Abstract
Measurement of gait is essential for identifying underlying deficits contributing to gait dysfunction, guiding clinical decisions and measuring rehabilitation outcomes. Velocity is commonly used to measure gait, however, its interpretation in patient populations is complicated by the confound of age. Gait symmetry may be an additional and valuable measure since it may not feature the same age-related changes as velocity. The purpose of this study was to determine if gait symmetry is related to age.
METHODS
Spatiotemporal gait parameters were recorded for 172 individuals with stroke and 81 healthy adults walking across a pressure sensitive mat at their preferred speed. Swing time, stance time and step length symmetry ratios were calculated. The relationship of age to velocity and symmetry was examined using Pearson correlations.
RESULTS
There was a significant negative association between velocity and age in the healthy group (r=−0.57, p<0.01). There were no significant relationships between age and any of the three symmetry ratios for either the stroke or healthy groups.
CONCLUSIONS
The main finding of the current study is that gait symmetry ratios are not significantly associated with age in either a healthy or a post-stroke group. Gait symmetry ratios may therefore, allow the clinician and the researcher to make judgments about the effects of disease (such as stroke) on the control of an individual’s gait without the confound of age.
INTRODUCTION
Gait is a major determinant of independence, quality of life and participation1 and is frequently impaired by a variety of musculoskeletal and neurological conditions or diseases (e.g. osteoarthritis, lower limb amputation, multiple sclerosis, stroke). Improvement of walking function is a common goal stated by patients undergoing rehabilitation. In stroke, for example, it is the number one stated goal by patients2. Due to its importance to patients and independent function, walking receives considerable attention in rehabilitation3. Development and testing of gait interventions is important, but progress will depend on more sensitive and objective measurements of gait. Gait measurement is essential for understanding the particular deficits exhibited by a patient, guiding clinical decision making, customizing treatment and monitoring the effectiveness of a gait intervention.
In the clinical setting, spatiotemporal gait parameters are the most common objective measurements performed. In particular, gait velocity is the most widely reported measure4 and is believed to be a good indicator of overall gait performance5, 6,7. Gait velocity may also be used as an indicator of fall risk8, fear of falling9 and the capacity for community ambulation10. Gait symmetry is another measure gaining acceptance and provides a vital complementary measure to gait velocity11. Gait symmetry can be considered an indicator of the degree of gait control since it is a measure of the parallels of spatiotemporal gait variables (i.e. swing time, stance time or step length) between the lower limbs, 11, 12. A comparison of the common expressions of gait symmetry reported in the literature, recommended that gait symmetry be expressed as a simple ratio of the right and left limb values for swing time, stance time and step length12. This work also provided normative cut off values for each of the three symmetry ratios based on the 95% confidence intervals for the mean symmetry values for a group of healthy adults12. These provide a reference point to which a clinician or researcher may compare symmetry ratios for a patient population in order to determine if those values represent normal, symmetric gait or asymmetric gait.
Clinically, reduction of asymmetry is commonly addressed by rehabilitation therapists particularly in the post-stroke population13. There are a number of proposed negative consequences associated with persisting gait asymmetry post-stroke which include: 1) challenges to dynamic balance control, 2) gait inefficiencies, 3) cumulative musculoskeletal injury to the non-paretic lower limb, 4) loss of bone mass density in the femoral neck of the paretic lower limb, and 5) reduced activity levels which may occur in response to any one or combination of the preceding consequences14,15. There is also evidence that gait asymmetry may worsen over time post-stroke11, 16.While direct links between gait asymmetry and these negative consequences have not yet been established, several lines of indirect evidence provide support to these hypotheses although an in-depth discussion of this evidence is beyond the scope of the current study. However, given the clinical relevance, it is important to establish the usefulness of symmetry ratios as complementary gait measures to velocity.
The interpretation of gait velocity in patient populations such as osteoarthritis and stroke, has always been complicated by the confound of age. The relationship of age and gait velocity has been previously investigated. Bohannon17 described a negative relationship of velocity with age (r=−0.21). Himann and coauthors18 (1988) reported a critical age of decline for gait velocity (62 years), after which age becomes a significant predictor of velocity accounting for 37% of the variance of speed observed in a group of healthy individuals18. After the age of 62 years, gait velocity declines at a rate of 16% and 12%, per decade of life for men and women respectively18. Taking these results into account, decreased velocity in an older adult is challenging to interpret since it is difficult to separate the effects of ageing from the effects of the disease, especially when the prevalence or risk of the disease increases with age (such as osteoarthritis19 and stroke20).
It is possible that gait symmetry does not manifest the same age-related changes as velocity. Symmetrical gait is considered to be the most efficient walking pattern21. Symmetry is also unrelated to velocity in healthy individuals: healthy individuals walk symmetrically regardless of the speed at which they walk22. Barring some unilateral condition (e.g. stroke, arthritis), we have not seen any evidence that would lead us to suspect that the symmetrical gait pattern should change with age alone. If this is the case, gait symmetry could prove a valuable complementary measurement to velocity. A symmetry ratio that is outside the range for healthy adults could be attributed to changes associated with the disease without concern that an individual’s age has also had some influence.
The purpose of this study was to determine if gait symmetry is related to age. We compared the associations of age with velocity and three symmetry ratios (step length, swing time and stance time) in a group of healthy individuals. In order to verify that age and symmetry are not related in the presence of a disease that affects gait, we also compared associations of age with velocity and symmetry in a group of individuals with stroke.
METHODS
Data was abstracted from an ongoing standardized database that includes healthy individuals and those with stroke recruited from two tertiary referral centres of the University of X. The study was approved by the Research Ethics Boards at both institutions and all participants provided written informed consent prior to participation in the study.
Participants were selected from this database if they had a history of a single unilateral stroke (hemorrhagic or ischemic) and had completed an over-ground, preferred-pace walking task without assistance or their customary gait aid (if one was used). We restricted our analysis to walking trials performed without a gait aid since it has been shown that assistive devices can improve gait symmetry ratios in individuals with stroke who are asymmetric23. The use of the aid allows individuals to compensate for gait deficits which could lead to an underestimation of the extent of walking dysfunction in the study group. A total of 172 individuals with stroke and 81 healthy individuals were included in the present analysis.
Measurements
The database contained data from a standardized assessment that included both clinical and laboratory gait assessments.
Clinical Assessment
The clinical assessment consisted of two stroke-specific measures: the National Institutes of Health Stroke Scale (NIHSS) and the Chedoke McMaster Stroke Assessment (CMSA). The NIHSS is a standardized assessment of the severity of stroke-related neurologic deficit with higher scores indicating worse impairment24. The leg and foot dimensions of the CMSA (each measured with a 7-point scale) were used as a measure of motor impairment. Lower scores indicate greater motor impairment. The CMSA has good intra- and inter-rater reliability as well as good concurrent validity with the Fugl-Meyer25, 26.
Spatiotemporal Gait Measures
Spatiotemporal parameters of gait were measured using a pressure sensitive mat (GaitRite™, CIR Systems, Clifton, NJ). The GaitRite™ mat is 366cm in length and 81cm in width and contains a grid of 48 by 288 sensors (total of 13824 sensors) arranged 1.27 cm on center. Data were sampled at 30Hz and stored in a personal computer that calculated spatial and temporal parameters using application software. All individuals performed a standardized walking task: three trials of walking at their preferred speed, across a level 10m walkway which included the pressure sensitive mat in the middle. Stroke participants were requested to perform the walking trials without their customary gait aid but were permitted to wear a splint if needed.
Data and Statistical Analysis
All calculations and statistical analyses were performed using SAS 9.1 software (SAS Institute Inc, Cary, NC).
Descriptive Statistics
Group means and standard deviations (SD) were calculated for age, velocity, step length symmetry ratio, swing time symmetry ratio and stance time symmetry ratio. Group means for all these variables were compared between the stroke and healthy groups using an unpaired t-tests.
Calculation of symmetry ratios
Calculation of spatiotemporal parameters was performed using the GaitRite™ system. All values were averaged over the three trials for each individual and average data were used for calculation of the symmetry ratios. Calculation of step length symmetry, swing time symmetry and stance time symmetry has been described in detail previously12 but will be described briefly here. The left and right average values of step length, swing time and stance time were each used in a ratio with the largest value in the numerator so that all values for every individual were >1.0. A ratio value of 1.0 denotes perfect symmetry. (It should be noted that since swing time on one limb equates to single limb stance time on the opposite limb, swing time symmetry could also be called single limb stance symmetry.) The mean and standard deviation (SD) of velocity, step length symmetry, swing time symmetry and stance time symmetry were calculated for both the stroke and healthy groups.
Relationship of age with velocity and symmetry
The relationship of age with velocity and the three symmetry ratios within the stroke and healthy groups were determined using Pearson correlations. A Fisher’s transformation was used to convert the r values to z values and 95% confidence intervals (CI) were calculated for each r value.
RESULTS
The database contained data from 193 individuals. The number of individuals excluded and the reasons for exclusion were as follows: 8 because of a second stroke, 2 because no walking trials without a gait aid were available, 5 because age was not available and 6 because they did not have a confirmed diagnosis of stroke. Of the remaining 172 individuals, 39 had more than one visit recorded in the database. A single visit was randomly selected for each of these 39 individuals. In total, 172 visits (from 172 unique participants) were used in the analysis. The mean (SD) age was 63.2 (13.2) years and time post-stroke for the group was 23.5 (31.3) months. Sixty-one participants (35.5%) were women and 82 (47.7%) of the group had right-side hemiparesis. X (x%) participants regularly used a gait aid but were able to complete walking trials without their customary aid. The post-stroke group had a mean NIHSS score of 2.6 (2.4) and CMSA leg and foot scores of 5.0 (1.4) and 4.8 (1.5) respectively. A convenience sample of 81 healthy participants was also recruited. The mean age for the control group was 64.2 (22.4) years and 43 participants (53.8%) were women. The mean ages were not significantly different between the two groups.
The mean (SD) values for velocity, step length, swing time and stance time ratios for the stroke and healthy groups are summarized in Table 1. The two groups were significantly different in velocity, step length symmetry, swing time symmetry and stance time symmetry with the stroke group walking more slowly and with greater asymmetry than the healthy group (all p<0.01). All three mean symmetry values for the stroke group were above the normative cut-offs reported in previous work12 indicating that on average the stroke group exhibited asymmetric gait.
Table 1.
Group mean gait velocity and symmetry values.
| Healthy | Stroke | |
|---|---|---|
| Velocity (cm/s) | 113.79 (23.34) | 73.89 (33.30)* |
| Step length symmetry ratio | 1.03 (0.02) | 1.13 (0.25)* |
| Swing time symmetry ratio | 1.02 (0.02) | 1.23 (0.33)* |
| Stance time symmetry ratio | 1.02 (0.02) | 1.09 (0.10)* |
The mean (SD) values for gait velocity and three symmetry ratios for both the stroke and healthy groups are provided. Variables for the stroke group that are significantly different from those of the healthy group are denoted by an asterisk (*) (p<0.01).
Relationship of age with velocity and symmetry
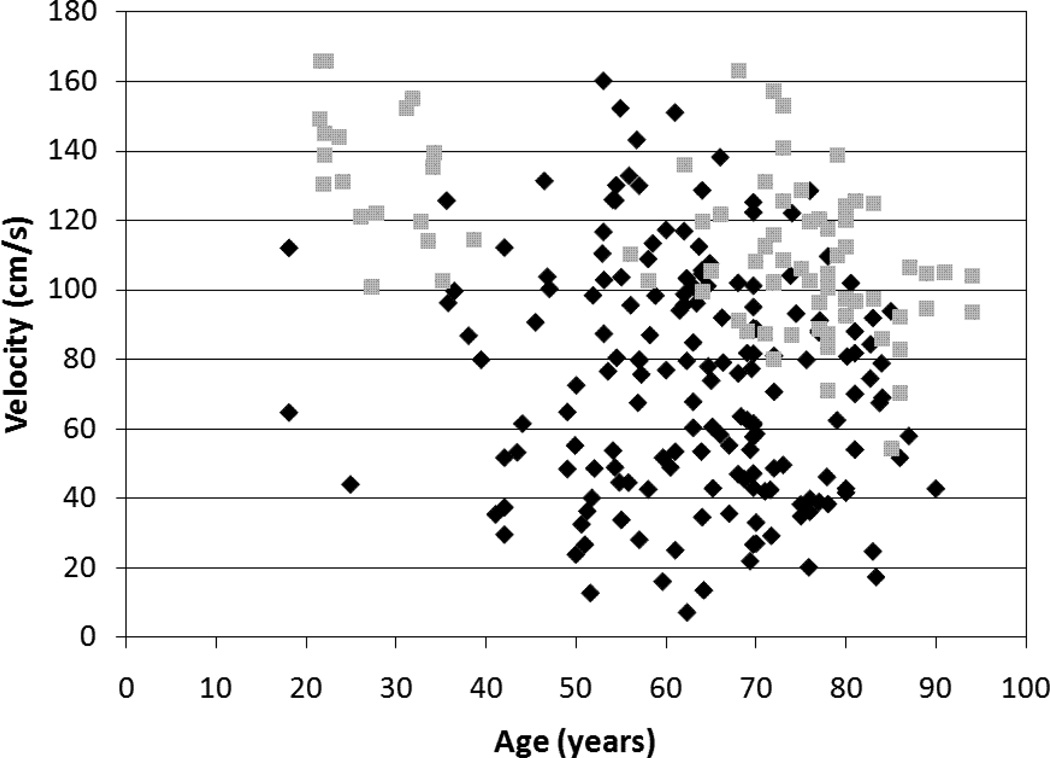
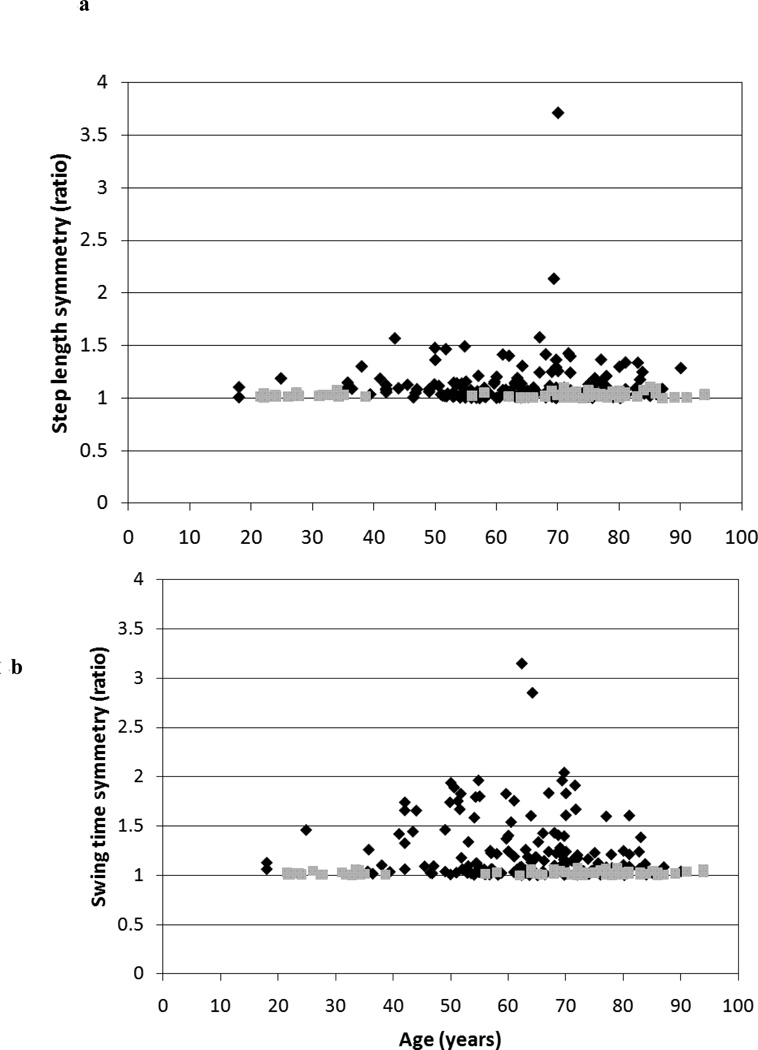
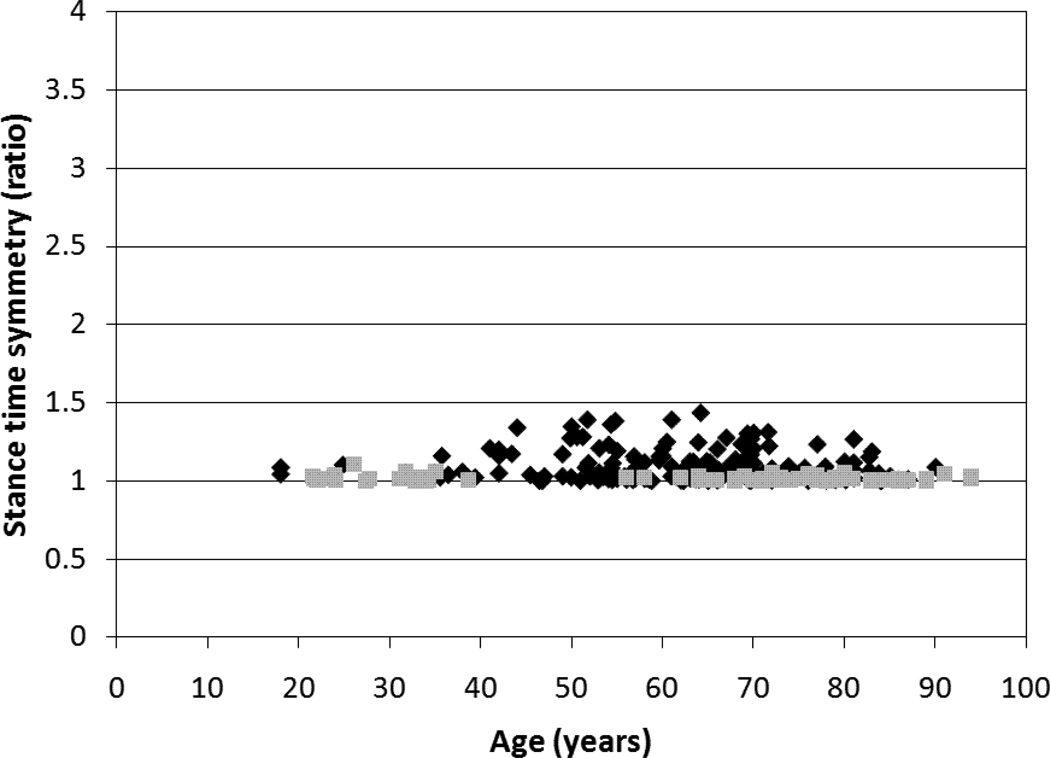
The correlation coefficients and 95% confidence intervals (CI) for the relationship of age with velocity and symmetry are included in Table 2. The only significant association was between velocity and age in the healthy group (r=−0.57, p<0.01). Gait symmetry was not significantly associated with age in the healthy group or the stroke group. All 95% CIs for the various r values contained zero except for the CI for the association between age and velocity in the healthy group. A comparison of the relationship between velocity and age for the stroke and healthy groups is included in Figure 1. A comparison of the relationship between age and each of the three symmetry ratios for the stroke and healthy groups is included in Figure 2.
Table 2.
Correlation coefficients for the association between age and gait velocity and symmetry values
| Gait Variable Association with Age |
Healthy | Stroke | ||||
|---|---|---|---|---|---|---|
| R value | 95% CI | R value | 95% CI | |||
| Velocity (cm/s) | −0.57 (p<0.001) | −0.70 to | −0.40 | −0.14 (p=0.07) | −0.28 to | 0.01 |
| Step length symmetry ratio | 0.17 (p=0.12) | −0.05 to 0.38 | 0.04 (p=0.61) | −0.11 to 0.19 | ||
| Swing time symmetry ratio | 0.13 (p=0.24) | −0.09 to 0.34 | −0.13 (p=0.09) | −0.28 to 0.02 | ||
| Stance time symmetry ratio | −0.13 (p=0.25) | −0.34 to 0.09 | −0.14 (p=0.07) | −0.28 to 0.01 |
The Pearson r values for age and gait velocity and three symmetry ratios are provided for both the stroke and healthy groups. Also reported are the 95% confidence intervals (CI) for the r values.
Figure 1. Relationship between age and gait velocity.
A between subject comparison illustrating the relationship between age and gait velocity in a group of healthy individuals (n=81, grey squares) (r= 0.57, p<0.01) and a group of individuals with stroke (n=172, black diamonds) (r=−0.14, p=0.07).
Figure 2. Relationship between age and gait symmetry.
Between subject comparisons illustrating the independence between age and (a) step length symmetry, (b) swing time symmetry and (c) stance time symmetry in a group of healthy individuals (n=81, grey squares) and a group of individuals with stroke (n=172, black diamonds).
DISCUSSION
The main finding of the present study is gait symmetry ratios are not significantly associated with age. This differs from velocity which is significantly associated with age in a group of healthy adults. Our results regarding velocity and age in the healthy group are consistent with previous reports of the negative relationship between velocity and age17, 18. In the current study, our correlation coefficient (r= −0.57) for the association between age and velocity in the healthy group (mean age 64.2y) is comparable to that reported by Himann and coauthors18 for a group of individuals older than 62 years (r= −0.56).
In this study, there was no significant relationship between age and velocity in the stroke group and our results are in line with previous reports. Goldie and colleagues27 reported only a very weak correlation between age and velocity (r= −0.11) and noted that age was only a weak predictor of the gait velocity achieved by patients after 8 weeks of rehabilitation. The current results may have differed from those of Goldie and coauthors because of the difference in chronicity between the two study groups. The current study examined the relationship within a group of stroke individuals with a median time post onset of 11 months, whereas the study by Goldie and colleagues featured a group with a median time post onset of 31 days27. Regardless of the differences between the two studies, taken together, these results indicate that gait velocity post-stroke is likely determined by a number of different factors (e.g. postural control, motor impairment, sensory impairment) but that age may have some limited influence.
More importantly, the present results indicate that gait symmetry ratios lack a significant relationship with age in both the stroke and healthy groups, indicating that the symmetrical nature of the gait pattern remains regardless of an individual’s age. Although the correlations between age and the swing and stance symmetry ratios approached significance in the stroke group, both of the corresponding 95% CIs contained zero. Therefore, we can safely state that symmetry and age are not associated. This is significant as it implies that an asymmetric ratio obtained from the gait analysis of an individual with a disease (such as stroke) can be interpreted as being related to the impairments resulting from the disease, without the concern for the influence of age. Previous work12 has identified cut off values for the step length, swing time and stance time symmetry ratios based on a 95% confidence interval of this group of healthy individuals (1.08, 1.06 and 1.05 respectively). The current work demonstrates that a clinician or researcher may interpret any symmetry ratio exceeding the corresponding cut off value as indicative of asymmetrical gait resulting from the impairments associated with disease and not age.
We believe the current results lend further support to the importance of symmetry measures for the study and rehabilitation of gait. In addition to not having the same age-confound as velocity, symmetry can provide insight about the control of walking. Symmetry may provide unique information, complementing the more conventional measure of velocity, and could have a role in guiding the clinician’s treatment decisions. Finally, previous work has demonstrated the potential for asymmetry ratios to worsen in the long-term post-stroke11. Interestingly, velocity remained constant over the same time period. This further supports the proposition that velocity and symmetry measure separate features of gait and that including a measure of symmetry in the assessment of gait provides additional information not attained with a measure of velocity alone.
In conclusion, measuring step length, swing time and stance time symmetry ratios allow the clinician or researcher to make judgements about the effects of a disease on a patient’s gait control without the confound of age. We believe gait symmetry is important to measure and address because it provides unique insight about gait separate from other measures such as velocity.
Acknowledgment
Funding Support:
The authors acknowledge the support of the Toronto Rehabilitation Institute which receives funding under the Provincial Rehabilitation Research Program from the Ministry of Health and Long-Term Care in Ontario. Equipment and space have been funded with grants from the Canada Foundation for Innovation, Ontario Innovation Trust and the Ministry of Research and Innovation. Support was also provided by the Heart and Stroke Foundation Centre for Stroke Recovery, Heart and Stroke Foundation of Ontario (72029807) and the Canadian Institutes of Health Research (MOP-62957). K Patterson was also supported with funding from Ontario Graduate Scholarship and Physiotherapy Foundation of Canada. N. Nadkarni was supported by the Pittsburgh Pepper Older American Independence Center (NIA grant: P30 AG024827). The views expressed do not necessarily reflect those of the funding agencies.
Contributor Information
Kara K. Patterson, University of Western Ontario, London, Ontario, Canada.
Neelesh K. Nadkarni, University of Pittsburgh, Pittsburgh, PA, USA.
Sandra E. Black, University of Toronto, Toronto, Ontario, Canada.
William E. McIlroy, University of Waterloo, Waterloo, Ontario, Canada.
REFERENCES
- 1.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38:2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 2.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1988;11:181–183. [Google Scholar]
- 3.Latham NK, Jette DU, Slavin M, et al. Physical therapy during stroke rehabilitation for people with different walking abilities. Arch Phys Med Rehabil. 2005;86(12) supplement 2:S41–S50. doi: 10.1016/j.apmr.2005.08.128. [DOI] [PubMed] [Google Scholar]
- 4.Mudge S, Stott NS. Outcome measures to assess walking ability following stroke: a systematic review of the literature. Physiotherapy. 2007;93:189–200. [Google Scholar]
- 5.Brandstater ME, deBruin H, Gowland C, Clarke BM. Hemiplegic gait: Analysis of temporal variables. Arch Phys Med Rehabil. 1983;64:583–587. [PubMed] [Google Scholar]
- 6.Bowden MG, Balasubramanian CK, Behrman AL, Kautz SA. Validation of a speed-based classification system using quantitative measures of walking performance poststroke. Neurorehabil Neural Repair. 2008;22(6):672–675. doi: 10.1177/1545968308318837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roth EJ, Merbitz C, Mroczek K, Dugan SA, Suh WW. Hemiplegic gait: Relationships between walking speed and other temporal parameters. Am J Phys Med Rehabil. 1997;76(2):128–133. doi: 10.1097/00002060-199703000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Montero-Odasso M, Schapira M, Soriano ER, et al. Gait velocity as a single predictor of adverse events in healthy seniors aged 75 years and older. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2005;60(10):1304. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- 9.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. Journal of the American geriatrics society. 1997;45(3):313. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 10.Lord SE, McPherson K, McNaughton HK, Rochester L, Weatherall M. Community ambulation after stroke: How important and obtainable is it and what measures appear predictive? Arch Phys Med Rehabil. 2004;85:234–239. doi: 10.1016/j.apmr.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Changes in gait symmetry and velocity after stroke: a cross sectional study from weeks to years after stroke. Neurorehabil Neural Repair. 2010;24(9):783–790. doi: 10.1177/1545968310372091. [DOI] [PubMed] [Google Scholar]
- 12.Patterson KK, Gage WH, Brooks D, Black SE, McIlroy WE. Evaluation of gait symmetry after stroke: a comparison of current methods and recommendations for standardization. Gait Posture. 2010;31(2):241–246. doi: 10.1016/j.gaitpost.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Wall JC, Turnbull GI. Gait asymmetries in residual hemiplegia. Arch Phys Med Rehabil. 1986;67:550–553. [PubMed] [Google Scholar]
- 14.Patterson KK, Parafianowicz I, Danells CJ, et al. Gait asymmetry in community-ambulating stroke survivors. Arch Phys Med Rehabil. 2008;89:304–310. doi: 10.1016/j.apmr.2007.08.142. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen L, Crabtree NJ, Reeve J, Jacobsen BK. Ambulatory level and asymmetrical weight bearing after stroke affects bone loss in the upper and lower part of the femoral neck differently: bone adaptation after decreased mechanical loading. Bone. 2000;27(5):701–707. doi: 10.1016/s8756-3282(00)00374-4. [DOI] [PubMed] [Google Scholar]
- 16.Turnbull GI, Wall JC. Long-term changes in hemiplegic gait. Gait Posture. 1995;3(4):258–261. [Google Scholar]
- 17.Bohannon RW. Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age and Ageing. 1997;26:15–19. doi: 10.1093/ageing/26.1.15. [DOI] [PubMed] [Google Scholar]
- 18.Himann JE, Cunningham DA, Rechnitzer PA, Paterson DH. Age-related changes in speed of walking. Med Sci Sports Exerc. 1988;20(2):161. doi: 10.1249/00005768-198820020-00010. [DOI] [PubMed] [Google Scholar]
- 19.Lethbridge-Cejku M, Tobin JD, Scott WW, Jr, Reichle R, Plato CC, Hochberg MC. The relationship of age and gender to prevalence and pattern of radiographic changes of osteoarthritis of the knee: data from Caucasian participants in the Baltimore Longitudinal study of Aging. Aging. 1994;6(5):353–357. doi: 10.1007/BF03324264. [DOI] [PubMed] [Google Scholar]
- 20.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 21.Draper ERC. A treadmill-based system for measuring symmetry of gait. Med Eng Phys. 2000;22:215–222. doi: 10.1016/s1350-4533(00)00026-6. [DOI] [PubMed] [Google Scholar]
- 22.Titianova EB, Tarkka IM. Asymmetry in walking performance and postural sway in patients with chronic unilateral cerebral infarction. Journal of Rehabilitation Research and Development. 1995;32(3):236–244. [PubMed] [Google Scholar]
- 23.Beauchamp MK, Skrela M, Southmayd D, et al. Immediate effects of cane use on gait symmetry in individuals with subacute stroke. Physiotherapy Canada. 2009;61(3):154–160. doi: 10.3138/physio.61.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 25.Gowland C, Stratford P, Ward M, et al. Measuring physical impairment and disability with the Chedoke-McMaster stroke assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 26.Gowland C, VanHullenaar S, Torresin W. Chedoke-McMaster Stroke Assessment: Development, validation and administration manual. Hamilton, ON: Mc-Master University; 1995. [Google Scholar]
- 27.Goldie PA, Matyas TA, Kinsella GJ, Galea MP, Evans OM, Bach TM. Prediction of gait velocity in ambulatory stroke patients during rehabilitation. Arch Phys Med Rehabil. 1999;80:415–420. doi: 10.1016/s0003-9993(99)90278-2. [DOI] [PubMed] [Google Scholar]