the recognition that these interactions are embedded in a microbial ecosystem that has a determining influence on infectious interplays [2,3]. Moreover, the discovery of the mucosal microbiome and its role in host-environment interactions substantiated the basic impact of microbes on the onset and chronification of non-communicable inflammatory diseases [4].
Medical microbiologists, immunologists, and other biomedical scientists are challenged by the avalanche of data derived from this new dimension and have answered the question of how to integrate this new microbial scenario at the body surfaces into common concepts of disease development. Recent investigations of the gut's microbial world have changed our view on the immunological network that protects against infection and autoimmune reactions. Whereas formerly students learned that this network is governed mainly by lymph nodes, spleen, and tonsils, we now recognize that the gut and the gut's colonizing microflora are powerful players in this body-wide immune system. Moreover, these new insights give an idea about many links between metabolic and immune reactions, and their benefits for health [5].
Here we will outline the basic principles of how the human microbiota contributes to metabolic homeostasis, early programming of the immune system, and the maintenance of health.
The metabolic character of the human microbiome
The human microbiome is defined as the collective genomes of the complete microbiota present in a human body. The trillions of cells that make up the human microbiota represent a complex microbial community of bacterial, fungal, and possibly viral species. When the microbiome between individuals is compared, it can be stated that there is only one similarity: each microbiome is different. Despite this enormous inter-individual variety, the symbiosis between microbes and humans results in a stable and common metabolic pattern and a well-balanced physiological homeostasis. Data from the human microbiome project (HMP) clearly showed that metagenomic carriage of metabolic pathways is stable among individuals despite variation in taxonomic community structure [6].
Phylogenetically, the human body was occupied selectively by microorganisms from the environment in a co-evolutionary process. Following the principles of ecology, species from nearly all taxa of the microbial world (including archeae and eubacteria) adapted to the specific physicochemical conditions of habitats and niches of the human mucosal surfaces [7,8]. Those microbes were highly successful and add physiological benefits to the human host's physiology and the microbial community as well. This occurred namely in the gut, the core organ to degrade macronutrients and to absorb microelements, microbial metabolic pathways that support host digestion and primary catabolic pathways that favor symbiotic interactions and colonization [5]. Recently, the MetaHIT-Consortium (Metagenomics of the Human Intestinal Tract) identified three robust metabolic clusters that were microbiome enterotypes characterized by distinct primary metabolic pathways and products: the first enterotype harbors microbes belonging mainly to the genera Bacteroides/Parabacteroides, characterized by a broad and efficient saccharolytic potential via glycolysis and pentose phosphate cycle; the second enterotype is dominated by genus Prevotella/Desulfovibrio, known to degrade glycoproteins from the mucus layer; and the third enterotype is mainly composed of Ruminococcus/Akkermansia species, capable of cleaving glycoscaccharides from the mucus barrier. In a following study, it could be demonstrated that enterotypes are associated with long-term dietary pattern. A diet based on animal protein and fat (characteristic of Western lifestyles) was strongly associated with the Bacteroides enterotype, while a vegetarian carbohydrate-based diet was linked to the Prevotella enterotype [9]. These patterns seemed to be stable against short-term changes in dietary habits, indicating resilience and the ability to withstand exogenous ad hoc disturbance of the microbiota of adult subjects [10]. The associations between dietary habits and the bacterial enterotype give rise to further questions. Are subjects harboring the Bacteroides enterotype more susceptible to metabolic and/or cardiovascular diseases or obesity? Is a long-term switching from the Bacteroides enterotype to other enterotypes possible, and might a switch to a long-term carbohydrate-based diet reduce the risk for metabolic and cardiovascular diseases by changing the microbial enterotype?
A later study in Danish non-obese and obese study participants performed by La Chatelier et al. pointed out that subjects harboring a gut microbiota that is characterized by a low diversity of species are more often affected by obesity and low-grade inflammation than individuals whose microbiome shows a high microbial richness [11]. Moreover, dietary habits might be a predictor for the microbial complexity of the gut-associated microbiota. In a study with overweight French subjects, the subgroup with a low bacterial richness of the gut microbiota had an increased risk for metabolic deviation compared to those overweight persons bearing a complex gut mircobiota. In addition, these individuals were more susceptible to dietary intervention by increasing the microbial diversity in the gut [12]. Taken together, these data suggest that high microbial richness of the gut microbiome may be an indicator for metabolic homeostasis and a protecting factor against metabolic deviation and disease.
The crosstalk between the microbiome and the immune system
Interestingly, the intimate relationship between the microbial diversity of the gut microbiome and the metabolic homeostasis is paralleled by the interaction between microbes and the host's immune response. Bacterial diversity was recently found to be a fundamental factor in the establishment of a tolerogenic immune response [13]. Besides these metabolic aspects, the immunologic response is a major determinant in the interplay between humans and microbes. In parallel to the metabolic adaptation, the microbial species of the microbiome as well the host developed a complex and effective system of pattern recognition and tolerogenic signal cascades. Within the co-evolutionary development of the immune response, the host acquired mechanisms to distinguish between microbial friends and foes at the epithelial interfaces of the gut and other mucosal layers [14]. Early exposure to a rich microbial flora, as found in prototypic environments such as traditional farming sites, has been shown to be protective against the development of chronic inflammatory conditions such as allergic outcomes [15]. Though the adaptability of the human microbiota might decrease with age, the first ontogenetic developmental phase is characterized by high changeability of species from the environment to colonize the gut [16].
The neonatal intestinal colonization is initiated by the confrontation with the maternal vaginal microbiota during birth and followed by bacterial colonization associated with breast-feeding and digestion of the mother's milk [17]. It is not surprising that vaginally delivered and breastfed infants have a denser and more complex gut microbiota compared to children born by cesarean section (C-section) and fed with formulated milk [18,19]. Namely the numbers and variety of intestinal lactic acid bacteria are lower in babies born by C-section and/or fed with formulated milk during the first weeks of life. The early colonization seems to have sustainable effects on the further development of microbiome as well as on the maturation of immune response [20,21]. The symbiotic relationship between early colonization with lactic acid bacteria and the infant's maturing immune system seems to give an advantage to well-balanced and tolerogenic immune response and might thereby prevent later inflammatory conditions and their chronification. This notion is supported by a plethora of observational as well as interventional prospective studies [22]. Neonatal activation of the infant's gut colonization and prenatal stimulation of the maternal mircobiota and the immune system might enrich the infant's mircobiota and support prevention of later chronic inflammatory conditions. In this regard, maternal lifestyle risk factors during pregnancy might have an influence on the maternal microbiome as well as on the developing fetal immune system (See Figure 1) [4].
Figure 1. Pre- and postnatal environmental factors pave the way for later chronic inflammatory disease through the microbiome.
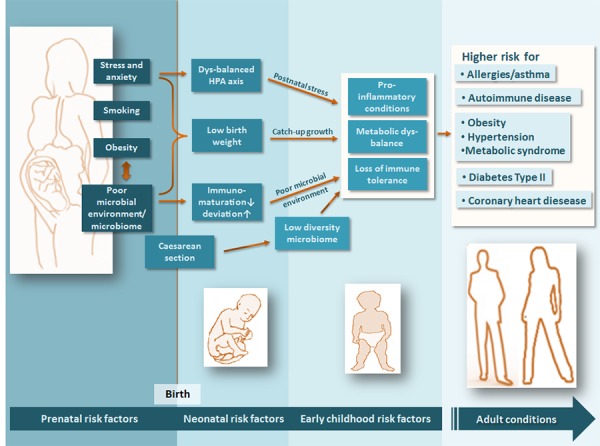
Maternal lifestyle factors such as dietary habits/obesity, smoking, a poor microbial environment (e.g. urban lifestyle), and chronic stress during pregnancy might lower the diversity of maternal mircobiota and decelerate the fetal immune development. Mode of delivery and immune maturation at birth might have an impact on the diversity and properties of the neo-/postnatal bacterial colonization. Neo-/postnatal nutrition, early chronic stressful events, and low microbial diversity of the living environment (e.g. exaggerated hygiene, frequent and early use of antibiotics) might hamper the development of a diverse and adaptive mircobiota, which favors a non-enriched mircobiota accompanied by pro-inflammatory conditions at the mucosal interfaces, a metabolic imbalance, and the loss of immune tolerance. This might result in a higher risk for chronic inflammatory and metabolic diseases in later life.
Early postnatal disturbance of the stepwise development of the fragile infant's microbiota (e.g. by use of antibiotics or excessive hygiene) might result in the dysbiosis of the microbiota (an imbalance between symbiotic and potentially pathogenic bacteria), a condition which subsequently fosters a disequilibrium of the immune response from regulation towards inflammation. In later childhood, malnutrition (Westernized dietary lifestyle) and stressful events might be powerful determinants that influence the equilibrium of the human microbiota and the capability to maintain immune tolerance [23-27]. Symbionts as well as pathogenic bacteria from the environment steadily migrate into the intestinal compartment. Hence, it is not surprising that potential pathogens (e.g. Clostridia spp. or Klebsiella spp.) are a common part of the normal microbiota of healthy beings. Under the control of a regulatory immune system, the host is capable of containing these strains [19]. Loss of a certain regulatory capacity will result in a dysbiosis between symbionts and pathogens, leading to an excess of pro-inflammatory pathogens with the consequence of inflammation. The thesis is supported by the occurrence of pseudomembranous colitis, a life-threatening diarrhea caused by Clostridium difficile. This strain is a common colonizer of the normal flora, whose numbers could tremendously increase under antibiotic regimens. A substantial loss of symbiotic bacteria of the gut as a consequence of antibiosis results in a dyscontainment of this pathogen and severe consequences for the host [28].
Mechanisms by which symbionts of the gut microbiome instruct the immune system
Both the innate as well as the adaptive immune system were shown to be shaped and instructed by bacterial species that colonize the gut. A growing body of evidence that comes from animal models indicates that tolerogenic dendritic cells (DCs) and T regulatory (Treg) cells play a fundamental role in the development of a well-balanced immune response that is maintained by the symbionts of the gut microbiota [14]. Treg cells play a major role in the control of pro-inflammatory Th1, Th2, and Th17 subsets that drive chronic inflammatory diseases, such as allergies and autoimmune diseases. Treg cells are directly addressed by symbiotic bacteria of the human microbiota [29] as recently shown for the symbiont Bacteroides fragilis, a frequent colonizing bacterium in the gut. In a basic experiment, Dennis Kasper's group [30] inoculated germ-free mice with the bacterium that is known to produce polysaccharide A (PSA). Release of PSA at the epithelial layer of the gut is taken up by DCs, which present PSA to naive CD4+ T cells by migrating into the mesenteric lymph nodes. T cells are thereby instructed to differentiate into Treg cells and to produce anti-inflammatory cytokines such as tumor growth factor β (TGF-β) and interleukin (IL)-10. These cytokines are capable of suppressing pro-inflammatory responses that occur in intestinal inflammation such as inflammatory bowel disease (IBD). An adequate model to mimic human IBD in mice is the infection of the intestine with Helicobacter hepaticus. Infection with H. hepaticus is followed by the recruitment of Th17 cells, which release pro-inflammatory cytokines IL-17, IL-23, and tumor necrosis factor-alpha (TNF-α) at the epithelial barrier and foster intestinal inflammation. Mice colonized primarily with B. fragilis could suppress the production of pro-inflammatory cytokines IL-17, IL-23, and TNF-α when infected with H. hepaticus and prevent the intestine from inflammation (See Figure 2) [31].
Figure 2. The role of the mircobiota in the establishment of a tolerogenic or pro-inflammatory immune response of the host.
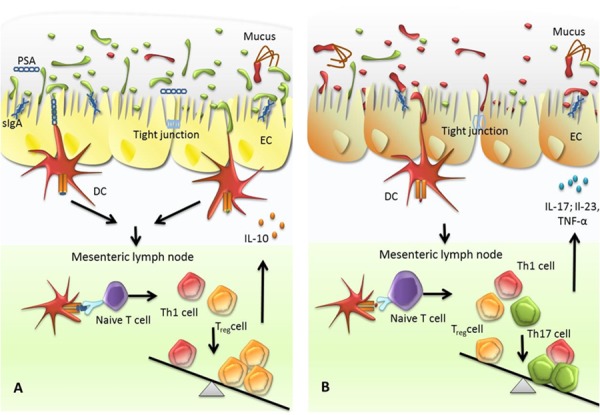
(A) Dominance of symbiotic bacteria within the gut microbiota favors a tolerogenic immune response. The gut microbiota is composed of both symbiotic/commensal bacteria and pathogen bacteria. A majority of symbiotic bacteria are able to control excessive multiplying of pathogenic species. Release of secretory IgA (sIgA) stabilizes tight junctions between cells of the epithelial layer and hampers pathogens as well as symbionts to invade into deeper layers. sIgA promotes the formation of biofilms by symbionts at the epithelial layer and the integrity of mucus layer. Mucus-derived glycans degraded by symbiotic bacteria, polysaccharides produced by symbiotic bacteria, and bacteria adhered at epithelial cells are sensed by dendritic cells (DCs) and presented at major histocompatibility complexes class II (MHCII). Antigen-presenting DCs migrate to the mesenteric lymph nodes and to present antigens to naive T cells. T cells are instructed to differentiate into T helper 1 (Th1) and T regulatory (Treg) cells with a predominance of the latter. As a consequence, T cells are released that predominantly produce anti-inflammatory cytokines such as interleukin-10 (IL-10), supporting tolerance against the microbiota. (B) A loss of symbiotic bacteria promotes growth of pathogenic bacteria. These bacteria are sensed by DCs that subsequently migrate to mesenteric lymph nodes to promote naive T cells to differentiate into Th1 and Th17 cells. As a result, these cells produce pro-inflammatory cytokines such as IL-17, IL-23, and tumor necrosis factor-alpha (TNFα) to increase the mucosal defence against enteric pathogens.
Investigations at the mucus layer of epithelial interfaces point out a number of symbiotic bacteria colonizing the different sections of the mucus, such as Akkermansia spp. or Bacteroides thetaiotaomicron [32,33]. These bacteria harbor mucus-degrading enzymes and release metabolic products that might support the human metabolism and stimulate the regulatory immune response [34] (See Figure 2).
Recent findings point out the crucial role of secretory IgA (sIgA) [35] in immune exclusion of pathogenic bacteria. sIgA exported into the lumen acts at the front line of the host defense by preserving the local homeostasis and as a neutralizing agent. Enhanced interaction between sIgA-coated symbiotic bacteria and the epithelium reinforces the tight junctions and results in an overproduction of polymeric immunoglobulin receptor (PIGR) and reduction in nuclear factor “kappa-light-chain-enhancer” of activated B cells (NF-κB). These mechanisms are preventive against epithelial pro-inflammatory signals and protect the epithelial barrier [36]. M cells are specialized to take up sIgA immune complexes with symbiont, and/or pathogenic bacteria, wherein they are targeted to myeloid DCs, which in consequence results in a downregulation of local pro-inflammatory responses. This was shown for the pathogen Shigella flexneri coated with sIgA [37]. Favoring biofilm formation of non-pathogenic bacteria and excluding pathogenic bacteria from the epithelial surface through encapsulation within mucus sIgA might provide a selective role against gut-colonizing bacteria [38,39]. There is growing evidence that symbiotic bacteria are triggering the release of sIgA [40]. In a mouse model using germ-free animals infected with the symbiont, B. thetaiotaomicron was identified as an inducer of sIgA and a regulator of bacterial growth, pro-inflammatory signaling, and oxidative burst suppression [41] (See Figure 2).
The intestinal microbiome and other organs – is there an axis between the gut, the lung, and the brain?
Most studies on the human microbiome focus on the intestinal compartment. This is not only reasoned by the fact that gut microbiome is readily accessible via stool assessment. Successful postnatal oral probiotic intervention against pulmonary diseases such as allergies has underlined the fundamental role of the gut microbiome in inflammatory conditions of the lung [42], but the underlying mechanism by which the gut microbiome instructs inflammatory conditions in other organs is far from being unraveled. Though there exists a body-wide lymphatic network, it is not clear which factors are responsible for these long-distance effects. It seems clear that there is a certain “window of opportunity” around birth that allows host-microbial interactions, which shape the immune system systematically. Our group could demonstrate that environmental microbes are protective against airway inflammation when given locally in the lung as well as intragastric during the perinatal life span [43]. In line with our findings, Olszak et al. showed that selective neonatal gut colonization of germ-free animals suppresses IBD as well as asthma, and was associated with a significant reduction of invariant natural killer cells (iNKT) in the gut and lung mucosal epithelium [44].
Based on observations that changes in the gut microbiota are associated with modifications in the central nervous system (CNS) and behavioral parameters, the question of whether a direct axis links the gut and brain arises [45]. Endocrine pathways such as the hypothalamic-pituitary-adrenal (HPA) axis act as links between the nervous and the immune system via cortisol. In turn, the nervous system is linked to the gut by the vagus nerve as a feedback system for the autonomous gut innervation. In addition, cytokine patterns might play a crucial role in the psychoneuroimmunlogical interplay between gut and brain. These patterns might be altered by cortisol shifts as observed under chronic stress conditions. The immunosuppressive effect of cortisol might also affect the conditions at the gut epithelium as well as gut bacterial composition [46]. Repeated stressful events were associated with changes in the Bacteroides spp. population at the gut mucosa [47] with consequences for the cytokine release by immune and epithelial cells of the gut. Altered cytokines of the gut compartment might be released into blood circulation and could pass the blood-brain-barrier and might affect brain function and inflammatory condition in the CNS [48]. In addition, some cytokines have been shown to possess additional properties when released in the brain. For example, IL-6 is recognized as a pivotal player in the neurogenesis of the hippocampus, a basic mechanism in cognitive and emotional behavior [49]. Disturbance of neurogenesis by altered cytokine levels might contribute to affective disorders such as major depression [50]. There are a number of hints from animal models that gut microbiota is linked to functional trades of the CNS [51-53]. To verify that the gut microbiome is closely linked to the cognitive and behavioral capacity of the CNS, more detailed research is needed to better understand the molecular, cellular, and biochemical principles of this putative communication.
Future perspectives
We are still at the beginning of a comprehensive view of the various impacts of the microbial world that colonizes the human body. The challenges for further research are manifold. The findings presented here underline that the gut microbiome is causatively involved in the development of inflammation. Nevertheless, the “hen-egg-question”, addressing the option that reduced diversity might precede clinical phenotypes, is still unanswered. Chronic inflammatory processes, as those that occur in asthma or IBD, modify the habitat of the mircobiota by remodeling the epithelial tissues. Remodeling of colonized surfaces might have a changing impact on the composition of the microbiota and the inflammatory development.
Another future focus will be laid on the characterization of lung microbiome. So far, the lower airways have been considered a sterile organ, but there is evidence that not only the upper but also the lower airways might be colonized by a plethora of microbes [54]. The role of microbial communities in communicable and non-communicable respiratory disease is still not unraveled.
Designing novel approaches in data management and analyses will take a great deal of effort. The technical possibilities to assess microbial communities will grow rapidly, and novel and deeper sequencing techniques will produce even more complex data sets [55]. Flexible and sophisticated statistical approaches will be needed to utilize these data in answering the question about how the microbiome shapes health and disease.
At least the microbiome offers a new dimension for interventional strategies on the preventive and therapeutic level. Personalized analysis and manipulation of microbiome might be a long-term perspective in the treatment of infectious and inflammatory diseases.
Acknowledgments
The authors thank Michaela Gnittka for critical revision of the manuscript.
Abbreviations
- CNS
central nervous system
- DC
dendritic cell
- IBD
inflammatory bowel disease
- IL
interleukin
- PSA
polysaccharide A
- sIgA
secretory IgA
- spp
species
- Th
T helper
- TNF-α
tumor necrosis factor-alpha
- Treg
T regulatory
Disclosures
The authors declare that they have no competing interests.
Grant information
The authors declared that no grants were involved in supporting this work.
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/6/11
References
- 1.Shanahan F. The host-microbe interface within the gut. Best Pract Res Clin Gastroenterol. 2002;16:915–31. doi: 10.1053/bega.2002.0342. [DOI] [PubMed] [Google Scholar]
- 2.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–4. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718240416
- 3.Weinstock GM. Genomic approaches to studying the human microbiota. Nature. 2012;489:250–6. doi: 10.1038/nature11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prescott SL. Early-life environmental determinants of allergic diseases and the wider pandemic of inflammatory noncommunicable diseases. J Allergy Clin Immunol. 2013;131:23–30. doi: 10.1016/j.jaci.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Sommer F, Bäckhed F. The gut microbiota--masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 6.Human Microbiome Project Consortium A framework for human microbiome research. Nature. 2012;486:215–21. doi: 10.1038/nature11209. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718046907
- 7.Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717952553
- 8.Wylie KM, Truty RM, Sharpton TJ, Mihindukulasuriya KA, Zhou Y, Gao H, Sodergren E, Weinstock GM, Pollard KS. Novel bacterial taxa in the human microbiome. PLoS ONE. 2012;7:e35294. doi: 10.1371/journal.pone.0035294. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240431
- 9.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto J, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–80. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/10015956
- 10.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334:105–8. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240432
- 11.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto J, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, et al. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–6. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718089027
- 12.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto J, Renault P, Doré J, Zucker J, Clément K, Ehrlich SD, Blottière H, Leclerc M, Juste C, de Wouters T, Lepage P, Fouqueray C, Basdevant A, Henegar C, Godard C, Fondacci M, Rohia A, Hajduch F, et al. Dietary intervention impact on gut microbial gene richness. Nature. 2013;500:585–8. doi: 10.1038/nature12480. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718089024
- 13.Ege MJ, Mayer M, Normand A, Genuneit J, Cookson WOCM, Braun-Fahrländer C, Heederik D, Piarroux R, Mutius E von. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/8846957
- 14.Garn H, Neves JF, Blumberg RS, Renz H. Effect of barrier microbes on organ-based inflammation. J Allergy Clin Immunol. 2013;131:1465–78. doi: 10.1016/j.jaci.2013.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutius E von, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–8. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 16.Martin R, Nauta AJ, Ben Amor K, Knippels LMJ, Knol J, Garssen J. Early life: gut microbiota and immune development in infancy. Benef Microbes. 2010;1:367–82. doi: 10.3920/BM2010.0027. [DOI] [PubMed] [Google Scholar]
- 17.Sanz Y. Gut microbiota and probiotics in maternal and infant health. Am J Clin Nutr. 2011;94:2000S–2005S. doi: 10.3945/ajcn.110.001172. [DOI] [PubMed] [Google Scholar]
- 18.Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107:11971–5. doi: 10.1073/pnas.1002601107. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240433
- 19.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–21. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718240434
- 20.Fallani M, Amarri S, Uusijarvi A, Adam R, Khanna S, Aguilera M, Gil A, Vieites JM, Norin E, Young D, Scott JA, Doré J, Edwards CA. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology (Reading, Engl) 2011;157:1385–92. doi: 10.1099/mic.0.042143-0. [DOI] [PubMed] [Google Scholar]
- 21.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5:e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1157369
- 22.Pfefferle PI, Prescott SL, Kopp M. Microbial influence on tolerance and opportunities for intervention with prebiotics/probiotics and bacterial lysates. J Allergy Clin Immunol. 2013;131:1453–63. doi: 10.1016/j.jaci.2013.03.020. quiz 1464. [DOI] [PubMed] [Google Scholar]
- 23.Pérez-Cobas AE, Gosalbes MJ, Friedrichs A, Knecht H, Artacho A, Eismann K, Otto W, Rojo D, Bargiela R, von Bergen M, Neulinger SC, Däumer C, Heinsen F, Latorre A, Barbas C, Seifert J, Dos Santos VM, Ott SJ, Ferrer M, Moya A. Gut microbiota disturbance during antibiotic therapy: a multi-omic approach. Gut. 2013;62:1591–601. doi: 10.1136/gutjnl-2012-303184. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718158970
- 24.Laitinen K, Collado MC, Isolauri E. Early nutritional environment: focus on health effects of microbiota and probiotics. Benef Microbes. 2010;1:383–90. doi: 10.3920/BM2010.0045. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718240440
- 25.O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A, Quigley EMM, Cryan JF, Dinan TG. Early life stress alters behavior, immunity, and microbiota in rats: implications for irritable bowel syndrome and psychiatric illnesses. Biol Psychiatry. 2009;65:263–7. doi: 10.1016/j.biopsych.2008.06.026. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/7985958
- 26.Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X, Kubo C, Koga Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol (Lond) 2004;558:263–75. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1019368
- 27.García-Ródenas CL, Bergonzelli GE, Nutten S, Schumann A, Cherbut C, Turini M, Ornstein K, Rochat F, Corthésy-Theulaz I. Nutritional approach to restore impaired intestinal barrier function and growth after neonatal stress in rats. J Pediatr Gastroenterol Nutr. 2006;43:16–24. doi: 10.1097/01.mpg.0000226376.95623.9f. [DOI] [PubMed] [Google Scholar]
- 28.de La Cochetière MF, Durand T, Lalande V, Petit JC, Potel G, Beaugerie L. Effect of antibiotic therapy on human fecal microbiota and the relation to the development of Clostridium difficile. Microb Ecol. 2008;56:395–402. doi: 10.1007/s00248-007-9356-5. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718240459
- 29.Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204–9. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/3790975
- 30.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1111083
- 31.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–18. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/14064
- 32.Derrien M, Vaughan EE, Plugge CM, Vos WM de. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54:1469–76. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 33.Sonnenburg JL, Xu J, Leip DD, Chen C, Westover BP, Weatherford J, Buhler JD, Gordon JI. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science. 2005;307:1955–9. doi: 10.1126/science.1109051. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1025863
- 34.Derrien M, van Baarlen P, Hooiveld G, Norin E, Müller M, Vos WM de. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front Microbiol. 2011;2:166. doi: 10.3389/fmicb.2011.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240460
- 35.Mantis NJ, Rol N, Corthésy B. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240461
- 36.Mathias A, Duc M, Favre L, Benyacoub J, Blum S, Corthésy B. Potentiation of polarized intestinal Caco-2 cell responsiveness to probiotics complexed with secretory IgA. J Biol Chem. 2010;285:33906–13. doi: 10.1074/jbc.M110.135111. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240467
- 37.Sansonetti PJ, Arondel J, Cantey JR, Prévost MC, Huerre M. Infection of rabbit Peyer's patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun. 1996;64:2752–64. doi: 10.1128/iai.64.7.2752-2764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240468
- 38.Bollinger RR, Everett ML, Palestrant D, Love SD, Lin SS, Parker W. Human secretory immunoglobulin A may contribute to biofilm formation in the gut. Immunology. 2003;109:580–7. doi: 10.1046/j.1365-2567.2003.01700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bollinger RR, Everett ML, Wahl SD, Lee Y, Orndorff PE, Parker W. Secretory IgA and mucin-mediated biofilm formation by environmental strains of Escherichia coli: role of type 1 pili. Mol Immunol. 2006;43:378–87. doi: 10.1016/j.molimm.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Geuking MB, McCoy KD, Macpherson AJ. Metabolites from intestinal microbes shape Treg. Cell Res. 2013;12:1339–40. doi: 10.1038/cr.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peterson DA, McNulty NP, Guruge JL, Gordon JI. IgA response to symbiotic bacteria as a mediator of gut homeostasis. Cell Host Microbe. 2007;2:328–39. doi: 10.1016/j.chom.2007.09.013. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718240475
- 42.Forsythe P. Probiotics and lung diseases. Chest. 2011;139:901–8. doi: 10.1378/chest.10-1861. [DOI] [PubMed] [Google Scholar]
- 43.Conrad ML, Ferstl R, Teich R, Brand S, Blümer N, Yildirim AO, Patrascan CC, Hanuszkiewicz A, Akira S, Wagner H, Holst O, Mutius E von, Pfefferle PI, Kirschning CJ, Garn H, Renz H. Maternal TLR signaling is required for prenatal asthma protection by the nonpathogenic microbe Acinetobacter lwoffii F78. J Exp Med. 2009;206:2869–77. doi: 10.1084/jem.20090845. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1442956
- 44.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, Glickman JN, Siebert R, Baron RM, Kasper DL, Blumberg RS. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/14248956
- 45.Dinan TG, Cryan JF. Melancholic microbes: a link between gut microbiota and depression? Neurogastroenterol. Motil. 2013;25:713–9. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 46.Montiel-Castro AJ, González-Cervantes RM, Bravo-Ruiseco G, Pacheco-López G. The microbiota-gut-brain axis: neurobehavioral correlates, health and sociality. Front Integr Neurosci. 2013;7:70. doi: 10.3389/fnint.2013.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain Behav Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718240476
- 48.Miller AH, Haroon E, Raison CL, Felger JC. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress Anxiety. 2013;30:297–306. doi: 10.1002/da.22084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Erta M, Quintana A, Hidalgo J. Interleukin-6, a major cytokine in the central nervous system. Int J Biol Sci. 2012;8:1254–66. doi: 10.7150/ijbs.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718240477
- 51.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–5. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/13131956
- 52.Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, Macqueen G, Sherman PM. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60:307–17. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/6142957
- 53.Neufeld KM, Kang N, Bienenstock J, Foster JA. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255–64. doi: 10.1111/j.1365-2982.2010.01620.x. e119. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/11073958
- 54.Huang YJ, Charlson ES, Collman RG, Colombini-Hatch S, Martinez FD, Senior RM. The role of the lung microbiome in health and disease. A National Heart, Lung, and Blood Institute workshop report. Am J Respir Crit Care Med. 2013;187:1382–7. doi: 10.1164/rccm.201303-0488WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teeling H, Glöckner FO. Current opportunities and challenges in microbial metagenome analysis--a bioinformatic perspective. Brief. Bioinformatics. 2012;13:728–42. doi: 10.1093/bib/bbs039. [DOI] [PMC free article] [PubMed] [Google Scholar]


