Abstract
Natural antimicrobial peptides (AMPs) are gene-coded defense molecules discovered in all the three life domains: Eubacteria, Archaea, and Eukarya. The latter covers protists, fungi, plants, and animals. It is now recognized that amino acid composition, peptide sequence, and post-translational modifications determine to a large extent the structure and function of AMPs. This article systematically describes post-translational modifications of natural AMPs annotated in the antimicrobial peptide database (http://aps.unmc.edu/AP). Currently, 1147 out of 1755 AMPs in the database are modified and classified into more than 17 types. Through chemical modifications, the peptides fold into a variety of structural scaffolds that target bacterial surfaces or molecules within cells. Chemical modifications also confer desired functions to a particular peptide. Meanwhile, these modifications modulate other peptide properties such as stability. Elucidation of the relationship between AMP property and chemical modification inspires peptide engineering. Depending on the objective of our design, peptides may be modified in various ways so that the desired features can be enhanced whereas unwanted properties can be minimized. Therefore, peptide design plays an essential role in developing natural AMPs into a new generation of therapeutic molecules.
Keywords: Chemical modification, database, peptide selectivity, peptide stability, peptide engineering, structural diversity
Introduction
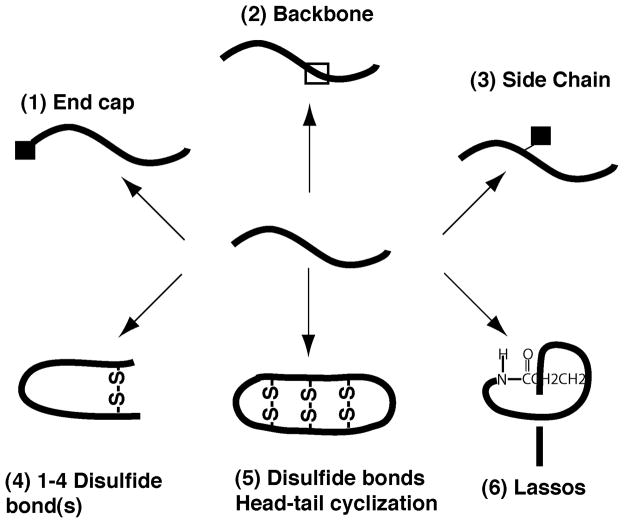
In an era of antibiotics, one of the powerful approaches for developing novel antimicrobial agents is to borrow the wisdom of nature. Natural antimicrobial peptides (AMPs) are universal host defense molecules of all living organisms. They play a key role in warding off invading pathogens to keep the host healthy [1–7]. These molecules are normally gene-coded and expressed either constitutively or upon pathogen invasion. A few bacterial antibiotics are synthesized by multi-enzyme systems. To date, more than 1750 such peptides have been registered into the updated antimicrobial peptide database (APD) [8, 9]. Database analysis reveals that AMPs possess a broad range of properties in terms of net charge, length, hydrophobic residue%, amino acid composition signature, three-dimensional structure, and mechanisms of action [10]. The majority of AMPs are basic (89%), with only a small population neutral (6%) or acidic (5%). The length of these AMPs ranges from 5 to 100. The upper limit was arbitrarily defined in our database as a cutoff for peptides. The hydrophobic residues% of the peptides varies from 2% to >90%. Examples for the former are Gly-rich AMPs and for the latter are channel-forming gramicidins. The amino acid composition signature is a plot of amino acid occurring frequency versus the 20 common residues. As a characteristic feature for each AMP, the amino acid composition signature determines many aspects of peptide structure and function. The 3D structures of natural AMPs have recently been classified into four broad families: α, β, αβ, and non-αβ [10]. The α family consists of α-helical AMPs, whereas the β family contains a collection of AMPs with β-sheet structures, usually stabilized by disulfide bonds. AMPs in the αβ family possess both α and β structures, which may and may not pack into a single fold. In this structural family, disulfide bonds are also critical to stabilize the AMP fold. The non-αβ peptides do not have clearly defined α nor β structures. If the formation of disulfide bonds is treated as one special type of chemical modifications, it is evident that post-translational modifications are an important factor that diversifies the structures of AMPs (Figure 1). The diversity of structural scaffolds of natural AMPs may enable them to recognize a variety of cellular targets such as cell wall, membranes, proteins, and nucleic acids [10–12]. A combination of the above parameters leads to a remarkable number of distinct peptide sequences. In addition, these sequences can be further modified in different manners, further enriching the chemical space for peptide design. This article discusses post-translational modifications observed in natural AMPs. Elucidation of the relationship between post-translational modifications and peptide properties has inspired peptide engineering. After engineering, the peptide templates should have desired properties such as selective killing and stability to proteases.
Figure 1.
Post-translational modifications diversify the structural scaffolds of natural antimicrobial peptides. A simple modification (1. Terminal capping such as C-terminal amidation; 2. Backbone modifications such as D-amino acids; and 3. Side chain modifications such as bromination) may, and may not, cause conformational alteration. Multiple modifications are critical for generating unique polypeptide folds (4. In the simple case, the peptide chain forms a loop due to at least one disulfide bond between side chains; in a more complicated case, the peptide adopts the folds of α or β-defensins. 5. θ-Defensins with a cyclic backbone and further stabilized by three disulfide bonds; and 6. An amide bond forms between peptide side chain and the N-terminus followed by the chain going through the ring, thereby named lassos).
1. Major Types of Chemical Modifications in Natural AMPs
The information on post-translational modifications of natural AMPs is scattered in the literature. To advance the field, we started to enter such information into the APD (URL: http//aps.unmc.edu/AP) in 2008 [9]. As of June 2011, 1147 modified AMPs (17 types) have been annotated, accounting for 65.5% of the entries in the APD. The number of peptides with a defined type of chemical modifications is provided in Table 1. Such information can be searched in the name field of the APD by using search keys also listed in Table 1. In the following, I briefly describe these chemical modifications and their effects on peptide properties.
Table 1.
Types of Post-translational Modifications in Natural AMPs
| Post-translational Modifications | Search Key1 | Number of Peptides | Application to Peptide Design? |
|---|---|---|---|
| N-terminal acetylation | XXE | 11 | Yes |
| N-terminal cyclic glutamate | XXQ | 13 | |
| C-terminal amidation | XXA | 324 | Yes |
| C-terminal iron- binding moieties | XXB | 4 | |
| C-terminal Rana Box | XXU | 235 | |
| Carboxylic-acid-containing unit | XXF | 1 | |
| D-amino acids | XXD | 9 | Yes |
| Glycosylation | XXG | 9 | |
| Halogenation (Cl, Br) | XXH | 7 | Yes, but usually F |
| Hydroxylation | XXK | 6 | |
| Oxidation | XXO | 9 | |
| Phosphorylation | XXP | 2 | |
| Reduction | XXR | 1 | |
| Sulfation | XXS | 1 | |
| Disulfide bridge | Structure search2 | 474 | Yes |
| Thioether bridge | XXT | 42 | |
| Cyclization | XXC | 148 | Yes |
To view peptide entries with a specific modification, enter the search key into the NAME field of the search interface of the APD. Data were obtained from the APD website on June 6, 2011 (http://aps.unmc.edu/AP). A partial list was published previously solely for the recognition of this phenomenon [9, 10].
This number was obtained by searching for disulfide bond-containing AMPs classified as “Bridge”, “β structure”, and “αβ structure” families, respectively. The “bridged” AMPs are known to have disulfide bonds but unknown 3D structure. Beta structures without disulfide bonds were excluded by including “c” as a sequence search term. For the αβ structures, only the packed one-domain defensins were counted.
1.1. Terminal capping
Although N-terminal acetylation is frequently used in synthetic peptides, this modification is only found in a few natural AMPs from bacteria and earthworms. Another N-terminal modification is cyclization of glutamine residues to become pyroglutamates. This modification has been detected in AMPs isolated from plants, spiders, insects, amphibians, and reptiles. The biological function of such a modification was found to be unimportant in the case of crab hyperglycemic neuropeptide [13]. However, N-terminal pyroglutamate is important in maintaining structural integrity of the N-terminal helix, which is required for the catalytic activity of adjacent histidine of bullfrog ribonuclease 3 [14].
C-terminal amidation is very common in natural AMPs. The amidation group corresponds to a glycine residue in the translated peptide sequence. Many linear peptides (324 in the APD) are C-terminally amidated. For some short AMPs such as anoplin [15], C-terminal amidation is critical for antimicrobial activity. This effect is not universal because not all amidated peptides display increased activity after amidation [16]. C-terminal rana box consists of a pair of Cys residues connected by a disulfide bond. This box may provide an alternative approach to stabilizing the C-terminal structure of many amphibian AMPs (235 entries in the APD) [17].
1.2. D-amino acids
D-amino acids were first identified in bombinins from amphibians [17]. They have also been found in bacteriocins. Cotter et al. [18] found an enzyme that converts a dehydrated L-Ser to D-Ala. Such enzymes may be harnessed to incorporate D-amino acids into bacterially expressed polypeptides.
1.3. Halogenation (Br or Cl)
In some AMPs, the Trp side chain is halogenated (usually brominated). The residue Trp2 in the sequence of styelin D from sea squirt was brominated at position 6 of the aromatic ring. A similar modification might exist in cathelicidins from hagfish, hedistin, and centrocins [19–21]. A synthetic hedistin analog without bromination was found to be as active as the modified natural form, indicating bromination is not critical for antimicrobial activity [20]. In lantibiotic Microbisporicin A1, Trp4 is unprecedentedly chlorinated at position 5 [22]. Fluorination does not occur in natural AMPs, but has been utilized to improve the desired properties of synthetic peptides (see below).
1.4. Hydroxylation
Styelin D also possesses other chemical modifications. Residues Arg, Lys, and Tyr were found to be hydroxylated. Such modifications appear to be essential for the peptide to remain active at high salt concentrations. Indeed, the native peptide is more active than a synthetic peptide without those modifications [23]. However, hydroxylation of Trp showed little effect on antimicrobial activity of mussel defensin MGD-1 or piscidin 4 [24, 25]. The only difference between microbisporicins A1 and A2 is that Pro14 is monohydroxylated in A2 and dihydroxylated in A1 [22]. Because these two peptides showed similar antibacterial activity, the extent of proline hydroxylation appeared to be unimportant. In lantibiotic duramycin B, an Asp residue at position 15 was found to be hydroxylated [26]. Thus, five amino acid residues (Arg, Lys, Tyr, Trp, and Asp) have been found to be hydroxylated in some natural AMPs.
1.5. Oxidation
A few AMPs from bacteria and plants were found to contain oxidized residues. For kalata B1, B2, and B10, Trp could be oxidized to oxindolylalanine, N-formylkynurenine, or kynurenine [27]. The effect on peptide activity was not reported. Carnobacteriocin B1 has the same amino acid sequence as carnobacteriocin BM1 except for Met41 oxidation. The sulfoxide formation in carnobacteriocin B1 reduced peptide activity by a factor of 4–8 [28]. Trp oxidation of divercin V41, a class IIa bacteriocin from Gram-positive bacteria, led to a loss of activity [29]. It is likely that such oxidation influences the binding of the peptide to its target (e.g. membranes). Wilson-Stanford et al. [30] found that oxidation of lantibiotics such as nisin A disrupted antibacterial activity. It was proposed that the oxidized form of sulfur is unable to bind to lipid II of the cell wall. Therefore, this type of modification should be avoided during peptide production, purification, formulation, transport or storage.
1.6. Phosphorylation
Although phosphorylation is common in signal-transducing proteins from prokaryotes to eukaryotes, only two phosphorylated AMPs exist in the APD database. Cattle chrombacin is phosphorylated at Ser10 [31], whereas human histatin 1 is phosphorylated at Ser2 [32]. Whether these peptides participate in signal transduction remains to be elucidated.
1.7. Glycosylation
Several Pro-rich AMPs, mainly from insects, were found to be glycosylated. These include formaecin 1, pyrrhocoricin, drosocin, heliocin, 3 lebocins, drosophila diptericin, and MPAC. In several cases, attachment of N-acetylgalactosamine to a conserved Thr residue enhanced peptide activity by several fold [33, 34]. However, the effect on conformation of drosocin was found to be minimal [35]. Unlike many membrane-targeting AMPs, Pro-rich peptides tend to bind to internal targets such as heat shock proteins [36, 37].
1.8. Sulfation
Tyrosine sulfation is ubiquitous in proteins synthesized in the secretory pathway. Such a modification could enhance protein-protein interaction, including receptor binding [38]. Tyrosine sulfation of human chemokine receptors is prevalent and influences the binding of chemokine ligands that trigger specific signal transduction directly related to human immune diseases [39]. Also, tyrosine sulfation of the N-terminal region of human P-selectin glycoprotein ligand-1 (PSGL-1) is critical for the entry and replication of enterovirus E71 in leukocytes [40]. However, sulfated AMPs are rarely reported. Chrombacin [31] provides the only example in the APD. In this peptide, sulfation occurs at Tyr8.
1.9. Reduction
Reduction is yet another modulator that regulates the activity of AMPs in biological systems. A recent study by Schroeder et al. provides an excellent example for this type of regulation [41]. They found that human β-defensin 1 (hBD-1) was not bactericidal before reduction, but became active under reducing conditions where the protein became unfolded as a result of the loss of disulfide bonds. Wu et al. [42] investigated the functional roles of disulfide bonds of human β-defensin 3 (hBD-3). While disulfide bonds were not absolutely required for antimicrobial activity, these bridges are important for chemotactic activity that involves AMP binding to receptors.
1.10. Disulfide bridges
Disulfide bonds are key structural elements of numerous AMPs (474 in the APD). Defensins contain 3–5 pairs of disulfide bonds, which enable the formation of different polypeptide folds. Human defensins contain three disulfide bonds: which are CysI–CysVI, CysII–CysIV, and CysIII–CysV in α-defensins, and CysI–CysV, CysII–CysIV, and CysIII–CysVI in β-defensins, where the Cys residues are numbered from I to VI based on the order they appear in the sequence [7, 41, 42]. As mentioned above, it is likely that the folded and unfolded defensins conduct distinct biological functions. Note that many AMPs in the β-structure family possess only one or two disulfide bonds. For examples, visit the APD [9].
Disulfide bonds, however, also occur in helical AMPs. The rana box formation is a special case where one intramolecular disulfide bond confers advantage to amphibian peptides. In the case of distinctin, one intermolecular disulfide bond occurs between the two peptide chains, leading to an increase in peptide stability (due to the formation of a helix bundle structure in water) but not antimicrobial activity [43]. A recent study provides a novel example for helical AMPs with two disulfide bonds, which lock the two adjacent helices into a hairpin structure [44]. In addition, three disulfide bonds exist in the 10 saposin-like AMPs that form helix bundle structures [10].
1.11. Thioether bridges
In lantibiotics (bacteriocins from Gram-positive bacteria), Ser and Thr residues are rich prior to post-translational modifications [10]. Some of these residues are dehydrated and form a thioether ring with a Cys residue [5]. The discovery of the broad substrate specificity of the nisin modification enzymes [45] may open the door to enzyme-mediated introduction of thioether rings into a peptide template for required biological activity or structural stability.
1.12. Cyclization
The APD analysis reveals that circular AMPs have been found in bacteria, plants, and animals [9]. The majority of the 145 circular AMPs in the database possess a peptide bond that connects the N- and C-termini of the peptide. Enterocin AS-48 is the first circular bacteriocin identified in bacteria [46]. It turned out that circularization is required for peptide structure rather than bactericidal activity [47]. Kalata B1 is the first circular AMP identified in plants [48]. While the cyclic form is HIV-1 inhibitory, acyclic kalata B1 was found to be inactive due to the loss of cyclization as well as hydrogen bonding network [49]. This peptide can tolerate heat and detergents. At present, more than 150 plant cyclotides have been reported. A large reservoir of 50,000 such peptides is estimated in plants, providing a great opportunity for research and development [50]. In the kingdom of animals, a third type of defensins called θ-defensins (Figure 1) has been found primarily from primates [7, 51]. These minidefensins are small circular peptides of only 18 residues. The peptide scaffold is further stabilized by three disulfide bonds, leading to a fascinating ladder-like structure highly resistant to proteases [52]. Interestingly, this unique group of natural AMPs is made of only a few amino acid residues, including Cys (33.33%), Arg (25%), Gly (11.11%), Thr (5.5%), Ile (4.62%), Val (10.64%), Leu (3.7%), and Phe (6.01%) [8, 9].
Not all peptides were cyclized in the same way, however. For microcin J25, a ring structure was formed between the backbone amide of residue Gly1 and the side chain of Glu8 [53]. In the three-dimensional structure, the tail was found to pass the polypeptide ring forming a lasso structure (Figure 1) [54].
1.13. Targeting moieties
Attachment of a bacterial receptor-recognizable moiety allows micocins to be smuggled into the bacterium via the “Trojan horse” trick. MccC7 contains a phosphoramidate linkage to adenosine monophosphate at the C-terminus. The added C-terminal moiety was cleaved within the cell. The resulting peptide targets tRNA synthetase and inhibits protein synthesis [55]. Microcin E492 possesses a ferric-binding moiety, which enables it to be smuggled into enterobacteria that express siderophore uptake pump. The polypeptide core of Microcin E492 stably associates with the mannose permease and interferes with mannose metabolism [56].
In summary, post-translational modifications are important for both structure and function of AMPs. Such modifications lead to diverse structural scaffolds. The stability of cyclotides and lantibiotics to proteases make them attractive templates for peptide engineering [10]. Post-translational modifications also tune peptide activities. For example, reduction of hBD-1 made the peptide antimicrobial under reduced conditions [41].
2. Peptide Engineering and Stability
2.1. Modifications of Linear Peptide Templates
One of the requirements for therapeutic peptides is that the peptide should be stable so that the compound has a sufficient time to complete its targeted mission before being destroyed. Some post-translational modification strategies have found use in peptide engineering (Table 1). The simplest and most commonly used approach is to include N-terminal acetylation and C-terminal amidation. C-terminal amidation may, and may not, increase peptide stability. For Trp- and Arg-rich peptide analogs, amidation did not increase peptide stability in human serum. In contrast, N-terminal acetylation improved peptide stability to proteases [57]. While natural AMPs may be brominated or occasionally chlorinated, chemists prefer fluorinated amino acids to improve stability of synthetic peptides [58, 59]. The enhanced peptide stability may result from increase in size or hydrophobicity due to fluorination [60, 61]. Incorporation of D-amino acids provides another useful approach for improvement of peptide stability. One of the early demonstrations was the synthesis of all-D-peptide analogs by Merrifield et al [62]. Alternatively, partial incorporation of D-amino acids may also improve peptide stability. Using helical peptides as models, Shai and colleagues introduced D-amino acids at every 2–3 residues to improve peptide selectivity [63, 64]. By incorporating D-amino acids at positions 20, 24, and 28 of GF-17 (as numbered in LL-37), we obtained a human LL-37-derivative that was toxic to bacteria but not human cells. Structural analysis revealed a non-classical amphipathic structure [65]. Similarly, Hong et al. [66] found that incorporation of D-amino acids at the terminal regions did not disrupt the antibacterial activity of the peptide, but improved peptide stability in serum. All these results add to our knowledge on peptide engineering.
2.2. Cyclization of Polypeptide Chains
As described above, three cyclization patterns are known in natural AMPs, which can be backbone to backbone, backbone to side chain, or side chain to side chain (Table 2). Cyclization made the peptide chain more compact and less exposed, thereby becoming more resistant to proteases. This strategy has been applied to peptide design. Dathe et al. [67] achieved cyclization of an arginine- and tryptophan-rich peptide RRWWRF-NH2 using method I (Table 2). They found that the cyclized peptide had higher antimicrobial activity and cell selectivity than the linear counterpart. Shai and colleagues produced cyclization by Method III. While the cyclic melittin analog displayed increased antibacterial activity but decreased hemolytic activity, cyclic magainin 2 had a marked decrease in both antibacterial and hemolytic activity [68]. Based on a U-shaped NMR structure, Rozek et al. [69] introduced a disulfide bond to lock the peptide termini. The cyclized form not only retained antimicrobial activity but also showed higher stability to trypsin than its parent molecule. Vogel and colleagues found that disulfide cyclization of a hexamer peptide resulted in higher activity, but backbone cyclization led to peptides that are more resistant to proteases. For an 11-residue peptide, backbone cyclization did not show stability improvement [57]. Collectively, it appears that the effect of cyclization on the properties of linear peptides depends on both the peptide sequence and cyclization method.
Table 2.
Cyclization Methods Observed in Natural AMPs
2.3. Target-recognition motifs
Bacteria-targeting signals attached to microcins are inspiring [55, 56]. It constitutes the prototype for designing target-specific AMPs. Eckert et al. [70] succeeded in enhancing the antimicrobial activity of novispirin G10 by adding a Psuedomonas-specific targeting moiety. In this case, additional engineering is needed since the peptide became inactive in sputum due to the action of serine proteases [71]. Another issue with peptide attachment is potential loss of antimicrobial activity. In the work of Eckert et al. [70], a relatively short peptide-targeting moiety was attached to the AMP separated by a flexible linker. However, an AMP may become inactive when the targeting moiety is large (i.e. a protein). This is not surprising because AMPs are expressed as precursor proteins in vivo to diminish unwanted toxic effects to the host. This strategy is borrowed to express AMPs in bacteria as fusion proteins followed by enzyme or chemical cleavage [10]. It appears that an AMP could remain to be active if it is properly attached to a small targeting moiety. The game could be different in the case of AMPs with folded structure. Some defensins such as hBD-2 and mBD-4 are known to be bactericidal even when fused to another protein [72]. In these cases, the defensin and its linked partner both appear to behave as independent domains in the fusion protein. This may be a useful construct to deliver antimicrobial defensins to a defined target recognized by its binding partner.
3. Therapeutic Potentials of AMPs
Natural AMPs have a great potential to be developed into novel agents for various applications, including therapeutic use. First, the in vivo efficacy of AMPs has been tested in different models. According to the APD [9], more than a dozen of peptides have been expressed in plants to reduce or avoid infection. In addition, lactoferricin, distinctin, ranalexin, halocidin, polybia-MPI, chicken cathelicidin 1 (fowlicidin-1), bacterial mutacin B-Ny266, and bacterial ABP-118 have been tested in mice models [73–80]. Other engineered peptides or AMP mimics that have been evaluated in animal models include D, L-peptides, WLBU2, Pro-rich A3-APO, and acyl-lysyl oligomers [81–84]. Although topical treatments are preferred, an acyl-lysyl compound showed systemic efficacy in a mouse model [84]. These studies demonstrate that in vivo efficacy of AMPs is achievable topically or systemically. Second, the AMP research has gone beyond the traditional approach for antibiotic development. For example, the elucidation of the link between the expression of human host defense cathelicidin LL-37 in vivo and the light therapy (that causes vitamin D conversion) is inspiring. In other words, it is possible to augment host defense by applying vitamin D or its analogs without directly applying antimicrobial agents, thereby offering novel therapeutic strategies for AMPs [85, 86]. Third, the therapeutic values of AMPs are not limited to antimicrobial properties. AMPs such as human cathelicidin and defensins are known to possess other biological functions such as chemotaxis and wound healing. As a consequence, there is great interest in developing AMPs into immune modulating agents as well [87]. Because these peptides do not work on bacteria directly, it is unlikely that bacteria will develop resistance.
4. Concluding Remarks and Future Directions
Post-translational modifications are common in natural AMPs (Table 1). Such modifications are key to structural and functional diversity of natural AMPs (Figure 1). Thioether bond, disulfide bond, and head-to-tail cyclization are so critical to peptide identity and structure, they may be regarded as an integral part of the peptide sequence. Usually, the first clue to a potential sequence modification stems from the observation that antimicrobial activity of an isolated AMP differs from its synthetic counterpart. A difference in mass between the two forms provides further evidence. When there is no mass difference, one may ask whether the peptide contains D-amino acid. Caution should be taken in characterizing D-amino acids to avoid potential artifacts from peptide purification or characterization [10]. It is also important to ask whether the identified modification results from a chemical reaction during peptide purification. This becomes important especially when certain residues are very susceptible to oxidation, hydration, and deamidation. Once a bona fide chemical modification is established, one may ask how such a modification impacts peptide structure and function. This can be achieved by carefully comparing the modified and unmodified versions of the same peptide. The elucidation of chemical modifications will enable us to better mimic the functional roles of natural AMPs and to utilize the scheme for peptide design. While terminal capping, selective D-amino acid incorporation, disulfide bond formation, and head-to-tail cyclization have been applied to rational design of therapeutic peptides, other modifications such as phosphorylation and glycosylation are probably created mainly for specific functional roles of polypeptides only (Table 1). Some type of chemical modifications such as oxidation during peptide storage, however, should be avoided, since it could reduce peptide activity [29, 30].
Chemical modifications may also confer stability to AMPs so that they can be stored for rapid host response and defense. Different strategies have been observed in natural AMPs. Distinctin is stabilized by forming a helix bundle structure in water [55]. Defensins and cyclotides are stabilized by multiple disulfide bonds, leading to unique folds. In addition, cyclic defensins (θ-defensins) and cyclotides are further stabilized by peptide head-to-tail cyclization (Figure 1). These modifications make such natural AMPs stable. Peptide stability is an important factor in developing novel antimicrobials based on natural AMPs. Some of the post-translational strategies, such as C-terminal amidation, cyclization, D-amino acid incorporation, and halogenation, have been adopted in laboratories to enhance peptide stability to proteases. As a consequence, several engineered peptides have been demonstrated to be able to keep animal healthy [73–84].
Finally, one should ask whether the engineered peptide could be produced in an acceptable cost. While short AMPs with simple modifications can be synthesized chemically, it is challenging to obtain complex peptides such as lantibiotics in the same manner. A better choice is to harness natural systems. A bacterial genome contains a gene cluster that encodes a particular AMP, processing enzymes, transporters, and immunity proteins [5]. It is conceivable that the future peptide production line will incorporate such natural processing units so that AMPs such as lantibiotics could be readily produced to benefit human kinds. Indeed, nisin is already in use as a food preservative.
In summary, this article will serve as a starting point for those who are interested in exploring post-translational modifications of natural AMPs. Elucidation of the relationship between AMP property and chemical modification inspires peptide engineering. Thus, future research on novel post-translational modification schemes will enrich our toolbox for peptide engineering summarized in Table 1. It is also important to elucidate the relationship between post-translational modifications and biological functions of AMPs. In particular, some pathogenic microbes may circumvent host defense via counteracting chemical modifications in the host. Therefore, it is necessary to extend the scope of chemical modifications to other proteins/receptors related to infection. In this regard, understanding post-translational modifications of the host-pathogen system may lead to novel strategies to combat infection. Continued studies are also needed for peptide engineering. For AMPs that lack the desired properties such as stability, future research will offer novel methods that could well carry the lead compound into practical applications. For those AMPs such as cyclotides and lantibiotics, which are sufficiently stable, future research should focus on identification of novel candidates with unique features and development of innovative technologies for large-scale peptide production. All these efforts will eventually remove the hurdles that limit the use of AMPs. In addition, novel therapeutic strategies are actively sought in the laboratories around the world [10, 88, 89]. It is my hope that natural post-translational modifications and the peptide engineering strategies of AMPs summarized here are inspiring to colleagues who are working diligently to bring their favorite candidates into the market in the near future.
Acknowledgments
The author appreciates NIH funding (R56AI081975 and R21AI082689) during this study.
References
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Shai Y. Mode of action of membrane active antimicrobial peptides. Biopolymers. 2002;66:234–48. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 3.Boman HG. Antibacterial peptides: basic facts and emerging concepts. J Int Med. 2003;254:197–215. doi: 10.1046/j.1365-2796.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 4.Brogden KA, Guthmiller JM, Salzet M, Zasloff M. The nervous system and innate immunity: the neuropeptide connection. Nat Immunol. 2005;6:558–64. doi: 10.1038/ni1209. [DOI] [PubMed] [Google Scholar]
- 5.Cotter PD, Hill C, Ross RP. Bacteriocins: developing innate immunity for food. Nature Rev Microbiol. 2005;3:777–788. doi: 10.1038/nrmicro1273. [DOI] [PubMed] [Google Scholar]
- 6.Hancock REW, Sahl H. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotech. 2006;24:1551–7. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 7.Lehrer RI. Immunology: peptide gets in shape for self-defense. Nature. 2011;469:309–10. doi: 10.1038/469309a. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z, Wang G. APD: the antimicrobial peptide database. Nucleic Acids Res. 2004;32 (Database issue):D590–D592. doi: 10.1093/nar/gkh025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang G, Li X, Wang Z. APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 2009;37 (Database issue):D933–D937. doi: 10.1093/nar/gkn823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang G. Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies. CABI; England: 2010. [Google Scholar]
- 11.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochim Biophys Acta. 1999;1462:11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 12.Tossi A, Sandri L. Molecular diversity in gene-coded, cationic antimicrobial polypeptides. Curr Pharm Des. 2002;8:743–761. doi: 10.2174/1381612023395475. [DOI] [PubMed] [Google Scholar]
- 13.Chung JS, Webster SG. Does the N-terminal pyroglutamate residue have any physiological significance for crab hyperglycemic neuropeptides? Eur J Biochem. 1996;240:358–64. doi: 10.1111/j.1432-1033.1996.0358h.x. [DOI] [PubMed] [Google Scholar]
- 14.Lou YC, Huang YC, Pan YR, Chen C, Liao YD. Roles of N-terminal pyroglutamate in maintaining structural integrity and pKa values of catalytic histidine residues in bullfrog ribonuclease 3. J Mol Biol. 2006;355:409–21. doi: 10.1016/j.jmb.2005.10.069. [DOI] [PubMed] [Google Scholar]
- 15.Dos Santos Cabrera MP, Arcisio-Miranda M, et al. Study of the mechanism of action of anoplin, a helical antimicrobial decapeptide with ion channel-like activity, and the role of the amidated C-terminus. J Pept Sci. 2008;14:661–669. doi: 10.1002/psc.960. [DOI] [PubMed] [Google Scholar]
- 16.Dennison SR, Harris F, Bhatt T, Singh J, Phoenix DA. The effect of C-terminal amidation on the efficacy and selectivity of antimicrobial and anticancer peptides. Mol Cell Biochem. 2009;332:43–50. doi: 10.1007/s11010-009-0172-8. [DOI] [PubMed] [Google Scholar]
- 17.Simmaco M, Kreil G, Barra D. Bombinins, antimicrobial peptides from Bombina species. Biochim Biophys Acta. 2009;1788:1551–1555. doi: 10.1016/j.bbamem.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Cotter PD, O’Connor PM, Draper LA, et al. Posttranslational conversion of L-serines to D-alanines is vital for optimal production and activity of the lantibiotic lacticin 3147. Proc Natl Acad Sci USA. 2005;102:18584–9. doi: 10.1073/pnas.0509371102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uzzell T, Stolzenberg ED, Shinnar AE, Zasloff M. Hagfish intestinal antimicrobial peptides are ancient cathelicidins. Peptides. 2003;24:1655–67. doi: 10.1016/j.peptides.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 20.Tasiemski A, Schikorski D, Le Marrec-Croq F, et al. Hedistin: A novel antimicrobial peptide containing bromotryptophan constitutively expressed in the NK cells-like of the marine annelid, Nereis diversicolor. Dev Comp Immunol. 2007;31:749–62. doi: 10.1016/j.dci.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Li C, Haug T, Moe MK, Styrvold OB, Stensvåg K. Centrocins: isolation and characterization of novel dimeric antimicrobial peptides from the green sea urchin, Strongylocentrotus droebachiensis. Dev Comp Immunol. 2010;34:959–68. doi: 10.1016/j.dci.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Castiglione F, Lazzarini A, Carrano L, et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multi-resistant pathogens. Chem Biol. 2008;15:22–31. doi: 10.1016/j.chembiol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 23.Taylor SW, Craig AG, Fischer WH, Park M, Lehrer RI. Styelin D, an extensively modified antimicrobial peptide from ascidian hemocytes. J Biol Chem. 2000;275:38417–26. doi: 10.1074/jbc.M006762200. [DOI] [PubMed] [Google Scholar]
- 24.Yang YS, Mitta G, Chavanieu A, et al. Solution structure and activity of the synthetic four-disulfide bond Mediterranean mussel defensin (MGD-1) Biochemistry. 2000;39:14436–47. doi: 10.1021/bi0011835. [DOI] [PubMed] [Google Scholar]
- 25.Noga EJ, Silphaduang U, Park NG, Seo JK, Stephenson J, Kozlowicz S. Piscidin 4, a novel member of the piscidin family of antimicrobial peptides. Comp Biochem Physiol B Biochem Mol Biol. 2009;152:299–305. doi: 10.1016/j.cbpb.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 26.Fredenhagen A, Fendrich G, Märki F, et al. Duramycins B and C, two new lanthionine containing antibiotics as inhibitors of phospholipase A2. Structural revision of duramycin and cinnamycin. J Antibiot (Tokyo) 1990;43:1403–12. doi: 10.7164/antibiotics.43.1403. [DOI] [PubMed] [Google Scholar]
- 27.Plan MR, Göransson U, Clark RJ, Daly NL, Colgrave ML, Craik DJ. The cyclotide fingerprint in oldenlandia affinis: elucidation of chemically modified, linear and novel macrocyclic peptides. Chembiochem. 2007;8:1001–11. doi: 10.1002/cbic.200700097. [DOI] [PubMed] [Google Scholar]
- 28.Quadri LE, Sailer M, Roy KL, Vederas JC, Stiles ME. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem. 1994;269:12204–11. [PubMed] [Google Scholar]
- 29.Bhugaloo-Vial P, Douliez JP, Moll D, Dousset X, Boyaval P, Marion D. Delineation of key amino acid side chains and peptide domains for antimicrobial properties of divercin V41, a pediocin-like bacteriocin secreted by Carnobacterium divergens V41. Appl Environ Microbiol. 1999;65:2895–900. doi: 10.1128/aem.65.7.2895-2900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson-Stanford S, Kalli A, Håkansson K, Kastrantas J, Orugunty RS, Smith L. Oxidation of lanthionines renders the lantibiotic nisin inactive. Appl Environ Microbiol. 2009;75:1381–7. doi: 10.1128/AEM.01864-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor SW, Sun C, Hsieh A, Andon NL, Ghosh SS. A sulfated, phosphorylated 7 kDa secreted peptide characterized by direct analysis of cell culture media. J Proteome Res. 2008;7:795–802. doi: 10.1021/pr7006686. [DOI] [PubMed] [Google Scholar]
- 32.Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J Biol Chem. 1988;263:7472–7. [PubMed] [Google Scholar]
- 33.Bulet P, Dimarcq JL, Hetru C, et al. A novel inducible antibacterial peptide of Drosophila carries an O-glycosylated substitution. J Biol Chem. 1993;268:14893–7. [PubMed] [Google Scholar]
- 34.Mackintosh JA, Veal DA, Beattie AJ, Gooley AA. Isolation from an ant Myrmecia gulosa of two inducible O-glycosylated proline-rich antibacterial peptides. J Biol Chem. 1998;273:6139–43. doi: 10.1074/jbc.273.11.6139. [DOI] [PubMed] [Google Scholar]
- 35.McManus AM, Otvos L, Jr, Hoffmann R, Craik DJ. Conformational studies by NMR of the antimicrobial peptide, drosocin, and its non-glycosylated derivative: effects of glycosylation on solution conformation. Biochemistry. 1999;38:705–14. doi: 10.1021/bi981956d. [DOI] [PubMed] [Google Scholar]
- 36.Otvos L, Jr, OI, Rogers ME, et al. Interaction between heat shock proteins and antimicrobial peptides. Biochemistry. 2000;39:14150–9. doi: 10.1021/bi0012843. [DOI] [PubMed] [Google Scholar]
- 37.Szabo D, Ostorhazi E, Binas A, et al. The designer proline-rich antibacterial peptide A3-APO is effective against systemic Escherichia coli infections in different mouse models. Int J Antimicrob Agents. 2010;35:357–61. doi: 10.1016/j.ijantimicag.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 38.Bundgaard JR, Vuust J, Rehfeld JF. New consensus features for tyrosine O-sulfation determined by mutational analysis. J Biol Chem. 1997;272:21700–5. doi: 10.1074/jbc.272.35.21700. [DOI] [PubMed] [Google Scholar]
- 39.Liu J, Louie S, Hsu W, Yu KM, Nicholas HB, Jr, Rosenquist GL. Tyrosine sulfation is prevent in human chemokine receptors important in lung disease. Am J Respir Cell Mol Biol. 2008;38:738–43. doi: 10.1165/rcmb.2007-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishimura Y, Wakita T, Shimizu H. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS Pathog. 2010;6:e1001174. doi: 10.1371/journal.ppat.1001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schroeder BO, Wu Z, Nuding S, et al. Reduction of disulphide bonds unmasks potent antimicrobial activity of human β-defensin 1. Nature. 2011;469:419–23. doi: 10.1038/nature09674. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Hoover DM, Yang D, Bouleque C, Santamaria F, Oppenheim JJ, Lubkowski J, Lu W. Engineering disulfide bridge to dissect antimicrobial and chemotactic activities of human beta-defensin 3. Proc Natl Acad Sci USA. 2003;100:8880–5. doi: 10.1073/pnas.1533186100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Batista CV, Scaloni A, Rigden DJ, et al. A novel heterodimeric antimicrobial peptide from the tree-frog Phyllomedusa distincta. FEBS Lett. 2001;494:85–9. doi: 10.1016/s0014-5793(01)02324-9. [DOI] [PubMed] [Google Scholar]
- 44.Nolde SB, Vassilevski AA, Rogozhin EA, Barinov NA, Balashova TA, Samsonova OV, Baranov YV, Feofanov AV, Egorov TA, Arseniev AS. Disulfide-stabilized helical hairpin structure and activity of a novel antifungal peptide EcANP1 from seeds of barnyard grass (Echinochloa crus-galli) J Biol Chem. 2011;286:25145–53. doi: 10.1074/jbc.M110.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rink R, Kuipers A, de Boef E, et al. Lantibiotic structures as guidelines for the design of peptides that can be modified by lantibiotic enzymes. Biochemistry. 2005;44:8873–82. doi: 10.1021/bi050081h. [DOI] [PubMed] [Google Scholar]
- 46.Galvez A, Maqueda M, Martínez-Bueno M, Valdivia E. Bactericidal and bacteriolytic action of peptide antibiotic AS-48 against gram-positive and gram-negative bacteria and other organisms. Res Microbiol. 1989;140:57–68. doi: 10.1016/0923-2508(89)90060-0. [DOI] [PubMed] [Google Scholar]
- 47.Montalbán-López M, Spolaore B, Pinato O, et al. Characterization of linear forms of the circular enterocin AS-48 obtained by limited proteolysis. FEBS Lett. 2008;582:3237–42. doi: 10.1016/j.febslet.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 48.Gran L. On the effect of a polypeptide isolated from “Kalata-Kalata” (Oldenlandia affinis DC) on the oestrogen dominated uterus. Acta Pharmacol. Toxicol. 1973;33:400–8. doi: 10.1111/j.1600-0773.1973.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 49.Ireland DC, Wang CKL, Wilson JA, Gustafson KR, Craik DJ. Cyclotides as natural anti-HIV agents. Biopolymers (Peptide Sci) 2008;90:51–60. doi: 10.1002/bip.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaas Q, Westermann J-C, Henriques ST, Craik DJ. In: Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies. Wang G, editor. Vol. 3. CABI; England: 2010. pp. 40–71. [Google Scholar]
- 51.Gallo SA, Wang W, Rawat SS, et al. Theta-defensins prevent HIV-1 Env-mediated fusion by binding gp41 and blocking 6-helix bundle formation. J Biol Chem. 2006;281:18787–18792. doi: 10.1074/jbc.M602422200. [DOI] [PubMed] [Google Scholar]
- 52.Daly NL, Chen YK, Rosengren KJ, et al. Retrocyclin-2: structural analysis of a potent anti-HIV theta-defensin. Biochemistry. 2007;46:9920–8. doi: 10.1021/bi700720e. [DOI] [PubMed] [Google Scholar]
- 53.Rosengren KJ, Clark RJ, Daly NL, Göransson U, Jones A, Craik DJ. Microcin J25 has a threaded sidechain-to-backbone ring structure and not a head-to-tail cyclized backbone. J Am Chem Soc. 2003;125:12464–74. doi: 10.1021/ja0367703. [DOI] [PubMed] [Google Scholar]
- 54.Rosengren KJ, Craik DJ. How bugs make lassos. Chem Biol. 2009;16:1211–2. doi: 10.1016/j.chembiol.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Roush RF, Nolan EM, Löhr F, Walsh CT. Maturation of an Escherichia coli ribosomal peptide antibiotic by ATP-consuming N-P bond formation in microcin C7. J Am Chem Soc. 2008;130:3603–9. doi: 10.1021/ja7101949. [DOI] [PubMed] [Google Scholar]
- 56.Biéler S, Silva F, Belin D. The polypeptide core of Microcin E492 stably associates with the mannose permease and interferes with mannose metabolism. Res Microbiol. 2010;161:706–10. doi: 10.1016/j.resmic.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 57.Nguyen LT, Chau JK, Perry NA, de Boer L, Zaat SA, Vogel HJ. Serum stabilities of short tryptophan- and arginine-rich antimicrobial peptide analogs. PLoS One. 2010;5(9):e12684. doi: 10.1371/journal.pone.0012684. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niemz A, Tirrell DA. Self-association and membrane-binding behavior of mellitins containing trifluorpleucine. J Am Chem Soc. 2001;123:7407–13. doi: 10.1021/ja004351p. [DOI] [PubMed] [Google Scholar]
- 59.Meng H, Kumar K. Antimicrobial activity and protease stability of peptides containing fluorinated amino acids. J Am Chem Soc. 2007;129:15615–922. doi: 10.1021/ja075373f. [DOI] [PubMed] [Google Scholar]
- 60.Lee KH, Lee HY, Slutsky MM, Anderson JT, Marsh EN. Fluorous effect in proteins: de novo design and characterization of a four-alpha-helix bundle protein containing hexafluoroleucine . Biochemistry. 2004;43:16277–84. doi: 10.1021/bi049086p. [DOI] [PubMed] [Google Scholar]
- 61.Gottler LM, Lee HY, Shelburne CE, Ramamoorthy A, Marsh EN. Using fluorous amino acids to modulate the biological activity of an antimicrobial peptide. Chembiochem. 2008;9:370–3. doi: 10.1002/cbic.200700643. [DOI] [PubMed] [Google Scholar]
- 62.Merrifield RB, Juvvadi P, Andreu D, Ubach J, Boman A, Boman HG. Retro and retroenantio analogs of cecropin-melittin hybrids. Proc Natl Acad Sci USA. 1995;92:3449–53. doi: 10.1073/pnas.92.8.3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharon M, Oren Z, Shai Y, Anglister J. 2D-NMR and ATR-FTIR study of the structure of a cell-selective diastereomer of melittin and its orientation in phospholipids. Biochemistry. 1999;38:15305–16. doi: 10.1021/bi991225t. [DOI] [PubMed] [Google Scholar]
- 64.Pouny Y, Shai Y. Interaction of D-amino acid incorporated analogues of pardaxinwith membranes. Biochemistry. 1992;31:9482–90. doi: 10.1021/bi00154a022. [DOI] [PubMed] [Google Scholar]
- 65.Li X, Li Y, Han H, Miller DW, Wang G. Solution structures of human LL-37 fragments and NMR-based identification of a minimal membrane-targeting antimicrobial and anticancer region. J Am Chem Soc. 2006;128:5776–85. doi: 10.1021/ja0584875. [DOI] [PubMed] [Google Scholar]
- 66.Hong SY, Oh JE, Lee KH. Effect of D-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem Pharmacol. 1999;58:1775–80. doi: 10.1016/s0006-2952(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 67.Dathe M, Nikolenko H, Klose J, Bienert M. Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry. 2004;43:9140–50. doi: 10.1021/bi035948v. [DOI] [PubMed] [Google Scholar]
- 68.Unger T, Oren Z, Shai Y. The effect of cyclization of magainin 2 and melittin analogues on structure, function, and model membrane interactions: implication to their mode of action. Biochemistry. 2001;40:6388–97. doi: 10.1021/bi0026066. [DOI] [PubMed] [Google Scholar]
- 69.Rozek A, Powers JP, Friedrich CL, Hancock RE. Structure-based design of an indolicidin peptide analogue with increased protease stability. Biochemistry. 2003;42:14310–8. doi: 10.1021/bi035643g. [DOI] [PubMed] [Google Scholar]
- 70.Eckert R, Qi F, Yarbrough DK, He J, Anderson MH, Shi W. Adding selectivity to antimicrobial peptides: rational design of a multidomain peptide against Pseudomonas spp. Antimicrob Agents Chemother. 2006;50:1480–8. doi: 10.1128/AAC.50.4.1480-1488.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eckert R, Mchardy I, Yarbrough DK, He J, Qi F, Anderson MH, Shi W. Stability and activity in sputum of G10KHc, a potent anti-Pseudomonas antimicrobial peptide. Chem Biol Drug Des. 2007;70:456–60. doi: 10.1111/j.1747-0285.2007.00580.x. [DOI] [PubMed] [Google Scholar]
- 72.Rohrl J, Yang D, Oppenheim JJ, Hehlgans T. Specific binding and chemotactic activity of mBD4 and its functional orthologues hBD2 to CCR6-expressing cells. J Biol Chem. 2010;285:7028–34. doi: 10.1074/jbc.M109.091090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berge G, Eliassen LT, Camilio KA, Bartnes K, Sveinbjornsson B, Rekdal O. Therapeutic vaccination against a murine lymphoma by intratumoral injection of a cationic anticancer peptide. Cancer Immunol Immunother. 2010;59:1285–94. doi: 10.1007/s00262-010-0857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cirioni O, Ghiselli R, Orlando F, Silvestri C, Luca S, Salzano AM, Mocchegiani F, Saba V, Scalise G, Scalono A, Giacometti A. Efficacy of the amphibian peptide distinctin in a neutropenic mouse model of staphylococcal sepsis. Crit Care Med. 2008;36:2629–33. doi: 10.1097/CCM.0b013e318184430d. [DOI] [PubMed] [Google Scholar]
- 75.Desbois AP, Gemmell CG, Coote PJ. In vivo efficacy of the antimicrobial peptide ranalexin in combination with endopeptidase lysostaphin against wound and systemic methicillin-resistant Staphylococcus aureus (MRSA) infections. Int J Antimicrob Agents. 2010;35:559–65. doi: 10.1016/j.ijantimicag.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 76.Lee YS, Shin YP, Shin SH, Park S, Kim MH, Lee IH. Therapeutic efficacy of halocidin-derived peptide HG1 in a mouse model of surgical wound infection with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:1296–9. doi: 10.1128/AAC.00948-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang KR, Zhang BZ, Zhang W, Yan JX, Li J, Wang R. Antitumor effects, cell selectivity and structure-activity relationship of a novel antimicrobial peptide polybia-MPI. Peptides. 2008;29:963–8. doi: 10.1016/j.peptides.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 78.Bommineni YR, Achanta M, Alexander J, Sunkara LT, Ritchey JW, Zhang G. A fowlicidin-1 analog protects mice from lethal infections induced by methicillin-resistant Staphylococcus aureus. Peptides. 2010;31:1225–30. doi: 10.1016/j.peptides.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 79.Mota-Meira M, Morency H, Lavoie MC. In vivo activity of mutacin B-Ny266. J Antimicrob Chemother. 2005;56:869–71. doi: 10.1093/jac/dki295. [DOI] [PubMed] [Google Scholar]
- 80.Corr SC, Li Y, Riedel CU, O’Toole PW, Hill C, Gahan CG. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. 2007;104:7617–21. doi: 10.1073/pnas.0700440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Braunstein A, Papo N, Shai Y. In vitro activity and potency of an intravenously injected antimicrobial peptide and its DL amino acid analog in mice infected with bacteria. Antimicrob. Agents Chemother. 2004;48:3127–9. doi: 10.1128/AAC.48.8.3127-3129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Skinner MC, Kiselev AO, Isaacs CE, Mietzner TA, Montelaro RC, Lampe MF. Evaluation of WLBU2 peptide and 3-O-octyl-sn-glycerol lipid as active ingredients for a topical microbicide formulation targeting chlamydia trachomatis. Antimicrob Agents Chemother. 2010;54:627–36. doi: 10.1128/AAC.00635-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ostorhazi E, Holub MC, Rozgonyi F, Harmos F, Cassone M, Wade JD, Otvos L., Jr Broad-spectgrum antimicrobial efficacy of peptide A3-APO in mouse models of multidrug-resistant wound and lung infections cannot be explained by in vitro activity against the pathogens involved. Int J Antimicrob Agents. 2011;37:480–4. doi: 10.1016/j.ijantimicag.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 84.Mor A. In: Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies, Wang G. Ed CABI: England. 2010;6:100–15. [Google Scholar]
- 85.White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–8. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 86.Bartley J. Vitamin D: emerging roles in infection and immunity. Expert Rev Anti Infect Ther. 2010;8:1359–69. doi: 10.1586/eri.10.102. [DOI] [PubMed] [Google Scholar]
- 87.Yeung AT, Gellatly SL, Hancock RE. Multifunctional cationic host defence peptides and their clinical applications. Cell Mol Life Sci. 2011;68:2161–76. doi: 10.1007/s00018-011-0710-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eckert R. Road to clinical efficacy: challenges and novel strategies for antimicrobial peptide development. Future Microbiol. 2011;6:635–51. doi: 10.2217/fmb.11.27. [DOI] [PubMed] [Google Scholar]
- 89.Fang W, Vega-Rodriguez J, Ghosh AK, Jacobs-Lorena M, Kang A, StLeger RJ. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science. 2011;331:1074–7. doi: 10.1126/science.1199115. [DOI] [PMC free article] [PubMed] [Google Scholar]