Abstract
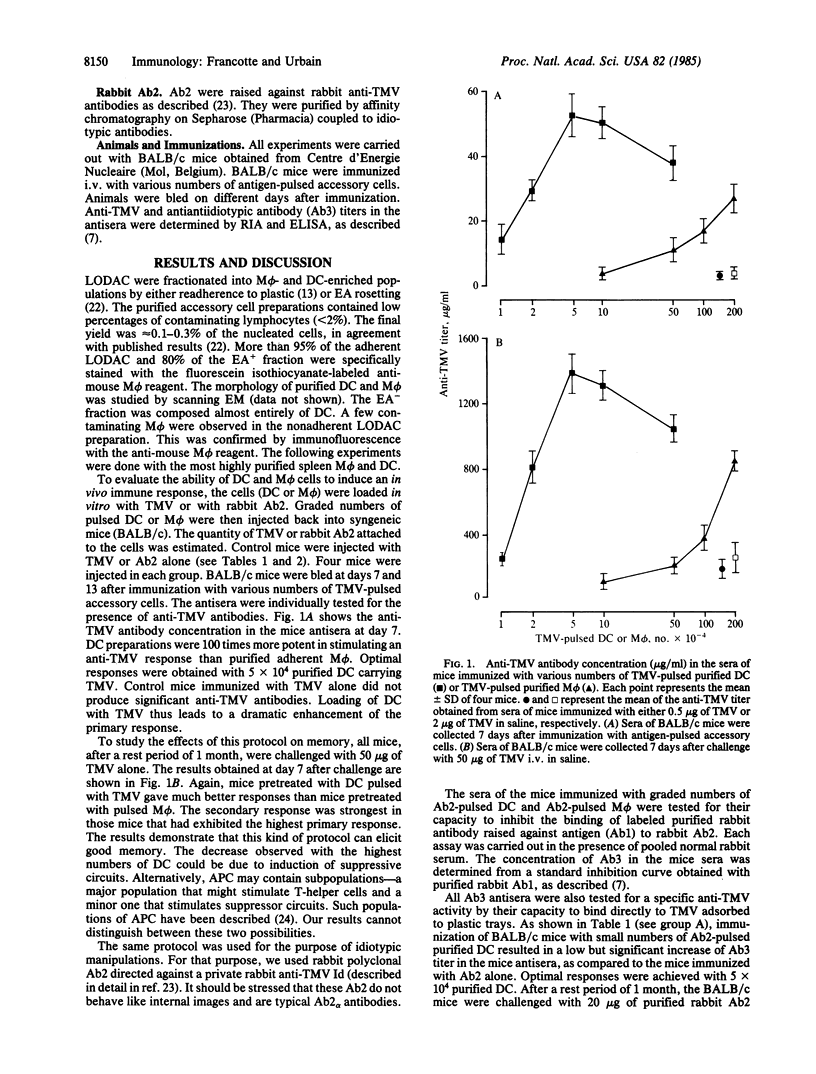
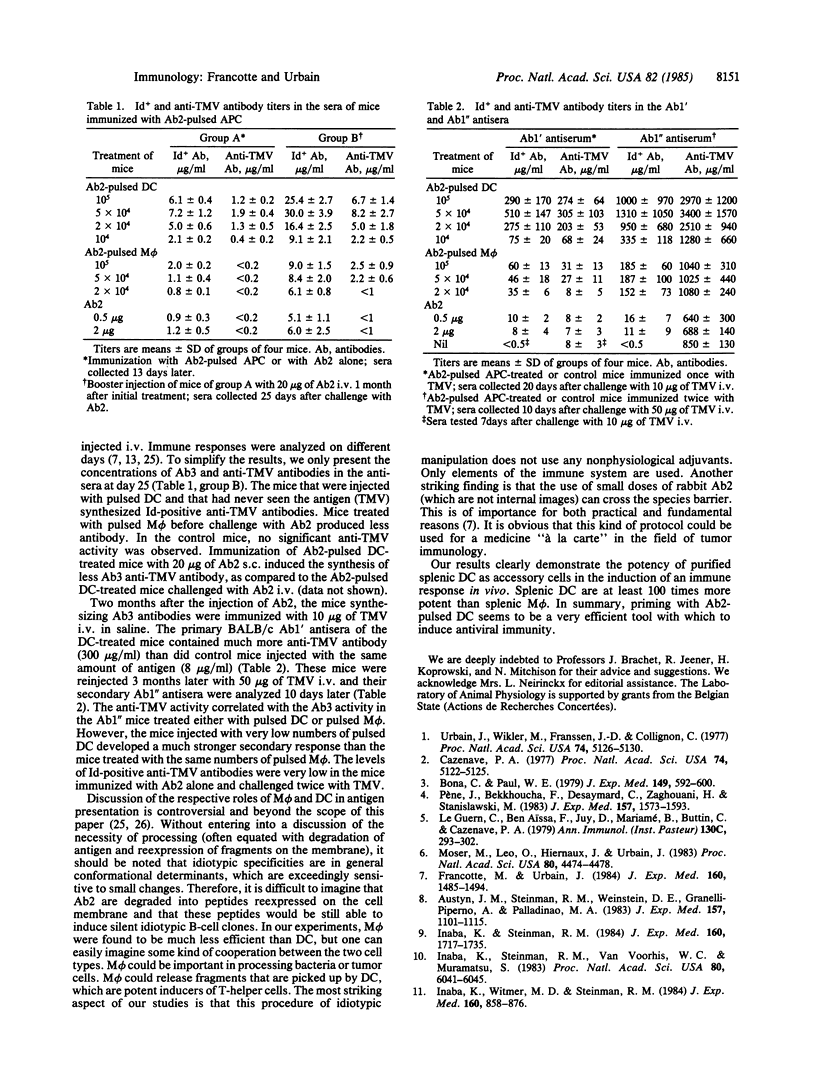
The role of splenic lymphoid dendritic cells (DC) and macrophages (M phi) from mice in induction of immune responses in vivo has been investigated. Varying numbers of purified DC and M phi pulsed in vitro with tobacco mosaic virus (TMV) or with rabbit antiidiotypic antibodies (Ab2) directed against a private rabbit anti-TMV idiotype were injected back into syngeneic mice. In both systems, DC appeared to strongly enhance the primary and secondary responses to the virus. Optimal responses were obtained with 5 X 10(4) purified DC carrying TMV or rabbit Ab2. In contrast, M phi were less efficient by a factor of at least 100. These results show the potency of lymphoid DC as inducing cells in T-dependent antibody responses in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austyn J. M., Steinman R. M., Weinstein D. E., Granelli-Piperno A., Palladino M. A. Dendritic cells initiate a two-stage mechanism for T lymphocyte proliferation. J Exp Med. 1983 Apr 1;157(4):1101–1115. doi: 10.1084/jem.157.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bona C., Paul W. E. Cellular basis of regulation of expression of idiotype. I. T-suppressor cells specific for MOPC 460 idiotype regulate the expression of cells secreting anti-TNP antibodies bearing 460 idiotype. J Exp Med. 1979 Mar 1;149(3):592–600. doi: 10.1084/jem.149.3.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz J. S., Askenase P. W., Ptak W., Steinman R. M., Gershon R. K. Specialized antigen-presenting cells. Splenic dendritic cells and peritoneal-exudate cells induced by mycobacteria activate effector T cells that are resistant to suppression. J Exp Med. 1982 May 1;155(5):1344–1356. doi: 10.1084/jem.155.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave P. A. Idiotypic-anti-idiotypic regulation of antibody synthesis in rabbits. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5122–5125. doi: 10.1073/pnas.74.11.5122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut R. W., Grey H. M. Antigen presenting cells and mechanisms of antigen presentation. Crit Rev Immunol. 1985;5(3):263–316. [PubMed] [Google Scholar]
- Francotte M., Urbain J. Induction of anti-tobacco mosaic virus antibodies in mice by rabbit antiidiotypic antibodies. J Exp Med. 1984 Nov 1;160(5):1485–1494. doi: 10.1084/jem.160.5.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstein R. D., Tominaga A., Mizel S. B., Parrish J. A., Greene M. I. Molecular signals in antigen presentation. II. Activation of cytolytic cells in vitro after ultraviolet radiation or combined gamma and ultraviolet radiation treatment of antigen-presenting cells. J Immunol. 1984 May;132(5):2210–2217. [PubMed] [Google Scholar]
- Inaba K., Granelli-Piperno A., Steinman R. M. Dendritic cells induce T lymphocytes to release B cell-stimulating factors by an interleukin 2-dependent mechanism. J Exp Med. 1983 Dec 1;158(6):2040–2057. doi: 10.1084/jem.158.6.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M. Resting and sensitized T lymphocytes exhibit distinct stimulatory (antigen-presenting cell) requirements for growth and lymphokine release. J Exp Med. 1984 Dec 1;160(6):1717–1735. doi: 10.1084/jem.160.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Van Voorhis W. C., Muramatsu S. Dendritic cells are critical accessory cells for thymus-dependent antibody responses in mouse and in man. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6041–6045. doi: 10.1073/pnas.80.19.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Witmer M. D., Steinman R. M. Clustering of dendritic cells, helper T lymphocytes, and histocompatible B cells during primary antibody responses in vitro. J Exp Med. 1984 Sep 1;160(3):858–876. doi: 10.1084/jem.160.3.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Guern C., Ben Aïssa F., Juy D., Mariamé B., Buttin G., Cazenave P. A. Expression and induction of MOPC-460 idiotopes in different strains of mice. Ann Immunol (Paris) 1979 Mar-Apr;130(2):293–302. [PubMed] [Google Scholar]
- Moser M., Leo O., Hiernaux J., Urbain J. Idiotypic manipulation in mice: BALB/c mice can express the crossreactive idiotype of A/J mice. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4474–4478. doi: 10.1073/pnas.80.14.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M. Contribution of dendritic cells to stimulation of the murine syngeneic mixed leukocyte reaction. J Exp Med. 1980 May 1;151(5):1196–1212. doi: 10.1084/jem.151.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig M. C., Steinman R. M., Gutchinov B., Cohn Z. A. Dendritic cells are accessory cells for the development of anti-trinitrophenyl cytotoxic T lymphocytes. J Exp Med. 1980 Oct 1;152(4):1070–1084. doi: 10.1084/jem.152.4.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak W., Rozycka D., Askenase P. W., Gershon R. K. Role of antigen-presenting cells in the development and persistence of contact hypersensitivity. J Exp Med. 1980 Feb 1;151(2):362–375. doi: 10.1084/jem.151.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pène J., Bekkhoucha F., Desaymard C., Zaghouani H., Stanislawski M. Induction of a cross-reactive idiotype dextran-positive antibody response in two IgH-Cb mouse strains treated with anti-J558 cross-reactive idiotype antibodies. J Exp Med. 1983 May 1;157(5):1573–1593. doi: 10.1084/jem.157.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röllinghoff M., Pfizenmaier K., Wagner H. T-T cell interactions during cytotoxic T cell responses. IV. Murine lymphoid dendritic cells are powerful stimulators for helper T lymphocytes. Eur J Immunol. 1982 Apr;12(4):337–342. doi: 10.1002/eji.1830120415. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. II. Functional properties in vitro. J Exp Med. 1974 Feb 1;139(2):380–397. doi: 10.1084/jem.139.2.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Kaplan G., Witmer M. D., Cohn Z. A. Identification of a novel cell type in peripheral lymphoid organs of mice. V. Purification of spleen dendritic cells, new surface markers, and maintenance in vitro. J Exp Med. 1979 Jan 1;149(1):1–16. doi: 10.1084/jem.149.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Witmer M. D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine G. H., Katz D. R., Czitrom A. A. Heterogeneity of stimulator cells in the murine mixed leukocyte response. Eur J Immunol. 1982 Jan;12(1):9–15. doi: 10.1002/eji.1830120105. [DOI] [PubMed] [Google Scholar]
- Sunshine G. H., Mitchell T. J., Czitrom A. A., Edwards S., Glasebrook A. L., Kelso A., MacDonald H. R. Stimulator requirements for primed alloreactive T cells: macrophages and dendritic cells activate T cells across all genetic disparities. Cell Immunol. 1985 Mar;91(1):60–74. doi: 10.1016/0008-8749(85)90032-2. [DOI] [PubMed] [Google Scholar]
- Unanue E. R., Beller D. I., Lu C. Y., Allen P. M. Antigen presentation: comments on its regulation and mechanism. J Immunol. 1984 Jan;132(1):1–5. [PubMed] [Google Scholar]
- Urbain J., Wikler M., Franssen J. D., Collignon C. Idiotypic regulation of the immune system by the induction of antibodies against anti-idiotypic antibodies. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5126–5130. doi: 10.1073/pnas.74.11.5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler M., Franssen J. D., Collignon C., Leo O., Mariamé B., van de Walle P., de Groote D., Urbain J. Idiotypic regulation of the immune system. Common idiotypic specificities between idiotypes and antibodies raised against anti-idiotypic antibodies in rabbits. J Exp Med. 1979 Jul 1;150(1):184–195. doi: 10.1084/jem.150.1.184. [DOI] [PMC free article] [PubMed] [Google Scholar]


