Abstract
Chronic diseases such as atherosclerosis and cancer are now the leading causes of morbidity and mortality worldwide. Inflammatory processes and oxidative stress underlie the pathogenesis of these pathological conditions. Bioactive peptides derived from food proteins have been evaluated for various beneficial effects, including anti-inflammatory and antioxidant properties. In this review, we summarize the roles of various food-derived bioactive peptides in inflammation and oxidative stress and discuss the potential benefits and limitations of using these compounds against the burden of chronic diseases.
1. Introduction
Chronic noncommunicable diseases such as cardiovascular diseases and cancer make up an increasing share of the global disease burden. With the increased longevity and improvement in global living standards, these “diseases of affluence” are now widespread in both developed and developing nations [1, 2]. Indeed, cardiovascular diseases such as atherosclerosis and its complications are now the leading cause of mortality and morbidity worldwide, closely followed by various cancers [3, 4]. Increased life spans have also meant corresponding increase in aging-related diseases in both developing and developed nations which may overwhelm their health care systems. Although atherosclerosis, cancers, and aging-related diseases can have diverse etiologies, they share many underlying pathological mechanisms including abnormalities in inflammatory responses and oxidative stress [5–7]. Thus targeting of the common pathological pathways has gained increasing attention in recent years for both prevention and treatment of chronic diseases.
While a number of commercially available anti-inflammatory and antioxidant drugs exist, none of these are free from side effects. Given the concerns about the side effects from prolonged usage of synthetic compounds, there is growing interest in the therapeutic applications of natural compounds and their derivatives as safer alternatives, either as functional foods or nutraceuticals. Food proteins from both plant and animal sources have been used to obtain a wide range of bioactive peptides [8]. Bioactive peptides are generally short peptides (3–20 amino acids) derived from proteins that can exert biological activities over and above their expected nutritional value [9]. These peptides are often functionally inactive within the native proteins and must be released by proteolysis (in vivo digestion, in vitro enzymatic hydrolysis, or bacterial fermentation) to achieve their specific “bioactive” roles. Many of these food-derived peptides demonstrate antihypertensive, anti-inflammatory, antidiabetic, and antioxidant properties under experimental conditions [10–12]. While some studies have observed the effects of single peptides, many others have examined protein hydrolysates composed of a mixture of diverse bioactive peptides [13–15]. Given their food-based sources and a perceived lack of serious side effects, bioactive peptides and peptide-rich protein hydrolysates can potentially provide a better alternative to synthetic pharmaceuticals for the prevention and treatment of chronic illnesses that affect an increasing number of people.
While bioactive peptides and peptide-rich protein hydrolysates can have a range of beneficial effects on diverse pathological conditions, this review would mainly focus on their anti-inflammatory and antioxidant actions. We would also discuss the potential challenges that may limit the use of these compounds as novel therapies against the global burden of chronic diseases.
2. Bioactive Peptides on Inflammation
2.1. Inflammation and Chronic Disease
Inflammation is the body's response to nonlethal injury which is characterized by increased endothelial permeability, leakage of protein-rich exudates, and infiltration of leukocytes into extravascular tissues. While inflammation is essential for resistance to microbial infections and wound healing, excessive and uncontrolled inflammatory changes often lead to chronic diseases. Indeed, vascular inflammation is an early event in the development of atherosclerosis and its complications such as myocardial infarction and stroke. Increasing evidence also links chronic inflammation to many types of cancer which further highlights its key role as a mediator of non-communicable illnesses. Despite the significance of inflammation, relatively few therapies have been devised to target the inflammatory component of cardiovascular and malignant diseases. The nonsteroidal anti-inflammatory drugs (NSAIDs) like aspirin are widely used to prevent and manage cardiovascular diseases, due to its antithrombotic as well as anti-inflammatory properties [16, 17]. Recent studies suggest that NSAIDs may also contribute to beneficial effects against cancers of the gastrointestinal system, further broadening the potential for anti-inflammatory therapies [18]. However, the presence of well-known side effects such as gastric bleeding and ulceration preclude the long-term use of NSAIDs for a large part of the population.
2.2. Pathways of Inflammatory Response
Inflammation is a complex and multisystem event affecting a wide range of cells, tissues, and organs. The vascular endothelium plays a key role as a gate keeper for the extravasation of leukocytes which is a hallmark of inflammation. However, tissue macrophages, epithelial cells, and fibroblasts are often involved in the generation of mediators which impinge upon and subsequently activate the endothelium through expression of leukocyte adhesion molecules like intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) which recruit leukocytes from the bloodstream and lead to their extravasation through a sequential cascade that involves tethering, rolling, activation, firm adhesion, and, finally, transmigration across the endothelial barrier. Mediators like various proinflammatory cytokines (such as tumor necrosis factor and interleukin (IL)-1β), chemokines (such as IL-8 and monocyte chemoattractant protein-1 (MCP-1)), and reactive oxygen species (ROS, such as superoxide and peroxynitrite) are involved in both the generation and propagation of the inflammatory response. A number of intracellular signaling pathways are activated in the participating cells which include nuclear factor-kappaB (NF-κB), mitogen activated protein (MAP) kinases, and activator protein-1 (AP-1), to name a few [19, 20]. Thus, the markers of inflammation include activation of proinflammatory signaling cascades, upregulation of leukocyte adhesion molecules, tissue infiltration of leukocytes, and increased levels of cytokines and chemokines in the circulation. Given the complexity and diversity of the inflammatory response, an investigation of potential anti-inflammatory agents involves the study of their effects on several of these markers, often using different cellular and intact animal systems for validation.
2.3. Bioactive Peptides on Inflammation in Cellular Systems
Much of the recent knowledge on bioactive peptides has been based on studies performed in cultured mammalian cells. Cell culture systems offer fast, economically feasible, and reproducible assays to analyze and validate the effects of many different compounds on a wide range of inflammatory markers. Peptides and protein hydrolysates derived from food sources such as milk, egg, fish, meat, and soybeans (to name a few) have all been tested for potential beneficial effects in these systems.
Bioactive peptides from milk have been among the first food-derived peptides studied. Milk is rich in caseins and whey proteins, both of which can give rise to a number of peptides with bioactive properties upon further processing such as enzymatic hydrolysis, digestion, and/or fermentation. The tripeptides VPP and IPP, derived from bacterial fermentation of casein, demonstrate inhibitory effects on angiotensin converting enzyme (ACE) in addition to stimulation of nitric oxide (NO) and bradykinin-mediated vasorelaxant pathways, thus suppressing the prohypertensive and proinflammatory mechanisms associated with hypertension and atherosclerosis [21]. Recently, a more direct anti-inflammatory role for VPP has been shown by its ability to attenuate leukocyte-endothelial interactions in vitro, largely through inhibition of proinflammatory c-Jun N-terminal kinase (JNK, a type of MAP kinase) pathway [22]. Casein hydrolysates generated by enzymatic digestion and containing a mixture of peptides have also been evaluated for anti-inflammatory properties. For example, digestion of casein with Corolase yields preparations that demonstrate anti-inflammatory effects on activated macrophages [23]. Hydrolysates of whey proteins also show promise in inhibition of inflammatory responses in respiratory and intestinal epithelial cells [24, 25]. Lactoferrin is a milk protein with antimicrobial properties which also exerts anti-inflammatory effects on activated macrophages [26]. Hydrolysis of lactoferrin yields the bioactive peptide lactoferricin, which demonstrates anti-inflammatory effects on human cartilage and synovial cells, suggesting potential benefits in arthritis management [27, 28]. In addition, both human and animal milk contain a number of anti-inflammatory and immunomodulatory compounds such as transforming growth factor-beta (TGF-beta), IL-10, and immunoglobulins which can further modulate the immune system of the gastrointestinal tract; however, these are not strictly “bioactive” peptides as they do not require processing from the native protein for their actions (reviewed in [11, 29, 30]).
Egg is another nutritious dietary component that is a source for many bioactive peptides [31]. Work from our lab has demonstrated the generation of egg tripeptides (IRW and IQW) from ovotransferrin (an egg white component) which are effective in the downregulation of cytokine-induced inflammatory protein expression in vascular endothelium, at least partly through the modulation of NF-κB pathway [32, 33]. These anti-inflammatory properties are also observed in conjunction with antioxidant and ACE inhibitory effects, further enhancing the beneficial actions [34]. Interestingly, these beneficial effects require the presence of an intact tripeptide as the corresponding dipeptides and constituent amino acids alone failed to replicate the anti-inflammatory functions, indicating a structure-function relationship between the tripeptide structure and blockade of inflammation [32].
Fish and meat are important sources of dietary protein. Recent findings suggest they also contribute to human health through generation of bioactive peptides; however, detailed studies at the cellular and molecular level are still quite sparse. A fish hydrolysate preparation has been shown to induce proliferation and migration in intestinal epithelial cells, which may contribute to anti-inflammatory and healing properties [13].
Plant-derived foods are another important source for bioactive compounds including many peptides and protein hydrolysates. Soybean hydrolysates have yielded several bioactive peptides with anti-inflammatory effects on macrophage cell lines, with preparations from germinated beans eliciting the stronger responses [35]. Chungkookjang, a fermented soybean product from Korea, is rich in bioactive peptides and shows anti-inflammatory effects in breast cancer cells by downregulation of cytokine/chemokines expression and activation of transforming growth factor (TGF)-beta signaling [36]. One of the soybean-derived peptides, lunasin, appears to exert widespread anti-inflammatory effects including suppression of NF-κB activity, reduced cytokine expression, and reduction in cyclooxygenase-2 (COX-2) levels in addition to its antioxidant and anticarcinogenic properties [37, 38]. The presence of an RGD motif in lunasin and similar peptides is believed to contribute to their anti-inflammatory effects, potentially involving antagonism of integrin signaling and downstream proinflammatory cascades [39].
2.4. Bioactive Peptides on Inflammation In Vivo
Based on the encouraging findings from cell-based studies, several bioactive peptides and hydrolysates have now been tested in animal models of human diseases. A number of different inflammatory models, typically experimentally induced colitis, arthritis, atherosclerosis, and respiratory tract inflammation, have been used. As much of this work has been performed only within the last few years, large-scale human trials are still lacking, although a few smaller studies on humans have shown some therapeutic promise.
Not surprisingly, milk-derived peptides have been in the forefront of in vivo studies of anti-inflammatory properties. The tripeptides VPP and IPP appear to be beneficial in a model of intestinal enterocolitis by their mediation of anti-inflammatory effects [40]. In addition, these peptides offer protection against the development of atherosclerotic changes in the apolipoprotein E (ApoE) knockout mice through a concerted action that involves modulation of both inflammatory and hypertensive pathways [41]. Hydrolysates of whey proteins can attenuate the dermatitis in NC/Nga mice [42], while casein hydrolysates (such as those produced by Aspergillus oryzae protease or fermentation with thermophilic lactobacilli) have shown promise in treating adjuvant arthritis in rats and chemically induced colitis in mice through modulation of both chronic and acute inflammatory responses [43, 44].
Other peptides and protein hydrolysates from animal sources have been used in several animal models of disease. In our lab, the egg-derived tripeptide IRW has shown promise in controlling both the hyperactive renin-angiotensin system (RAS) pathway as well as the exaggerated proinflammatory phenotype in spontaneously hypertensive rats (SHRs), a widely used model of hypertension and cardiovascular disease [45]. Fish protein hydrolysates have demonstrated protective effects on different murine models of colitis, including those induced by dextran sulphate as well as by chronic NSAID usage, suggesting their potential applications in human disease [46–48]. A similar preparation also reduced markers of inflammation and improved the plasma lipid profile in high fat-fed mice, with potential implications for obesity-induced inflammation and vascular disease [49]. Chicken collagen hydrolysate (CCH) containing an array of bioactive peptides has been used in rodent models of cardiovascular diseases. In the ApoE deficient mice, CCH administration successfully reduced the plasma levels of inflammatory cytokines in addition to improving the plasma lipid profile [50]. CCH given to SHRs reduced blood pressure and circulating inflammatory markers while increasing the bioavailability of the beneficial vasorelaxant NO [51]. A pilot study on human volunteers has also confirmed the antihypertensive effects of CCH although the potential anti-inflammatory mechanisms, if any, remain to be determined [52].
A number of plant-derived bioactive peptides and peptide-rich hydrolysates have also been tested by in vivo studies. Feeding of soy protein isolate to rodents (which presumably generates bioactive short peptides through intestinal digestion) has shown beneficial effects on experimentally induced arthritis [53] and genetically predisposed atherosclerosis [54], through the induction of protective anti-inflammatory effects. Soybean peptides such as VPY and others have shown promise in controlling cytokine/chemokines levels, reduction of oxidative stress, and reversal of the tissue damage observed in animal models of colitis, suggesting potential applications in treatment of inflammatory bowel diseases [55, 56]. Oral intake of a corn gluten hydrolysate also reduced inflammatory injury in a rat model of experimental colitis [57]. Similarly, ingestion of pyro-glutamyl leucine (a bioactive peptide from wheat gluten hydrolysate) was shown to protect against dextran sulphate-induced colitis in mice [58] and chemically induced hepatitis in rats [14], further supporting the in vivo anti-inflammatory functions of plant-derived peptides.
These anti-inflammatory effects of bioactive peptides and hydrolysates have been summarized in Table 1. A schematic diagram of the potential anti-inflammatory mechanisms of bioactive peptides is also shown (Figure 1) demonstrating the effects of these compounds on proinflammatory signaling kinases, pro- and anti-inflammatory cytokines, integrin-dependent signaling, ROS generation, and the renin-angiotensin system.
Table 1.
Food-derived anti-inflammatory peptides/hydrolysates in cell-based and in vivo systems.
| Protein source | Preparation | Active component | Cell/organism tested in | Observed effects | Reference |
|---|---|---|---|---|---|
| Casein | Bacterial fermentation | VPP | Endothelial cells and leukocytes | Reduced leukocyte recruitment | [22] |
| VPP, IPP | Murine colitis | Anti-inflammatory | [40] | ||
| Casein | Corolase hydrolysis |
Hydrolysate | Macrophages | Downregulation of COX-2, NF-κB inhibition |
[23] |
|
Aspergillus oryzae protease hydrolysis |
Hydrolysate | Rat adjuvant arthritis | Reduced arthritic score, anti-inflammatory | [43] | |
| Whey protein | Enzymatic hydrolysis | Hydrolysate | Epithelial cells | Reduced cytokine expression | [24, 25] |
| NC/Nga mouse | Reduced dermatitis | [42] | |||
| Lactoferrin |
Enzymatic hydrolysis | Lactoferricin | Synovial cells | Anti-inflammatory, antiarthritis | [27, 28] |
| Ovotransferrin | Thermolysin and pepsin hydrolysis | IRW, IQW | Human endothelial cells | Reduced ICAM-1/VCAM-1 with cytokine treatment | [32] |
| Fish protein | Enzymatic hydrolysis | Hydrolysate | Intestinal epithelial cells (human and rat) | Anti-inflammatory, increased proliferation | [13] |
| Murine colitis (DSS/NSAID induced) | Reduced cytokines, improved healing | [46] | |||
| High fat-fed mouse | Improved lipid profile |
[49] | |||
| Chicken collagen | Acid treatment followed by Aspergillus oryzae protease hydrolysis |
Hydrolysate | ApoE knockout mouse | Reduced cytokines, improved plasma lipid profile | [50] |
| SHR (rat) | Reduced ICAM-1 and decreased blood pressure | [51] | |||
| Soy protein | Fermentation | Chungkookjang | Breast cancer cells | Anti-inflammatory, increased TGF-beta | [36] |
| Soy protein | Enzymatic hydrolysis | Lunasin | Macrophage | Reduced cytokines, NF-κB inhibition | [37] |
| VPY | Murine colitis | Reduced cytokines, reduced oxidative stress, and improved histology | [55] | ||
| Wheat gluten |
Aspergillus oryzae protease hydrolysis and fractionation |
Pyro-glutamyl leucine | Rat hepatitis | Anti-inflammatory, improved hepatic enzyme profile | [14] |
| Mouse colitis | Improved mucosal histology and less weight loss | [58] |
Figure 1.
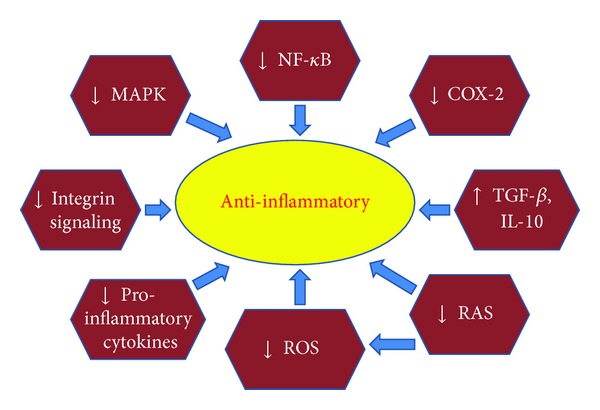
The potential mechanisms of action of anti-inflammatory bioactive peptides and peptide-rich protein hydrolysates. MAPK: mitogen activated protein kinase; NF-κB: nuclear factor-κB; COX-2: cyclo-oxygenase-2; TGF-beta: transforming growth factor-beta; IL-10: interleukin-10; RAS: renin-angiotensin system; ROS: reactive oxygen species.
3. Bioactive Peptides on Oxidative Stress
3.1. Oxidative Stress and Chronic Disease
The term ROS encompasses a range of oxygen-containing highly reactive species including free radicals superoxide (O2 −•) and hydroxyl radicals (HO•) as well as nonradical form like hydrogen peroxide (H2O2), hypochlorous acid (HOCl), singlet oxygen, and peroxynitrite (ONOO−) [59]. In low concentrations, ROS can be actually beneficial by induction of apoptosis in damaged/aged cells, detoxification of xenobiotics by cytochrome P450 system, and killing invading microorganisms by phagocytes and as regulatory mediators in cell signaling pathways [60, 61]. However, an excess of ROS, both due to excessive production or impaired antioxidant capacities or both, is harmful and leads to what is known as oxidative stress.
In pathological conditions, ROS attack nucleic acids (DNA or RNA), proteins, and unsaturated fatty acids and aggravate cellular damage (reviewed in [62, 63]). One example of DNA lesions is the conversion of guanine to 8-hydroxyguanine which affects the methylation of cytosine. Normal methylation of cytosine is considered as a critical step in regulation of gene expression and once it is altered, it may contribute to carcinogenesis [64]. Apart from DNA, peroxyl radicals (ROO•) can also initiate peroxidation of fatty acids. The final products of this reaction are malondialdehydes (MDA) which possess carcinogenic properties [65]. Proteins are another group of macromolecules affected by the ROS. Cleavage of the peptide bond, amino acid modification, and formation of cross-linked peptide aggregates happen during protein oxidation by ROS that leads to formation of protein derivatives possessing highly reactive carbonyl groups (ketones and aldehydes) which are involved in the complications of diabetes and many age-related diseases [66].
In addition to destructive effects on macromolecules, ROS also impair vasodilatory responses by reaction with NO. The reaction between NO and O2 − results in the production of peroxynitrite (ONOO−), which reduces the bioavailability of NO which is a potent vasorelaxant signaling messenger in vascular system. Increased oxidative stress and its downstream effects can lead to various conditions such as cardiovascular diseases [67], Alzheimer's disease [68], aging [69], and cancer [70]. Dietary intake of antioxidant compounds can reinforce the body's oxidant status and help to maintain a balanced condition in terms of oxidant/antioxidant in the body. Given this background, there is increasing interest in food proteins and their constituent peptides as potential candidates for use as antioxidants.
3.2. Bioactive Peptides as Antioxidants: Cell-Free Systems
Several chemical methods with different mechanisms of action have been developed to measure antioxidant potential of food proteins and peptides. This is because of complexity of oxidative reactions taking place in biological systems. Scavenging of stable free radicals 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid (ABTS) methods), reduction of metal ions (ferric ion reducing antioxidant power (FRAP) and cupric ion reducing antioxidant capacity (CUPRAC) methods), protecting a target molecule by inhibition of its consumption (oxygen radical absorbance capacity (ORAC) and total radical-trapping antioxidant potential (TRAP) assays), and inhibition of low density lipoprotein (LDL) oxidation are some of the common strategies used in chemical-based antioxidant assays (reviewed in [71, 72]). While the DPPH-based assay was among the first ones to be used extensively [73–76], many such assays have been widely used for screening antioxidant peptides.
A wide range of antioxidant peptides have been identified from marine organisms including oyster, shrimp, squid, blue mussel, and a variety of fish species (tuna, sardine, hoki, sole, and pacific hake) after hydrolysis with different enzymes. Puffer fish hydrolysate produced strong antioxidant action as shown by the ORAC assay compared to many other fish sources [77]. Both the salmon protein hydrolysate and peptide fractions inhibited the oxidation of linoleic acid [78]. Flounder fish muscle hydrolyzed with α-chymotrypsin has been also reported to possess strong antioxidant activities by scavenging free radicals in vitro [79]. Blue mussel (Mytilus edulis) is another source for the production of antioxidant peptides. Hydrolysis of this protein by the enzyme neutrase could scavenge 30% of DPPH radicals while further purification of this hydrolysate revealed the active peptide with the sequence of YPPAK with enhanced hydroxyl and superoxide anion radical scavenging activities [80].
Milk proteins also contribute much in the context of antioxidant peptides. YFYPEL, a hexapeptide isolated from pepsin hydrolysate of bovine casein, showed antioxidant activity by scavenging superoxide, DPPH, and hydroxyl radicals in vitro [81]. In a recent study, whey protein concentrates (WPC) hydrolyzed by Corolase or thermolysin were investigated for antioxidant activity by ORAC assay. Thermolysin-hydrolyzed WPC (8 hrs at 80°C, enzyme/substrate ratio: 0.10 w/w) was the most potent hydrolysate with radical scavenging activity and several peptides were identified in this hydrolysate [82]. Ovine κ-casein antioxidant activity also increased by 3-fold upon hydrolysis with pepsin, trypsin, and chymotrypsin. This casein hydrolysate further inhibited lipid peroxidation and several peptides contributing to antioxidant activity were identified [83]. Goat milk casein also exhibited enhanced free radical scavenging and metal ion chelating activity following hydrolysis by a combination of neutral and alkaline proteases. Further purification revealed five novel peptides in this hydrolysate with potential antioxidant properties [84].
Plants are known for antioxidant effects mostly because of their polyphenolic compounds. However recent research indicates the significance of many plant proteins and peptides as novel antioxidant agents. The potential of commercially available microbial proteases to enhance antioxidant potential of soy and corn proteins has been recently demonstrated [85, 86]. Corn protein hydrolysates were more effective than soy protein hydrolysate and inhibited lipid oxidation by 53% at lower incorporated dosage (200 μg/g) while soy protein hydrolysate reduced oxidation by 20% at much higher dosage (800 μg/g) [85]. In a recent study the antioxidant activity of chickpea albumin hydrolysate through in vitro radical scavenging and reducing power assays has been assayed. Further purification of the hydrolysate fraction with highest antioxidant activity identified RQSHFANAQP as the active component responsible [87]. All of the aforementioned peptides were evaluated for antioxidant activity through in vitro methods. Although these methods are good for screening and assessing preliminary data, there are drawbacks associated with these chemical-based assays including potential lack of relevance to biological systems and altered mechanisms of free radical generation [72, 88]. Therefore, it is preferable to use at least two different chemical assays prior to validation of antioxidant activity in more physiologically relevant systems like cells and whole organisms.
3.3. Bioactive Peptides as Antioxidants: Cellular Systems
Cell-based assays as intermediate methods have been used increasingly recently to evaluate the protective effects of antioxidants against oxidative stressors and to elucidate mechanism of action of peptides within cells [89]. Cell-based methods can be used to elucidate the mechanism/s of action of antioxidant agents within live cells. Moreover, cell culture assays are useful for the determination of peptide dosage to exert beneficial antioxidant effects without cytotoxicity for in vivo experiments. Among animal source peptides, those from flounder fish protein hydrolysates showed antioxidant and cytoprotective effects against 2,2′-azobis-(2-amidinopropane) dihydrochloride (AAPH) without cytotoxicity in the range of 12.5 to 200 μg/mL in Vero cells, a monkey kidney fibroblast line [79]. An antioxidant peptide from skin gelatin hydrolysate of hoki fish increased expression of cellular antioxidant enzymes including catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx) in human hepatoma cells (Hep3B) [90].
A recent study on bacterial fermentation in sourdough showed antioxidant effects on cultured fibroblasts [91]. Hempseed-derived antioxidant peptides also exhibited protective effects against oxidative apoptosis in rat pheochromocytoma line PC12 cells [92]. Similar effects were exerted by a hydrolysate of rice endosperm protein on mouse macrophages [93]. Results of these cell-based assays clearly show antioxidant potential of food peptides beyond the free radical scavenging in chemical assays.
3.4. Bioactive Peptides as Antioxidants: In Vivo Effects
In vivo studies such as animal experiments or human trials should be conducted after identification of an antioxidant peptide through in vitro and cell-based assays to demonstrate its antioxidant activity in an intact organism. Despite the importance of in vivo studies for verifying the antioxidant activity of bioactive peptides, only a few studies on the efficacy of antioxidant peptides have been conducted in animal models. Long-term administration (17 weeks) of egg white hydrolysate to SHRs has been shown to improve the plasma antioxidant capacity. Moreover MDA levels decreased significantly in the aortic tissue of rats receiving 0.5 g/kg/day and reverted to baseline levels five weeks after the treatment [94]. Similarly, recent studies on whey protein consumption also suggest a number of benefits including reduction of oxidative stress and better management of metabolic syndrome in both animal models and human subjects (reviewed in [12]). Among plant-derived sources, rapeseed crude protein hydrolyzed with Alcalase and Flavourzyme has shown to be a potential source for antioxidant peptides. Rapeseed hydrolysates were intraperitoneally injected to rats at 50 or 100 mg/kg/day for 16 days. At the end of the experiment, the serum was collected when 12.8% and 46.9% reduction in MDA levels were observed for 50 and 100 mg/kg/day dosages of rapeseed protein hydrolysate, respectively [95]. The findings from various cell-based and in vivo studies have been summarized in Table 2.
Table 2.
Food-derived antioxidant peptides/hydrolysates in cell-based and in vivo systems.
| Protein source | Preparation | Active component | Cell/organism tested in | Observed effects | Reference |
|---|---|---|---|---|---|
| Ovotransferrin | Thermolysin and pepsin hydrolysis | IRW, IQW | Endothelial cells (human) | Reduced cellular superoxide (dihydroethidium staining) | [32] |
| Chicken egg white | Hydrolysis with pepsin | Egg white hydrolysate | SHR (rat) | Increase in plasma radical scavenging, reduction in aorta MDA levels |
[94] |
| Hoki skin gelatin | Hydrolysis with trypsin | HGPLGPL | Hep3B (human hepatoma cells) | Increase in cellular antioxidant enzymes (catalase, SOD, GPx) | [90] |
| Flounder fish protein | Hydrolysis with α-chymotrypsin | CAAP, VCSV | Vero cells (monkey kidney fibroblast cell line) | Cytotoxic protective effects, scavenging intracellular ROS | [79] |
| Cereal flours | Fermentation of sourdough with lactic acid bacteria |
25 peptides (8–57 amino acid residues) | Mouse fibroblasts (Balb 3T3) | Protective effects against oxidative stress in fibroblasts | [91] |
| Hempseed | Alcalase hydrolysate of hempseed protein isolate |
NHAV HVRETALV |
Rat pheochromocytoma line PC12 cells | Protective effects against cell death/oxidative apoptosis | [92] |
| Rice endosperm protein | Neutrase hydrolysate of defatted rice endosperm protein |
FRDEHKK | Mouse macrophage (RAW 264.7) | Scavenging of intracellular ROS (DCFH-DA method) | [93] |
| Rapeseed | Hydrolysis with Alcalase and Flavourzyme | Rapeseed crude hydrolysate |
Wistar rat | 50% reduction in serum MDA levels | [95] |
4. Potential Challenges and Opportunities
4.1. Limitations and Risks
While the field of bioactive peptides is an exciting and growing area of research, there are a few risks and limitations before the widespread use of such peptides in the general population. As previously discussed, many of the studies are still at an early stage and more in vivo data will be needed before applications to human health. In the absence of solid pharmacokinetic data, proper dosage and frequency of administration may be impossible to determine, leading to wide variability in intake and biological effects [29, 96]. While these bioactive peptides are considered to be relatively safe, there is always the risk of potential side effects if too high a dose is consumed. For example, antioxidant vitamins were traditionally considered safe even in high doses; yet recent evidence suggests potential toxic effects on excessive consumption [97, 98]. Another potential risk could be due to the presence of immunogenic proteins and peptides within the protein hydrolysates, which may induce and/or exacerbate allergic reactions in a minority of users [99, 100]. Proper screening prior to the ingestion of such hydrolysates might be necessary in subjects prone to allergies. While this lack of knowledge about specific compounds and their potential side effects is a limitation, it also provides opportunities for future research in several directions.
4.2. Future Directions
Future studies in the field of bioactive peptides would likely involve detailed studies on animals and human volunteers to better understand the pharmacokinetics of these compounds, testing for potential immunogenicity (to prevent allergies), characterizing individual components of complex peptide-rich hydrolysates to tease out their specific actions as well as basic biomedical research directed towards identifying specific receptors and signaling pathways involved in mediating some of these beneficial anti-inflammatory and antioxidant actions. Indeed, outside the renin-angiotensin system [101, 102], few receptors have been identified as involved in bioactive peptide actions. Further work on identifying specific peptide sequences and their corresponding receptors may provide opportunities for better targeting of inflammation and oxidative stress in a tissue- and organ-specific manner.
5. Conclusions
Bioactive peptides and peptide-rich protein hydrolysates represent a new direction in functional foods and nutraceuticals. While both types of preparations have shown promise as potential anti-inflammatory and antioxidant agents, further research is still needed to verify these beneficial effects in order to successfully translate the research from bench to the bedside to effectively control the growing burden of chronic noncommunicable illnesses with minimal side effects.
Acknowledgments
The laboratory of Jianping Wu is supported by Grants from Natural Sciences and Engineering Research Council (NSERC) of Canada and Alberta Livestock and Meat Agency (ALMA).
References
- 1.Danaei G, Singh GM, Paciorek CJ, et al. The global cardiovascular risk transition: associations of four metabolic risk factors with national income, urbanization, and Western diet in 1980 and 2008. Circulation. 2013;127(14):1493–1502. doi: 10.1161/CIRCULATIONAHA.113.001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popkin BM. Understanding global nutrition dynamics as a step towards controlling cancer incidence. Nature Reviews Cancer. 2007;7(1):61–67. doi: 10.1038/nrc2029. [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Smaha LA, Smith SC, Jr., Mensah GA, Lenfant C. World Heart Day 2002: the international burden of cardiovascular disease: responding to the emerging global epidemic. Circulation. 2002;106(13):1602–1605. doi: 10.1161/01.cir.0000035036.22612.2b. [DOI] [PubMed] [Google Scholar]
- 4.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Annals of Tropical Medicine and Parasitology. 2006;100(5-6):481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 5.Gutowski M, Kowalczyk S. A study of free radical chemistry: their role and pathophysiological significance. Acta Biochimica Polonica. 2013;60(1):1–16. [PubMed] [Google Scholar]
- 6.Ramos AF, de Fuccio MB, Moretzsohn LD, et al. Cystic fibrosis, gastroduodenal inflammation, duodenal ulcer, and H. pylori infection: the “cystic fibrosis paradox” revisited. Journal of Cystic Fibrosis. 2013;12(4):377–383. doi: 10.1016/j.jcf.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Current Pharmaceutical Design. 2012;18(26):3831–3852. doi: 10.2174/138161212802083707. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikawa M, Fujita H, Matoba N, et al. Bioactive peptides derived from food proteins preventing lifestyle-related diseases. BioFactors. 2000;12(1–4):143–146. doi: 10.1002/biof.5520120122. [DOI] [PubMed] [Google Scholar]
- 9.Kitts DD, Weiler K. Bioactive proteins and peptides from food sources. Applications of bioprocesses used in isolation and recovery. Current Pharmaceutical Design. 2003;9(16):1309–1323. doi: 10.2174/1381612033454883. [DOI] [PubMed] [Google Scholar]
- 10.Erdmann K, Cheung BWY, Schröder H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. Journal of Nutritional Biochemistry. 2008;19(10):643–654. doi: 10.1016/j.jnutbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann R, Meisel H. Food-derived peptides with biological activity: from research to food applications. Current Opinion in Biotechnology. 2007;18(2):163–169. doi: 10.1016/j.copbio.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Sousa GTD, Lira FS, Rosa JC, et al. Dietary whey protein lessens several risk factors for metabolic diseases: a review. Lipids in Health and Disease. 2012;11:p. 67. doi: 10.1186/1476-511X-11-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald AJ, Rai PS, Marchbank T, et al. Reparative properties of a commercial fish protein hydrolysate preparation. Gut. 2005;54(6):775–781. doi: 10.1136/gut.2004.060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sato K, Egashira Y, Ono S, et al. Identification of a hepatoprotective peptide in wheat gluten hydrolysate against D-galactosamine-induced acute hepatitis in rats. Journal of Agricultural and Food Chemistry. 2013;61(26):6304–6310. doi: 10.1021/jf400914e. [DOI] [PubMed] [Google Scholar]
- 15.Izumi H, Ishizuka S, Inafune A, et al. α-lactalbumin hydrolysate stimulates glucagon-like peptide-2 secretion and small intestinal growth in suckling rats. Journal of Nutrition. 2009;139(7):1322–1327. doi: 10.3945/jn.109.106401. [DOI] [PubMed] [Google Scholar]
- 16.Davidge ST. Prostaglandin H synthase and vascular function. Circulation Research. 2001;89(8):650–660. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- 17.Fiorucci S, Distrutti E, Mencarelli A, et al. Cooperation between aspirin-triggered lipoxin and nitric oxide (NO) mediates antiadhesive properties of 2-(acetyloxy)benzoic acid 3-(nitrooxymethyl)phenyl ester (NCX-4016) (NO-aspirin) on neutrophil-endothelial cell adherence. Journal of Pharmacology and Experimental Therapeutics. 2004;309(3):1174–1182. doi: 10.1124/jpet.103.063651. [DOI] [PubMed] [Google Scholar]
- 18.Cook NR, Lee IM, Zhang SM, Moorthy MV, Buring JE. Alternate-day, low-dose aspirin and cancer risk: long-term observational follow-up of a randomized trial. Annals of Internal Medicine. 2013;159(2):77–85. doi: 10.7326/0003-4819-159-2-201307160-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Csiszar A, Wang M, Lakatta EG, Ungvari Z. Inflammation and endothelial dysfunction during aging: role of NF-κB. Journal of Applied Physiology. 2008;105(4):1333–1341. doi: 10.1152/japplphysiol.90470.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Ortega M, Lorenzo O, Egido J. Angiotensin III increases MCP-1 and activates NF-κB and AP-1 in cultured mesangial and mononuclear cells. Kidney International. 2000;57(6):2285–2298. doi: 10.1046/j.1523-1755.2000.00089.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirota T, Nonaka A, Matsushita A, et al. Milk casein-derived tripeptides, VPP and IPP induced NO production in cultured endothelial cells and endothelium-dependent relaxation of isolated aortic rings. Heart and Vessels. 2011;26(5):549–556. doi: 10.1007/s00380-010-0096-y. [DOI] [PubMed] [Google Scholar]
- 22.Aihara K, Ishii H, Yoshida M. Casein-derived tripeptide, Val-Pro-Pro (VPP), modulates monocyte adhesion to vascular endothelium. Journal of Atherosclerosis and Thrombosis. 2009;16(5):594–603. doi: 10.5551/jat.729. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen DS, Theil PK, Larsen LB, Purup S. Effect of milk hydrolysates on inflammation markers and drug-induced transcriptional alterations in cell-based models. Journal of Animal Science. 2012;90(supplement 4):403–405. doi: 10.2527/jas.53953. [DOI] [PubMed] [Google Scholar]
- 24.Iskandar MM, Dauletbaev N, Kubow S, Mawji N, Lands LC. Whey protein hydrolysates decrease IL-8 secretion in lipopolysaccharide (LPS)-stimulated respiratory epithelial cells by affecting LPS binding to Toll-like receptor 4. British Journal of Nutrition. 2013;110(1):58–68. doi: 10.1017/S0007114512004655. [DOI] [PubMed] [Google Scholar]
- 25.Piccolomini AF, Iskandar MM, Lands LC, Kubow S. High hydrostatic pressure pre-treatment of whey proteins enhances whey protein hydrolysate inhibition of oxidative stress and IL-8 secretion in intestinal epithelial cells. Food & Nutrition Research. 2012;56 doi: 10.3402/fnr.v56i0.17549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Håversen L, Ohlsson BG, Hahn-Zoric M, Hanson LÅ, Mattsby-Baltzer I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-κB. Cellular Immunology. 2002;220(2):83–95. doi: 10.1016/s0008-8749(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 27.Yan D, Chen D, Shen J, Xiao G, van Wijnen AJ, Im HJ. Bovine lactoferricin is anti-inflammatory and anti-catabolic in human articular cartilage and synovium. Journal of Cellular Physiology. 2013;228(2):447–456. doi: 10.1002/jcp.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JS, Ellman MB, Yan D, et al. Lactoferricin mediates anti-inflammatory and anti-catabolic effects via inhibition of IL-1 and LPS activity in the intervertebral disc. Journal of Cellular Physiology. 2013;228(9):1884–1896. doi: 10.1002/jcp.24350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutherfurd-Markwick KJ. Food proteins as a source of bioactive peptides with diverse functions. British Journal of Nutrition. 2012;108(supplement 2):S149–S157. doi: 10.1017/S000711451200253X. [DOI] [PubMed] [Google Scholar]
- 30.Kim YS, Young MR, Bobe G, Colburn NH, Milner JA. Bioactive food components, inflammatory targets, and cancer prevention. Cancer Prevention Research. 2009;2(3):200–208. doi: 10.1158/1940-6207.CAPR-08-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mine Y. Egg proteins and peptides in human health-chemistry, bioactivity and production. Current Pharmaceutical Design. 2007;13(9):875–884. doi: 10.2174/138161207780414278. [DOI] [PubMed] [Google Scholar]
- 32.Majumder K, Chakrabarti S, Davidge ST, Wu J. Structure and activity study of egg protein ovotransferrin derived peptides (IRW and IQW) on endothelial inflammatory response and oxidative stress. Journal of Agricultural and Food Chemistry. 2013;61(9):2120–2129. doi: 10.1021/jf3046076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang W, Chakrabarti S, Majumder K, Jiang Y, Davidge ST, Wu J. Egg-derived peptide IRW inhibits TNF-α-induced inflammatory response and oxidative stress in endothelial cells. Journal of Agricultural and Food Chemistry. 2010;58(20):10840–10846. doi: 10.1021/jf102120c. [DOI] [PubMed] [Google Scholar]
- 34.Huang W, Shen S, Nimalaratne C, Li S, Majumder K, Wu J. Effects of addition of egg ovotransferrin-derived peptides on the oxygen radical absorbance capacity of different teas. Food Chemistry. 2012;135(3):1600–1607. doi: 10.1016/j.foodchem.2012.05.093. [DOI] [PubMed] [Google Scholar]
- 35.Vernaza MG, Dia VP, Gonzalez de Mejia E, Chang YK. Antioxidant and antiinflammatory properties of germinated and hydrolysed Brazilian soybean flours. Food Chemistry. 2012;134(4):2217–2225. doi: 10.1016/j.foodchem.2012.04.037. [DOI] [PubMed] [Google Scholar]
- 36.Hwang JS, Yoo HJ, Song HJ, et al. Inflammation-related signaling pathways implicating TGFβ are revealed in the expression profiling of MCF7 cell treated with fermented soybean, Chungkookjang. Nutrition and Cancer. 2011;63(4):645–652. doi: 10.1080/01635581.2011.551987. [DOI] [PubMed] [Google Scholar]
- 37.de Mejia EG, Dia VP. Lunasin and lunasin-like peptides inhibit inflammation through suppression of NF-κB pathway in the macrophage. Peptides. 2009;30(12):2388–2398. doi: 10.1016/j.peptides.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Hernández-Ledesma B, Hsieh C-C, de Lumen BO. Antioxidant and anti-inflammatory properties of cancer preventive peptide lunasin in RAW 264.7 macrophages. Biochemical and Biophysical Research Communications. 2009;390(3):803–808. doi: 10.1016/j.bbrc.2009.10.053. [DOI] [PubMed] [Google Scholar]
- 39.Cam A, de Mejia EG. RGD-peptide lunasin inhibits Akt-mediated NF-κB activation in human macrophages through interaction with the αVβ3 integrin. Molecular Nutrition & Food Research. 2012;56(10):1569–1581. doi: 10.1002/mnfr.201200301. [DOI] [PubMed] [Google Scholar]
- 40.Chatterton DE, Nguyen DN, Bering SB, Sangild PT. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. The International Journal of Biochemistry & Cell Biology. 2013;45(8):1730–1747. doi: 10.1016/j.biocel.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura T, Hirota T, Mizushima K, et al. Milk-derived peptides, Val-Pro-Pro and Ile-Pro-Pro, attenuate atherosclerosis development in apolipoprotein e-deficient mice: a preliminary study. Journal of Medicinal Food. 2013;16(5):396–403. doi: 10.1089/jmf.2012.2541. [DOI] [PubMed] [Google Scholar]
- 42.Shimizu N, Dairiki K, Ogawa S, Kaneko T. Dietary whey protein hydrolysate suppresses development of atopic dermatitis-like skin lesions induced by mite antigen in NC/Nga mice. Allergology International. 2006;55(2):185–189. doi: 10.2332/allergolint.55.185. [DOI] [PubMed] [Google Scholar]
- 43.Hatori M, Ohki K, Hirano S-I, Yang X-P, Kuboki H, Abe C. Effects of a casein hydrolysate prepared from Aspergillus oryzae protease on adjuvant arthritis in rats. Bioscience, Biotechnology and Biochemistry. 2008;72(8):1983–1991. doi: 10.1271/bbb.70792. [DOI] [PubMed] [Google Scholar]
- 44.Pescuma M, Espeche Turbay MB, Mozzi F, Font de Valdez G, Savoy de Giori G, Hebert EM. Diversity in proteinase specificity of thermophilic lactobacilli as revealed by hydrolysis of dairy and vegetable proteins. Applied Microbiology and Biotechnology. 2013;97(17):7831–7844. doi: 10.1007/s00253-013-5037-0. [DOI] [PubMed] [Google Scholar]
- 45.Majumder K, Chakrabarti S, Morton JS, et al. Egg-derived Tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0082829.e82829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marchbank T, Elia G, Playford RJ. Intestinal protective effect of a commercial fish protein hydrolysate preparation. Regulatory Peptides. 2009;155(1–3):105–109. doi: 10.1016/j.regpep.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Marchbank T, Limdi JK, Mahmood A, Elia G, Playford RJ. Clinical trial: protective effect of a commercial fish protein hydrolysate against indomethacin (NSAID)-induced small intestinal injury. Alimentary Pharmacology and Therapeutics. 2008;28(6):799–804. doi: 10.1111/j.1365-2036.2008.03783.x. [DOI] [PubMed] [Google Scholar]
- 48.Hwang JW, Lee SJ, Kim YS, et al. Purification and characterization of a novel peptide with inhibitory effects on colitis induced mice by dextran sulfate sodium from enzymatic hydrolysates of Crassostrea gigas. Fish & Shellfish Immunology. 2012;33(4):993–999. doi: 10.1016/j.fsi.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 49.Bjorndal B, Berge C, Ramsvik MS, et al. A fish protein hydrolysate alters fatty acid composition in liver and adipose tissue and increases plasma carnitine levels in a mouse model of chronic inflammation. Lipids in Health and Disease. 2013;12(1):p. 143. doi: 10.1186/1476-511X-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Kouguchi T, Shimizu K, Sato M, Takahata Y, Morimatsu F. Chicken collagen hydrolysate reduces proinflammatory cytokine production in C57BL/6.KOR-ApoEsh1 mice. Journal of Nutritional Science and Vitaminology. 2010;56(3):208–210. doi: 10.3177/jnsv.56.208. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Y, Kouguchi T, Shimizu M, Ohmori T, Takahata Y, Morimatsu F. Chicken collagen hydrolysate protects rats from hypertension and cardiovascular damage. Journal of Medicinal Food. 2010;13(2):399–405. doi: 10.1089/jmf.2009.1246. [DOI] [PubMed] [Google Scholar]
- 52.Saiga-Egusa A, Iwai K, Hayakawa T, Takahata Y, Morimatsu F. Antihypertensive effects and endothelial progenitor cell activation by intake of chicken collagen hydrolysate in pre- and mild-hypertension. Bioscience, Biotechnology and Biochemistry. 2009;73(2):422–424. doi: 10.1271/bbb.80189. [DOI] [PubMed] [Google Scholar]
- 53.Mohammad Shahi M, Rashidi M-R, Mahboob S, Haidari F, Rashidi B, Hanaee J. Protective effect of soy protein on collagen-induced arthritis in rat. Rheumatology International. 2012;32(8):2407–2414. doi: 10.1007/s00296-011-1979-7. [DOI] [PubMed] [Google Scholar]
- 54.Nagarajan S, Burris RL, Stewart BW, Wilkerson JE, Badger TM. Dietary soy protein isolate ameliorates atherosclerotic lesions in apolipoprotein E-deficient mice potentially by inhibiting monocyte chemoattractant protein-1 expression. Journal of Nutrition. 2008;138(2):332–337. doi: 10.1093/jn/138.2.332. [DOI] [PubMed] [Google Scholar]
- 55.Kovacs-Nolan J, Zhang H, Ibuki M, et al. The PepT1-transportable soy tripeptide VPY reduces intestinal inflammation. Biochim Biophys Acta. 2012;1820(11):1753–1763. doi: 10.1016/j.bbagen.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 56.Young D, Ibuki M, Nakamori T, Fan M, Mine Y. Soy-derived di-and tripeptides alleviate colon and ileum inflammation in pigs with dextran sodium sulfate-induced colitis. Journal of Nutrition. 2012;142(2):363–368. doi: 10.3945/jn.111.149104. [DOI] [PubMed] [Google Scholar]
- 57.Mochizuki M, Shigemura H, Hasegawa N. Anti-inflammatory effect of enzymatic hydrolysate of corn gluten in an experimental model of colitis. Journal of Pharmacy and Pharmacology. 2010;62(3):389–392. doi: 10.1211/jpp.62.03.0015. [DOI] [PubMed] [Google Scholar]
- 58.Wada S, Sato K, Ohta R, et al. Ingestion of low dose pyroglutamyl leucine improves dextran sulfate sodium-induced colitis and intestinal microbiota in mice. Journal of Agricultural and Food Chemistry. 2013;61(37):8807–8813. doi: 10.1021/jf402515a. [DOI] [PubMed] [Google Scholar]
- 59.Lü J-M, Lin PH, Yao Q, Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. Journal of Cellular and Molecular Medicine. 2010;14(4):840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. International Journal of Biomedical Science. 2008;4(2):89–96. [PMC free article] [PubMed] [Google Scholar]
- 61.Poli G, Leonarduzzi G, Biasi F, Chiarpotto E. Oxidative stress and cell signalling. Current Medicinal Chemistry. 2004;11(9):1163–1182. doi: 10.2174/0929867043365323. [DOI] [PubMed] [Google Scholar]
- 62.Devasagayam TPA, Tilak JC, Boloor KK, Sane KS, Ghaskadbi SS, Lele RD. Free radicals and antioxidants in human health: current status and future prospects. Journal of Association of Physicians of India. 2004;52:794–804. [PubMed] [Google Scholar]
- 63.Brieger K, Schiavone S, Miller FJ, Jr., Krause KH. Reactive oxygen species: from health to disease. Swiss Medical Weekly. 2012;142:p. w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 64.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochemical Journal. 1996;313(1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Stadtman ER, Levine RL. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3-4):207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- 67.Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13(3):129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G. Oxidative stress in Alzheimer’s disease. Biochimica et Biophysica Acta. 2000;1502(1):139–144. doi: 10.1016/s0925-4439(00)00040-5. [DOI] [PubMed] [Google Scholar]
- 69.Hyun D-H, Hernandez JO, Mattson MP, de Cabo R. The plasma membrane redox system in aging. Ageing Research Reviews. 2006;5(2):209–220. doi: 10.1016/j.arr.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Kinnula VL, Crapo JD. Superoxide dismutases in malignant cells and human tumors. Free Radical Biology and Medicine. 2004;36(6):718–744. doi: 10.1016/j.freeradbiomed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 71.Niki E. Assessment of antioxidant capacity of natural products. Current Pharmaceutical Biotechnology. 2010;11(8):801–809. doi: 10.2174/138920110793262097. [DOI] [PubMed] [Google Scholar]
- 72.Carocho M, Ferreira IC. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food and Chemical Toxicology. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Nagai T, Egashira T, Yamanaka Y, Kohno M. The protective effect of glycyrrhizin against injury of the liver caused by ischemia-reperfusion. Archives of Environmental Contamination and Toxicology. 1991;20(3):432–436. doi: 10.1007/BF01064416. [DOI] [PubMed] [Google Scholar]
- 74.Rousseau-Richards C, Auclair C, Richard C, Martin R. Free radical scavenging and cytotoxic properties in the ellipticine series. Free Radical Biology and Medicine. 1990;8(3):223–230. doi: 10.1016/0891-5849(90)90067-s. [DOI] [PubMed] [Google Scholar]
- 75.Saito M. Polychlorinated biphenyls-induced lipid peroxidation as measured by thiobarbituric acid-reactive substances in liver subcellular fractions of rats. Biochimica et Biophysica Acta. 1990;1046(3):301–308. doi: 10.1016/0005-2760(90)90245-s. [DOI] [PubMed] [Google Scholar]
- 76.Smith RC, Reeves JC, Dage RC, Schnettler RA. Antioxidant properties of 2-imidazolones and 2-imidazolthiones. Biochemical Pharmacology. 1987;36(9):1457–1460. doi: 10.1016/0006-2952(87)90110-9. [DOI] [PubMed] [Google Scholar]
- 77.Harada K, Maeda T, Hasegawa Y, Tokunaga T, Tamura Y, Koizumi T. Antioxidant activity of fish sauces including puffer (Lagocephalus wheeleri) fish sauce measured by the oxygen radical absorbance capacity method. Molecular Medicine Reports. 2010;3(4):663–668. doi: 10.3892/mmr_00000313. [DOI] [PubMed] [Google Scholar]
- 78.Girgih AT, Udenigwe CC, Hasan FM, Gill TA, Aluko RE. Antioxidant properties of Salmon (Salmo salar) protein hydrolysate and peptide fractions isolated by reverse-phase HPLC. Food Research International. 2013;52(1):315–322. [Google Scholar]
- 79.Ko JY, Lee JH, Samarakoon K, Kim JS, Jeon YJ. Purification and determination of two novel antioxidant peptides from flounder fish (Paralichthys olivaceus) using digestive proteases. Food and Chemical Toxicology. 2013;52:113–120. doi: 10.1016/j.fct.2012.10.058. [DOI] [PubMed] [Google Scholar]
- 80.Wang B, Li L, Chi CF, Ma JH, Luo HY, Xu YF. Purification and characterisation of a novel antioxidant peptide derived from blue mussel (Mytilus edulis) protein hydrolysate. Food Chemistry. 2013;138(2-3):1713–1719. doi: 10.1016/j.foodchem.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Suetsuna K, Ukeda H, Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. Journal of Nutritional Biochemistry. 2000;11(3):128–131. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- 82.Contreras MDM, Hernández-Ledesma B, Amigo L, Martín-Álvarez PJ, Recio I. Production of antioxidant hydrolyzates from a whey protein concentrate with thermolysin: optimization by response surface methodology. LWT-Food Science and Technology. 2011;44(1):9–15. [Google Scholar]
- 83.Gómez-Ruiz JÁ, López-Expósito I, Pihlanto A, Ramos M, Recio I. Antioxidant activity of ovine casein hydrolysates: identification of active peptides by HPLC-MS/MS. European Food Research and Technology. 2008;227(4):1061–1067. [Google Scholar]
- 84.Li Z, Jiang A, Yue T, Wang J, Wang Y, Su J. Purification and identification of five novel antioxidant peptides from goat milk casein hydrolysates. Journal of Dairy Science. 2013;96(7):4242–4251. doi: 10.3168/jds.2012-6511. [DOI] [PubMed] [Google Scholar]
- 85.Zhou KQ, Sun S, Canning C. Production and functional characterisation of antioxidative hydrolysates from corn protein via enzymatic hydrolysis and ultrafiltration. Food Chemistry. 2012;135(3):1192–1197. doi: 10.1016/j.foodchem.2012.05.063. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Li J, Zhou K. Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresource Technology. 2010;101(7):2084–2089. doi: 10.1016/j.biortech.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 87.Kou XH, Gao J, Xue ZH, Zhang ZJ, Wang H, Wang X. Purification and identification of antioxidant peptides from chickpea (Cicer arietinum L.) albumin hydrolysates. LWT-Food Science and Technology. 2013;50(2):591–598. [Google Scholar]
- 88.Lopez-Alarcon C, Denicola A. Evaluating the antioxidant capacity of natural products: a review on chemical and cellular-based assays. Analytica Chimica Acta. 2013;763:1–10. doi: 10.1016/j.aca.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 89.Niki E. Assessment of antioxidant capacity in vitro and in vivo. Free Radical Biology and Medicine. 2010;49(4):503–515. doi: 10.1016/j.freeradbiomed.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 90.Mendis E, Rajapakse N, Kim S-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. Journal of Agricultural and Food Chemistry. 2005;53(3):581–587. doi: 10.1021/jf048877v. [DOI] [PubMed] [Google Scholar]
- 91.Coda R, Rizzello CG, Pinto D, Gobbetti M. Selected lactic acid bacteria synthesize antioxidant peptides during sourdough fermentation of cereal flours. Applied and Environmental Microbiology. 2012;78(4):1087–1096. doi: 10.1128/AEM.06837-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lu R-R, Qian P, Sun Z, et al. Hempseed protein derived antioxidative peptides: purification, identification and protection from hydrogen peroxide-induced apoptosis in PC12 cells. Food Chemistry. 2010;123(4):1210–1218. [Google Scholar]
- 93.Zhang J, Zhang H, Wang L, Guo X, Wang X, Yao H. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chemistry. 2010;119(1):226–234. [Google Scholar]
- 94.Manso MA, Miguel M, Even J, Hernández R, Aleixandre A, López-Fandiño R. Effect of the long-term intake of an egg white hydrolysate on the oxidative status and blood lipid profile of spontaneously hypertensive rats. Food Chemistry. 2008;109(2):361–367. doi: 10.1016/j.foodchem.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 95.Xue Z, Yu W, Liu Z, Wu M, Kou X, Wang J. Preparation and antioxidative properties of a rapeseed (Brassica napus) protein hydrolysate and three peptide fractions. Journal of Agricultural and Food Chemistry. 2009;57(12):5287–5293. doi: 10.1021/jf900860v. [DOI] [PubMed] [Google Scholar]
- 96.Cam A, de Mejia EG. Role of dietary proteins and peptides in cardiovascular disease. Molecular Nutrition and Food Research. 2012;56(1):53–66. doi: 10.1002/mnfr.201100535. [DOI] [PubMed] [Google Scholar]
- 97.Bast A, Haenen GRMM. The toxicity of antioxidants and their metabolites. Environmental Toxicology and Pharmacology. 2002;11(3-4):251–258. doi: 10.1016/s1382-6689(01)00118-1. [DOI] [PubMed] [Google Scholar]
- 98.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the beta-carotene and retinol efficacy trial. Journal of the National Cancer Institute. 1996;88(21):1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 99.Akiyama H, Sakata K, Yoshioka Y, et al. Profile analysis and immunoglobulin E reactivity of wheat protein hydrolysates. International Archives of Allergy and Immunology. 2006;140(1):36–42. doi: 10.1159/000092000. [DOI] [PubMed] [Google Scholar]
- 100.Franck P, Moneret Vautrin DA, Dousset B, et al. The allergenicity of soybean-based products is modified by food technologies. International Archives of Allergy and Immunology. 2002;128(3):212–219. doi: 10.1159/000064254. [DOI] [PubMed] [Google Scholar]
- 101.Yamada Y, Yamauchi D, Usui H, et al. Hypotensive activity of novokinin, a potent analogue of ovokinin(2-7), is mediated by angiotensin AT2 receptor and prostaglandin IP receptor. Peptides. 2008;29(3):412–418. doi: 10.1016/j.peptides.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Yoshikawa M. Isolation and characterization of ovokinin, a bradykinin B1 agonist peptide derived from ovalbumin. Peptides. 1995;16(5):785–790. doi: 10.1016/0196-9781(95)00054-n. [DOI] [PubMed] [Google Scholar]


