Abstract
Increasing age in a woman is a well-documented risk factor for meiotic errors, but the effect of paternal age is less clear. Although it is generally agreed that spermatogenesis declines with age, the mechanisms that account for this remain unclear. Because meiosis involves a complex and tightly regulated series of processes that include DNA replication, DNA repair, and cell cycle regulation, we postulated that the effects of age might be evident as an increase in the frequency of meiotic errors. Accordingly, we analyzed spermatogenesis in male mice of different ages, examining meiotic chromosome dynamics in spermatocytes at prophase, at metaphase I, and at metaphase II. Our analyses demonstrate that recombination levels are reduced in the first wave of spermatogenesis in juvenile mice but increase in older males. We also observed age-dependent increases in XY chromosome pairing failure at pachytene and in the frequency of prematurely separated autosomal homologs at metaphase I. However, we found no evidence of an age-related increase in aneuploidy at metaphase II, indicating that cells harboring meiotic errors are eliminated by cycle checkpoint mechanisms, regardless of paternal age. Taken together, our data suggest that advancing paternal age affects pairing, synapsis, and recombination between homologous chromosomes—and likely results in reduced sperm counts due to germ cell loss—but is not an important contributor to aneuploidy.
Keywords: meiosis, paternal age, recombination, checkpoint control
CHROMOSOME abnormalities are extraordinarily common in humans, with an estimated 10–30% of fertilized human eggs being aneuploid due to meiotic errors. Most cases of aneuploidy are maternally derived, and the risk of errors increases exponentially with advancing age such that the majority of eggs ovulated by women over the age of 40 are chromosomally abnormal (Hassold and Hunt 2001). The effect of maternal age on female meiosis is complex and likely mediated by events occurring at multiple points in the life cycle of the oocyte (Nagaoka et al. 2012). Intriguingly, from analyses of human trisomies it is clear that errors in meiotic recombination—an event that occurs in the fetal ovary—contribute to a large proportion of maternal nondisjunction events, with most being age-independent but some suggested as being age-dependent (Fisher et al. 1995; Hassold et al. 1995; Oliver et al. 2008).
By comparison with the female, our understanding of chromosome errors during spermatogenesis is limited. Although maternal errors account for the vast majority of human aneuploid conceptuses, paternal errors do occur and, for some chromosomes, predominate; in ∼10% of Down syndrome cases, the extra chromosome 21 is of paternal origin (Zaragoza et al. 1994) and, among sex chromosome aneuploidies, 50% of 47,XXY cases are attributable to a paternal error (Hassold et al. 1992). In addition, 47,XYY cases can only be of paternal origin, and they are not rare, with an incidence of 1 in 1000 male births (Hook and Hammerton 1977). However, relatively little is known about the association between paternally derived aneuploidy and advancing age. Analyses of autosomal and sex chromosome trisomies have been equivocal, with some reports but not others suggesting an effect of paternal age on the likelihood of nondisjunction (Sloter et al. 2004; Fonseka and Griffin 2011). Indeed, the most compelling case for an increased risk of meiotic errors with advancing paternal age comes from large analyses of human sperm by fluorescence in situ hybridization, with a number of studies demonstrating increased frequencies of sex chromosome disomy or disomy 21 with advancing paternal age (Griffin et al. 1995; Martin et al. 1995; Robbins et al. 1995; Rousseaux et al. 1998). The increase in risk is modest by comparison with the maternal age effect, with two- or threefold differences between males in their 20–30s and those 50 and older (e.g., Griffin et al. 1995). Furthermore, significant increases in sperm aneuploidy with advancing age have been reported in oligospermic men (e.g., Dakouane et al. 2005).
In addition to reports suggesting a small increase in meiotic segregation errors with age, other aspects of spermatogenesis appear to decline with age. For example, a study of testicular biopsies from men ranging from 29 to 102 years old suggested an age-related decline in the number of spermatogonia, spermatocytes, spermatozoa, and Sertoli cells (Dakouane et al. 2005), confirming reports from smaller studies focusing on younger men (Johnson et al. 1984, 1987, 1990). A significant increase in spermatocyte degeneration has also been reported in men of advanced age (Johnson et al. 1990) and, because meiotic errors are known to trigger meiotic arrest and cell death (Hunt and Hassold 2002; Burgoyne et al. 2009), increased cell death would be an expected consequence of an age-related increase in meiotic errors. Thus, the age-related decline in spermatogenesis appears to be due to changes in both the spermatogonial stem cells (SSCs) and the somatic environment: the SSCs become fewer in number, with compromised activity, and the somatic environment loses its ability to support spermatogenesis (Ryu et al. 2006; Zhang et al. 2006).
In addition, an increased risk of de novo gene mutations with advancing paternal age is well established in humans and is thought to contribute to both simple Mendelian and complex genetic traits (Goriely and Wilkie 2012). This has been postulated to result from a constellation of age-related changes that compromise DNA replication, DNA repair, cell cycle control, and epigenetic modifications in SSCs, leading to the accumulation of errors (Paul and Robaire 2013).
Because the onset of meiosis involves the accumulation and repair of programmed double-strand breaks, we postulated that an age-related increase in defects in DNA replication and repair would be evident as an increase in defects during meiotic prophase. Accordingly, we initiated studies to test the hypothesis that defects in meiotic prophase and/or metaphase are more common in older males. We examined male mice from different strains, asking whether age affected the incidence of defects in synapsis and recombination between homologs, the maintenance of connections between homologs at metaphase I (MI), or the incidence of abnormal numbers of chromatids or chromosomes at metaphase II (MII). We identified a surprising reduction in recombination levels in the first wave of spermatogenesis in the juvenile testis and, in older males, a slight increase in recombination as well as an increase in abnormalities at MI. However, regardless of age, our data suggest that meiotic checkpoints remain intact and that cells with meiotic errors are effectively eliminated. We conclude that, in the mouse, the incidence of meiotic errors increases with advancing paternal age, but spermatocytes with errors are effectively eliminated, preventing a corresponding increase in aneuploidy.
Materials and Methods
Animals
Wild-type male C57BL/6J (B6), C3H/HeJ (C3H) (Jackson Laboratory, Bar Harbor, ME), and ICR (CD-1) mice (Harlan Laboratories, Livermore, CA) were housed in ventilated rack caging in a pathogen-free facility. Three to 10 males from at least three different litters were analyzed for each age group, with the exception of the 2-year-old CD-1 group, which contained only two males. Male littermates not utilized for analysis at 20 days post-partum (dpp) were weaned and saved for later age analyses. At weaning, adult animals were housed individually and drinking water and chow (Purina Lab Diet, 5K52) were provided ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee at Washington State University, which is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Spermatocyte preparations
Males were killed and testes immediately dissected, weighed, and placed in PBS. Spermatocyte preparations were made according to the protocol of Peters et al. 1997, with one modification: a thin layer of 1% paraformaldehyde was applied to clean slides using a glass pipette, rather than by dipping the slide. After overnight incubation in a humid chamber, slides were dried, washed with 0.4% Photo-flo 200 solution (Kodak Professional), air-dried, and viewed on a Nikon Labophot-2 phase microscope. Two slides with spread cells were chosen for immediate staining, and the remaining slides were frozen at −20°.
Immunostaining
Slides were blocked for 1 hr in sterile-filtered antibody dilution buffer (ADB), consisting of 10 ml normal donkey serum (Jackson Immunoresearch), 3 g OmniPur BSA, Fraction V (EMD Millipore), 50 μl Triton X-100 (Alfa Aesar), and 990 ml PBS. MLH1 (Calbiochem, PC56, at 1:60) and RAD51 (Santa Cruz biotechnology, sc-8349, at 1:60) primary antibodies were diluted in ADB, and 60 μl of antibody solution was applied and covered with a 24 × 50-mm2 glass coverslip, sealed with rubber cement, and incubated overnight at 37°. Following incubation, slides were washed briefly in ADB, SYCP3 primary antibody (Santa Cruz biotechnology, sc-74569, at 1:300) was applied, a parafilm coverslip was added, and slides were incubated for 2 hr at 37°. Following incubation, slides were washed in two changes of ADB for at least 1 hr each. Alexa Fluor 488-conjugated AffiniPure donkey anti-rabbit secondary antibody (Jackson Immunoresearch Laboratories, Inc., 711-545-152, at 1:60) was applied to slides, a glass coverslip was added and sealed with rubber cement, and slides were incubated overnight at 37°. The next morning, slides were briefly washed in ADB, and Cy3-conjugated AffiniPure donkey anti-mouse secondary antibody (Jackson Immunoresearch Laboratories, Inc., 715-165-150, at 1:1000) was applied with a parafilm coverslip for 45 min at 37°. At the end of incubation, slides were washed in two changes of PBS for at least 1 hr each, and 20 μl of Prolong Gold antifade reagent with DAPI (Life Technologies, P36931) and coverslips were applied. Excess DAPI was blotted out with filter paper, and coverslip edges were sealed with rubber cement. Stained slides were stored at 4° in slide folders prior to analysis.
MLH1 and RAD51 analysis
For studies of recombination levels, pachytene-stage cells were scored for the number of MLH1 foci per cell. We scored a minimum of 25 cells per mouse and analyzed 3–10 mice for each age group (20 dpp, 12 weeks old, 1-year-old, and 1.5 years or 2 years old). For studies of the first wave of spermatogenesis, B6 males from a total of four litters were analyzed at 20, 30, and 35 dpp (three males in each group), and an additional group of 12-week-old B6 males was concurrently analyzed for comparison. Three to four males per age group were also analyzed for RAD51. The 15-dpp group for RAD51 consisted of three to four males from a minimum of three separate litters, used nowhere else in this study. Images of cells were captured on a Zeiss Axio Imager epifluorescence microscope. Three images were taken consecutively—SYCP3-TRITC, MLH1-FITC, and DAPI—and cell coordinates were recorded via England finder to allow relocation. Each image was adjusted uniformly using the Zeiss Axiovision software to reduce background and then saved without the DAPI channel for MLH1 and RAD51 foci analysis. Foci counts were determined for 12–30 zygotene-stage cells (RAD51) and 25–30 pachytene-stage cells (MLH1) per animal by two scorers who were blinded with regard to age group or strain of the animal. In the case of RAD51, scores were averaged. Minor scoring discrepancies for MLH1 were resolved between scorers, and cells with major discrepancies were discarded. Cells with poor staining or synaptic defects were excluded from foci number analysis.
Fluorescence in situ hybridization
Following initial capture of images of individual cells, the rubber cement was removed, and slides were soaked overnight in PBS at room temperature to remove the coverslip. Slides were hybridized with mouse whole-chromosome paint probes to chromosomes 1 and 11 (Applied Spectral Imaging) using the vendor’s protocol. Cells were then relocated on the Zeiss Axio Imager epifluorescence microscope using previously recorded coordinates and imaged using Zeiss Axiovision software.
Synaptic defects
The frequency of synaptic defects in 50 pachytene-stage cells from each animal was determined by two independent observers who were blinded with regard to age group. Pachytene cells were identified on the basis of SYCP3 immunostaining, and cells were scored into four categories: (1) perfect, if all homologs were fully synapsed and the sex chromosomes were closely associated; (2) major defects (asynapsis, partial asynapsis, nonhomologous synapsis); (3) minor defects (forks/bubbles/gaps or fragmentation in an otherwise normal pachytene cell); and (4) associations [nonhomologous end-to-end associations between two or more synaptonemal complexes (SCs)]. Individual defects were defined as follows—asynapsis: one or two pairs of homologs remaining unsynapsed for at least one-third the length of the SC in a cell that otherwise exhibited complete synapsis; nonhomologous synapsis: synapsis occurring between nonhomologous chromosomes; forks and bubbles: one or two pairs of homologs remaining unsynapsed at the end (fork) or internally on the SC (bubble) for less than one-third of the SC length; gaps: one or two gaps in SC staining that were longer than the width of an SC; and fragmentation: small segments of SC with colocalized DAPI staining that could not be identified as part of a pair of homologs. Cells with multiple defects could be scored in more than one defect category (e.g., a cell with both partial asynapsis and associations would be included in both categories). Because the sex chromosomes synapse later in pachytene and desynapse earlier in diplotene than autosomes (Solari 1989; Kauppi et al. 2011), they were excluded from synaptic defect analysis. However, sex chromosomes should be paired and in close proximity to one another, and therefore their failure to pair (but not synapse) was included in the defect analysis.
Analysis of synaptonemal complex length and MLH1 placement
To assess effects on the formation of the SC, SC lengths were measured from pachytene-stage cells used in recombination studies. The total synaptonemal complex length per cell was obtained by using Zeiss Axiovision measuring tools to measure the length of each of the 19 autosomal SCs. The sex chromosome bivalent was excluded. For chromosome-specific measurements, we used MicroMeasure v. 3.3 (Colorado State University), noting the position of MLH1 foci along the SC with respect to the centromere.
Diplotene-stage analysis
Fifty diplotene-stage cells were scored from three representative B6 males for each age group. Cells were scored on the basis of SYCP3 staining into four categories: (1) “all paired” if a physical connection was evident between all homologs in the cell, (2) “paired without obvious connections” if at least one pair of homologs had no obvious physical connection but remained aligned, (3) “unpaired” if at least one pair of homologs had no obvious physical connection and were neither aligned nor in close proximity, or (4) “unpaired sex chromosomes” if the X and Y were physically separated. A cell with unaligned homologs was placed in the third category even if other homologs in the cell had no obvious physical connections but were aligned. As in the analysis of synaptic defects in pachytene-stage cells, the sex chromosomes were excluded from analysis, except in the case where they were also unpaired (fourth category).
Air-dried preparations and analysis
MI and MII preparations were made from three 12-week-old and three 1.5-year-old C57BL6/J males using the air-dried method of Evans et al. 1964. Slides were scored by two independent observers who were blinded with respect to age. The frequency of univalents was determined for 25–40 MI cells per male, and the frequency of aneuploidy and the presence of monad chromosomes were determined for 15–25 MII cells per male.
Statistical analyses
Among-group differences in mean numbers of foci for MLH1 and RAD51 and in SC length were analyzed by one-way ANOVA. For statistically significant differences (P < 0.05), a Newman–Keuls post hoc test was performed to infer which groups differed. Linear regression analyses were performed to determine the correlation between MLH1 foci and SC lengths. Chi-square analyses were used to assess between-group differences in the frequency of defects at both the pachytene and the diplotene-stage and in univalents at metaphase I.
Results
During meiotic prophase, homologous chromosomes synapse and recombine, events that are essential for the completion of spermatogenesis and the production of genetically normal sperm. To assess the effect of age on these processes, surface-spread preparations of mouse spermatocytes were immunostained with antibodies specific for SYCP3, a component of the synaptonemal complex, and MLH1, a DNA mismatch repair protein known to localize to the vast majority of crossovers (Baker et al. 1996; Holloway et al. 2008). Males were analyzed at 20 dpp, 12 weeks, 1 year, and 1.5–2 years of age to assess successive life phases: juvenile, mature adult, middle-aged, and old, respectively (Flurkey et al. 2007). In mice, longevity varies with genetic background; accordingly, we analyzed two inbred strains (B6 and C3H) and one outbred (CD-1). Because of their comparatively long life span (Ray et al. 2010), CD-1 mice were aged to 2 years instead of 1.5 years. In addition to detecting differences with advancing age, our analysis provides evidence of an unexpected difference in recombination during the first wave of spermatogenesis in the testis of sexually immature males.
Recombination rate is lower in the first wave of spermatogenesis
Regardless of genetic background, the number of MLH1 foci per cell was significantly lower in 20-dpp males by comparison with any category of adult littermates (Table 1, P < 0.0001). For example, mean MLH1 foci counts per cell for 20-dpp-old males vs. 12-week-old adult littermates were 22.95 ± 0.16 and 24.02 ± 0.16 for B6, 21.73 ± 0.14 and 22.98 ± 0.16 for C3H, and 22.27 ± 0.12 and 24.26 ± 0.18 for CD-1. Thus, for all three strains the mean values for 20-dpp males were ∼5–8% lower than those of 12-week-old counterparts. In general, these reductions reflected decreases in SCs with two MLH1 foci and corresponding increases in those with a single focus. Pooled results for the different age groups are provided in Table 1, and data on individual animals are shown in Supporting Information, Figure S1. To determine if lower recombination levels were a reflection of sexual immaturity or a distinct feature of the first wave of cells to initiate meiosis, we compared MLH1 counts from sexually immature B6 males at 20, 30, and 35 dpp. As shown in Table 2, our data indicate that adult levels of recombination are attained prior to sexual maturation, with a significant increase in mean MLH1 foci evident by 30 dpp (P < 0.0001).
Table 1. Comparison of recombination and synaptonemal complex lengths with age.
| MLH1 foci per SC (%) | |||||||
|---|---|---|---|---|---|---|---|
| Strain | Age* | Mean MLH1 ± SEM** | 0 | 1 | 2 | 3 | Mean SC length (μm) ± SEM** |
| B6 | 20 dpp | 22.95 ± 0.16a | 0.4 | 78.8 | 20.5 | 0.3 | 154.20 ± 1.12a |
| 12 wk | 24.02 ± 0.16b | 0.6 | 72.8 | 26.3 | 0.3 | 167.97 ± 1.26b | |
| 1 yr | 24.48 ± 0.24b,c | 0.8 | 70.3 | 28.4 | 0.6 | 165.63 ± 1.40b | |
| 1.5 yr | 25.00 ± 0.25c | 0.6 | 67.3 | 31.3 | 0.8 | 172.47 ± 1.72c | |
| C3H | 20 dpp | 21.73 ± 0.14a | 0.2 | 85.3 | 14.2 | 0.2 | 151.56 ± 1.34 |
| 12 wk | 22.98 ± 0.16b | 0.1 | 78.9 | 20.7 | 0.2 | 147.87 ± 1.22 | |
| 1 yr | 23.99 ± 0.25c | 0.1 | 74.1 | 25.3 | 0.5 | 151.78 ± 1.55 | |
| CD-1 | 20 dpp | 22.27 ± 0.12a | 0.2 | 82.7 | 16.9 | 0.2 | 146.93 ± 1.18a |
| 12 wk | 24.26 ± 0.18b | 0.2 | 72.0 | 27.4 | 0.3 | 150.34 ± 1.18a | |
| 1 yr | 24.49 ± 0.18b | 0.3 | 70.6 | 28.9 | 0.2 | 149.22 ± 1.04a | |
| 2 yr | 24.50 ± 0.27b | 0.4 | 70.4 | 29.1 | 0.1 | 160.03 ± 1.82b | |
Data represent 3–10 males/group from a minimum of three litters, with the exception of 2-year-old CD-1, which represents 2 males from different litters.
For each strain, different age groups were compared by one-way ANOVA. Superscript letters denote statistically significant differences as determined by a Newman–Keuls post hoc test (at least P < 0.05); like letters indicate no differences.
Table 2. Analysis of recombination in first wave spermatocytes in B6 males.
| Age | n | Mean MLH1 ± SEM* |
|---|---|---|
| 20 dpp | 78 | 22.63 ± 0.20a |
| 30 dpp | 85 | 23.55 ± 0.23b |
| 35 dpp | 85 | 24.04 ± 0.24b |
| 12 wk | 77 | 23.96 ± 0.22b |
Data represent pachytene spermatocytes from three males. Age group data were compared by one-way ANOVA. Groups with different superscript letters are significantly different as determined by Newman–Keuls post hoc test (at least P < 0.05).
Age influences meiotic prophase events
To assess the effect of age on meiotic recombination, we compared MLH1 profiles from adult littermates analyzed at successive ages. As shown in Table 1, B6 and C3H males exhibited a steady increase in mean MLH1 counts with age, with a significant increase in recombination rate in aged males by comparison with young adults evident in both strains (P < 0.0001). The age-related increase was attributable to an increase in SCs with two recombination sites (from 20.5 to 31.3% in B6 and from 14.2 to 25.3% in C3H). The same trend was evident in CD-1 males, but the difference among groups did not reach significance (P > 0.05); mean MLH1 values at 12 weeks, 1 year, and 2 years were 24.26 ± 0.18 (n = 193 cells), 24.49 ± 0.18 (n = 140 cells), and 24.50 ± 0.27 (n = 56 cells), respectively. We further analyzed MLH1 placement on chromosomes 1 and 11 in B6 males and found no differences between age groups (Figure S2).
Because recombination has been positively correlated with SC length (Lynn et al. 2002; Gruhn et al. 2014), we measured total autosomal SC lengths in pachytene spermatocytes to determine if the age-related increase in recombination was accompanied by a corresponding increase in SC length (Table 1). Mean SC length, like recombination, exhibited an age-dependent increase on two of the three genetic backgrounds. As shown in Table 1, in B6 males, mean SC lengths increased from 154.20 ± 1.12 micrometer (n = 91 cells) at 20 dpp to 172.47 ± 1.72 μm (n = 85 cells) at 1.5 years old (P < 0.0001). Similarly, in CD-1 males, mean SC lengths increased from 146.93 ± 1.18 micrometer at 20 dpp to 160.03 ± 1.82 μm at 2 years old (P < 0.0001). In contrast, on the C3H background the age-related increase in recombination was not accompanied by an increase in SC length, as mean SC lengths did not deviate from ∼150 μm regardless of age (Table 1, P > 0.05). Nevertheless, in all three genetic backgrounds, an age-related increase in SC length variability was evident with an increase in the standard error of the mean with age in each strain (Table 1).
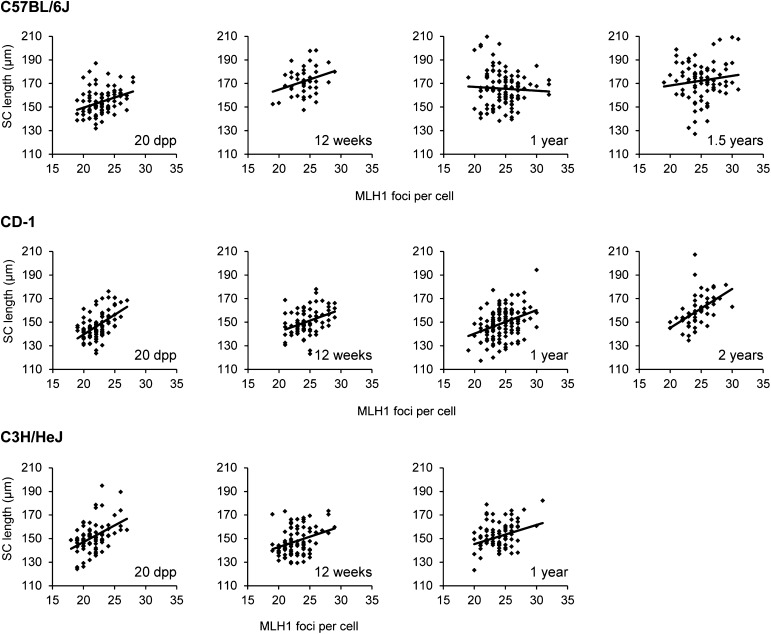
When recombination and SC length were correlated on a per-cell basis, a significant positive correlation was evident at all ages for both CD-1 and C3H males: the cells with the longest SCs had the most recombination (Figure 1). However, for the B6 strain, this relationship was observed for 20-dpp and 12-week-old mice, but not for 1-year and 1.5-year-old mice.
Figure 1.
Synaptonemal complex length and recombination correlation with age. Total MLH1 foci (x-axis) and corresponding SC length (y-axis) for pachytene-stage spermatocytes from juvenile and adult males. The Pearson correlation coefficients were calculated to determine the relationship between synaptonemal complex length and recombination in pachytene cells. For B6 males, the Pearson correlation coefficients were 0.34 (P < 0.001, n = 91) for 20 dpp, 0.23 (P < 0.05, n = 80) for 12-week-olds, 0.05 (P > 0.05, n = 112) for 1-year-olds, and 0.13 (P > 0.05, n = 85) for 1.5-year-olds. For CD-1 males, the Pearson correlation coefficients were 0.53 (P < 0.0001, n = 89) for 20 dpp, 0.37 (P < 0.0001, n = 79) for 12-week-olds, 0.35 (P < 0.0001, n = 140) for 1-year-olds, and 0.49 (P < 0.0001, n = 56) for 2-year-olds. For C3H males, the Pearson correlation coefficients were 0.45 (P < 0.0001, n = 84) for 20 dpp, 0.32 (P < 0.01, n = 90) for 12-week-olds, and 0.26 (P < 0.05, n = 82) for 1-year-olds.
Age-dependent differences in recombination occur downstream of double-strand breaks
Sex and strain-specific differences in recombination have been correlated with differences in RAD51 foci (Barlow et al. 1997; Lenzi et al. 2005; Oliver-Bonet et al. 2005; Gruhn et al. 2014). To determine if age-dependent differences in recombination are similarly correlated, we analyzed RAD51 foci in zygotene-stage cells (Table 3). Although significant differences were detected among some age groups, there was no clear pattern in RAD51 foci number with advancing age. For example, in B6 males, mean RAD51 counts were significantly different among age groups, but were not correlated with advancing age. Mean RAD51 jumped from 183.06 at 20 dpp to 230.73 at 12 weeks, but then decreased to 206.40 in 1-year-old males (P < 0.0001). For C3H and CD-1, there was no correlation with RAD51 counts and advancing age.
Table 3. Comparison of RAD51 in zygotene.
| Strain | n | Age | Mean RAD51 ± SEM* |
|---|---|---|---|
| B6 | 77 | 15 dpp | 183.06 ± 2.93a |
| 56 | 12 wk | 230.73 ± 3.88b | |
| 35 | 1 yr | 206.40 ± 5.07c | |
| C3H | 114 | 15 dpp | 199.18 ± 2.82 |
| 56 | 12 wk | 198.91 ± 3.20 | |
| 62 | 1 yr | 208.85 ± 2.84 | |
| CD-1 | 109 | 15 dpp | 171.30 ± 3.40 |
| 108 | 12 wk | 177.97 ± 2.38 | |
| 45 | 1 yr | 177.09 ± 4.45 |
Different ages within a background were compared by one-way ANOVA. Different letters represent groups that are statistically different as determined by Newman–Keuls post hoc test (at least P < 0.05).
An age-related increase in unpaired sex chromosomes
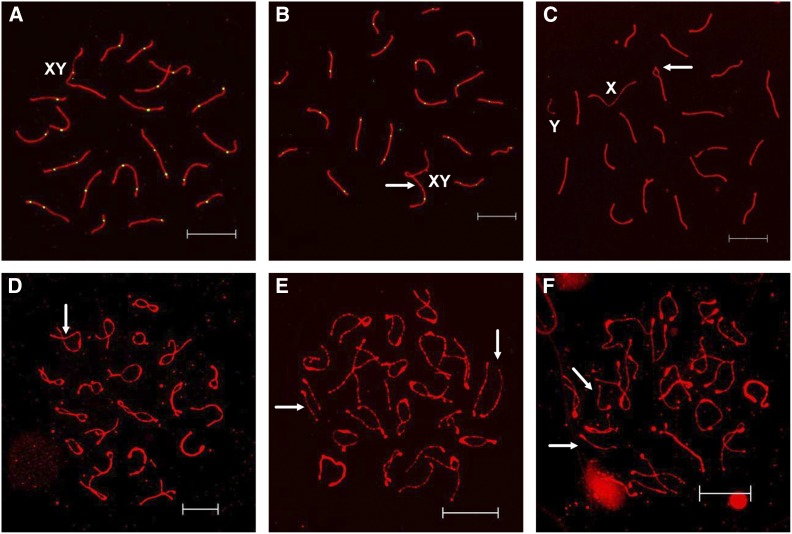
In addition to recombination analyses at mid-pachytene, we analyzed synaptic defects to determine if age influenced the frequency of errors. At mid-pachytene, homologs should be fully synapsed and the distal ends of the sex chromosomes should be associated (Figure 2A). With the exception of the C3H background, where an end-to-end association between an autosome and the X chromosome was frequently observed and increased in frequency with age (Figure 2B), there was no evidence for age-related synaptic defects (Table 4). However, an age-related increase in the frequency of sex chromosome pairing failure was evident in all strains (Table 4 and Figure 2C). B6 and CD-1 exhibited a steady, nonsignificant increase with age (e.g., for B6 1.7% of cells at 20 dpp and 6.7% in 1.5-year-old mice; for CD-1, 0.6% of cells at 20 dpp, and 5% of cells in 2-year-old males). In C3H males, sex chromosome pairing failure was not observed in 20 dpp and 12-week-old adults, but a 2% level was observed in 1-year-old males.
Figure 2.
Meiotic analyses of mouse spermatocytes at pachytene and diplotene. (A) Representative pachytene cell with normally synapsed sex chromosomes from a 12-week-old B6 male. (B) Pachytene cell with an association (arrow) between an autosome and X chromosome from a C3H male. (C) Pachytene cell with unpaired sex chromosomes and a synaptic defect (a bubble; arrow) from a 1.5-year-old B6 male. (D) Diplotene cell showing visible points of association (arrow) even as the SC breaks down for all bivalents from a 20-dpp B6 male. (E) Diplotene cell from a 1.5-year-old B6 male with homologs that have no obvious sites of connection, but remain closely aligned (arrows). (F) Diplotene cell with a pair of homologs that have no obvious sites of connection and are not in proximity to each other (arrows) from a 1.5-year-old B6 male. Bars, 10 μm.
Table 4. Analysis of synaptic defects in pachytene cells with age.
| Strain | Age | n | Perfect (%) | Major defects (%) | Minor defects (%) | Nonhomologous associations (%) | Unpaired XYa (%) |
|---|---|---|---|---|---|---|---|
| B6 | 20 dpp | 350 | 93.1 | 0.6 | 3.7 | 0.9 | 1.7 |
| 12 wk | 400 | 93.0 | 1.4 | 0.8 | 1.8 | 3.0 | |
| 1 yr | 200 | 93.5 | 0.5 | 3.0 | 0.5 | 2.5 | |
| 1.5 yr | 150 | 88.6 | 0.0 | 4.7 | 0.0 | 6.7 | |
| C3H | 20 dpp | 300 | 92.3 | 1.0 | 4.3 | 3.3 | 0.0 |
| 12 wk | 300 | 82.0 | 1.0 | 4.0 | 13.0 | 0.0 | |
| 1 yr | 150 | 76.7 | 1.3 | 7.3 | 18.0 | 2.0 | |
| CD-1 | 20 dpp | 500 | 91.0 | 1.4 | 2.6 | 4.4 | 0.6 |
| 12 wk | 400 | 90.3 | 0.8 | 6.0 | 2.3 | 1.3 | |
| 1 yr | 250 | 89.6 | 1.2 | 1.2 | 4.0 | 4.0 | |
| 2 yr | 100 | 88.0 | 0.0 | 5.0 | 2.0 | 5.0 |
The frequency of unpaired XY with age was individually tested for each strain by chi-square analysis.
Meiotic errors are effectively eliminated regardless of age
Sex chromosome pairing failure is known to interfere with meiotic sex chromosome silencing (MSCI), causing meiotic arrest and cell death (Royo et al. 2010). Furthermore, any cells that escape this fate and have unpartnered univalent chromosomes at metaphase I as a result of recombination failure would be expected to trigger meiotic arrest due to the actions of the spindle assembly checkpoint (Burgoyne et al. 2009). Therefore, we analyzed successive meiotic stages to examine the fate of cells with unpartnered sex chromosomes and determine if the efficacy of meiotic checkpoints diminishes with age. To minimize genetic variation, this analysis was performed on the B6 inbred strain and, as shown in Figure 2, age-dependent increases in failure to maintain connections between homologous chromosomes were evident at both diplotene and metaphase I.
At diplotene, SCs begin to desynapse, and sites of exchange become evident as persistent connections between homologs (Figure 2D). Unlike pachytene cells, the frequency of unpaired sex chromosomes was negligible at the diplotene-stage for any age group, ranging from 0 to 1.3% of cells, confirming the expectation that sex chromosome pairing failure would result in meiotic arrest and elimination of the cell at pachytene. Unexpectedly, however, we observed an increase in the frequency of autosomes that were unpaired at diplotene. The frequency of diplotene cells with visible sites of exchange between all homologs was 78.6, 59.3, and 54.7% at 20 dpp, 12 weeks, and 1.5 years old, respectively (Table 5 and Figure 2D). The remaining cells could be broken down into two categories: those in which a homologous pair without an observable connection remained in close proximity and loosely aligned (paired) (Figure 2E) and those in which homologs were clearly separated (unpaired) (Figure 2F). The frequency of cells with separated but paired homologs was 16.7, 33.3, and 34.7% at 20 dpp, 12 weeks, and 1.5 years old, respectively. The frequency of cells with clearly separated homologs, however, increased significantly with age from 4.7% in 20-dpp mice to 10.0% in 1.5-year-old males (χ2 = 21.5, P < 0.001).
Table 5. Analysis of diplotene-stage spermatocytes with age in B6 males.
| Age | All paired | Paired with no obvious connections | Unpaired | Unpaired sex chromosomes |
|---|---|---|---|---|
| 20 dpp | 118 (78.6) | 25 (16.7) | 7 (4.7) | 0 (0) |
| 12 wk | 89 (59.3) | 50 (33.3) | 9 (6.0) | 2 (1.3) |
| 1.5 yr | 82 (54.7) | 52 (34.7) | 15 (10.0) | 1 (0.6) |
Data represent 50 diplotene-stage cells from three B6 males per group. Significant differences among ages were tested for all catagories by chi-square analysis except “Unpaired sex chromosomes.” Numbers in parentheses are percentages.
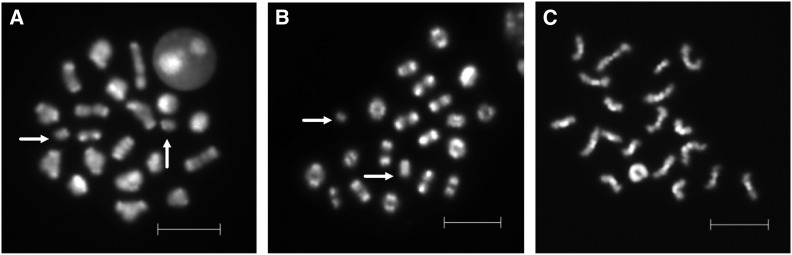
To further examine the frequency and the fate of unpaired homologs in different age groups, the frequency of univalents was determined for 25–40 MI cells, and the frequency of missing or additional chromatids or chromosomes was determined for 15–25 MII cells per B6 male. In 12-week-old males, only 4% of cells had univalents at MI, and, of these, 2.7% involved the sex chromosomes and 1.3% were autosomal. In 1.5-year-old males, the frequency of univalents increased to 3.7 and 7.4% for sex chromosomes and autosomes, respectively (Figure 3, A and B, and Table 6; P < 0.05). Because cells with univalents at MI should be eliminated by the spindle assembly checkpoint, we analyzed MII cells from the same males (Figure 3C). Neither the incidence of aneuploidy nor that of unpaired monads increased with age. Indeed, only a single abnormality was observed, and this was an apparent chromosome break in an MII cell from a 12-week-old male.
Figure 3.
Analyses of metaphase I and II B6 spermatocytes. (A) MI cell with autosomal univalents (arrows) from a 1.5-year-old B6 male. (B) MI cell with sex chromosome univalents (arrows) from a 1.5-year-old B6 male. (C) Normal MII cell from a 1.5-year-old B6 male. Bars, 10 μm.
Table 6. Analysis of air-dried MI and MII chromosome preparations in B6 males.
| MI analyses | MII analyses | ||||||
|---|---|---|---|---|---|---|---|
| Age | Normal | Abnormal | Univalent XY | Univalent autosome | Break | Normal | Abnormal |
| 12 wk | 72 (96) | 3 (4) | 2 (2.7) | 1 (1.3) | 0 (0) | 50 (98) | 1 (2) |
| 1.5 yr | 93 (86) | 15 (14) | 4 (3.7) | 8 (7.4) | 3 (2.8) | 73 (100) | 0 (0) |
The frequency of abnormal MI cells with age was individually tested by chi-square analysis. Numbers in parentheses are percentages.
Discussion
This study provides insight into an outstanding question in the field of reproductive aging: Does paternal age negatively impact spermatogenesis? Previous studies of paternal age effects on spermatogenesis have focused on the analysis of sperm and aneuploid offspring. However, because errors in synapsis and recombination are strongly selected against (Hunt and Hassold 2002), these studies may not accurately assess the effect of age on male meiosis. It has been postulated that age-related changes derive from compromised cellular functions such as DNA replication, repair, and cell cycle control (Paul and Robaire 2013). Because these functions are essential for meiosis, we directly analyzed spermatocytes from juvenile, young, middle age, and aged male mice on three different genetic backgrounds to assess the effect of age on male meiosis.
Three important findings derive from our studies. First, recombination is variable over the life span of the male, being comparatively lower in juvenile males and highest in males of advancing age. Second, the incidence of both synaptic defects and univalents at MI increases in aged mice, but the effects are limited to the most vulnerable chromosomes (the sex chromosomes and small autosomes). Third, even with advancing age, cells with errors are effectively eliminated at the metaphase/anaphase transition of MI, such that errors are not detected at MII.
Recombination levels vary with age
To our knowledge, this is the first study to demonstrate a significant reduction in recombination levels in the first wave of cells that initiate spermatogenesis. By comparison with the adult mouse testis, a number of factors differ in the sexually immature testis. Testis descent occurs prior to sexual maturation and is normally complete by 25 dpp in the mouse (O’Shaughnessy and Sheffield 1991). Thus, because the temperature in the scrotum is slightly lower than in the body, the first wave of spermatogenesis occurs at a higher temperature. In addition, first wave germ cells are supported by functionally immature Sertoli cells that have different gene expression profiles and lack the tight junctions responsible for the blood/testis barrier (Sharpe et al. 2003). Leydig cells are also maturing, and important hormonal changes occur, with a decline in responsiveness to follicle-stimulating hormone and a concurrent rise in testosterone production and responsiveness (Sharpe 1994). Thus, temperature and both the hormonal and paracrine environment differ markedly in the juvenile testis, and the possible influence of these environmental factors on recombination remains unknown.
However, because adult recombination levels were evident in the 30-dpp juvenile testis, our results suggest that lower recombination is not simply a reflection of sexual immaturity but rather a feature of the first cells that initiate meiosis. These cells are derived from gonocytes rather than from established spermatogonial stem cells and undergo fewer mitotic divisions before entering meiosis than cells in the adult testis (Yoshida et al. 2006; Drumond et al. 2011). Thus, it is possible that innate differences in germ cell origin affect the chromatin configuration of the meiocyte, leading to a reduction in recombination. The first wave of spermatogenesis is frequently utilized to obtain a synchronous population of spermatocytes to address questions about specific stages of spermatogenesis. Thus, our findings underscore the importance of recognizing that conclusions based on the study of the progenitors of the first population of cells that initiate meiosis may not accurately reflect the normal adult situation.
Recombination rate increased with advancing age in two of the three genetic backgrounds studied. This is similar to findings from early studies of chiasmata counts where an increase, although not statistically significant, was observed in 1.25-year-old B6, CBA, and Q strain males by comparison with younger adults (Speed 1977). Although an age-related increase in recombination was not evident in the outbred CD-1 background, these males as well as males of both inbred strains exhibited an age-related increase in the variance for both recombination and SC length (e.g., compare SEM for both in Table 1). We postulate that the age-related increase in variation reflects a weakening in the control in this tightly regulated process. Importantly, however, our data do not provide evidence of an increased risk of recombination failure with age (i.e., SCs lacking an MLH1 focus) for any strain.
Sex and strain-specific recombination differences have been correlated with changes in the number of double-strand breaks, as assessed by RAD51 foci (Moens et al. 2002). Our data, however, provide no evidence that age-related differences within a strain are mediated by changes in double-strand break formation. This is consistent with recent evidence suggesting that processes acting downstream of RAD51 can affect recombination levels (Cole et al. 2012). Thus, recombination levels appear to reflect a complex interaction of factors acting at multiple stages of meiotic prophase.
Currently, there is no evidence that recombination rate increases with age in humans, although the data bearing on this question are limited since most analyses have focused on young adult men and both extremes of the age spectrum (teenage males and men >65 years old) are not well represented. Furthermore, because linkage studies rely on the analysis of offspring, by comparison with direct analysis of pachytene spermatocytes, data for a given individual are extremely limited. Nonetheless, Kong et al. (2004) detected an increase in recombination rate variation in men 45 or older, which is consistent with the age-related increase in variance that we observed in mice. The development of a sensitive assay that allows for recombination studies of individual human sperm (Lu et al. 2012) provides a means of determining if a correlation between paternal age and recombination exists in humans.
Synaptic defect frequency increases with advancing age
We detected an age-related increase in unpaired sex chromosomes at the pachytene stage. The sex chromosomes are vulnerable to meiotic error in the male because synapsis and recombination are limited to the small pseudoautosomal region. In the mouse, sex chromosome pairing and synapsis are delayed by comparison to the autosomes, but synapsis involving the pseudoautosomal region is evident by early pachytene (Kauppi et al. 2011). Consistent with this, we rarely observed sex chromosome pairing failure in younger males, but an age-related increase was a feature of males on all three genetic backgrounds.
Failure of the X and Y chromosomes to pair interferes with MSCI, and the resultant misexpression of Zfy2 effectively triggers apoptosis (Royo et al. 2010). Consistent with this, we rarely observed unpartnered X and Y chromosomes at the diplotene stage, nor was an increase in sex chromosome univalents evident at metaphase I. This suggests that, although age increases errors in sex chromosome synapsis, the pachytene checkpoint control is unaffected by age and successfully prevents the progression of these cells.
Surprisingly, although our analyses did not reveal an increased incidence of sex chromosome univalents, an age-related increase in prematurely separated small autosomal chromosomes was evident at both diplotene and MI. Although univalents at MI are usually attributed to recombination failure, our pachytene analyses provide no evidence of an age-related increase in autosomal synaptic defects or recombination failure. This, coupled with the fact that six of the eight cases of univalents involved small chromosomes, suggests that these abnormalities reflect an age-related increase in premature loss of cohesion rather than recombination failure. Given the recent interest in loss of cohesion as an age-related mechanism of aneuploidy production in the female (Liu and Keefe 2008; Chiang et al. 2010; Lister et al. 2010), the finding of a similar, albeit subtle, age effect in the male is intriguing. Indeed, if a paternal age effect is confirmed in studies of carriers of mutations in cohesion genes, this would suggest that age-related cohesion loss may be driven, at least in part, by the physiological effects of aging (e.g., endocrine changes) rather than being a simple chronological effect as has been commonly assumed on the basis of studies in females.
The systematic analysis of successive meiotic stages illustrates the inherent risk of drawing conclusions from the analysis of a single meiotic stage. Specifically, our data suggest that the presence of “univalents” at the diplotene stage, which has been used in recent studies as a measure of recombination failure (e.g., Berkowitz et al. 2012), may not provide an accurate assessment. The lack of obvious connections between homologs at diplotene may be misleading. That is, ∼33% of cells from 12-week-old males had at least one pair of homologs without a visible site of connection. If these homologs truly lacked a physical connection, a comparable number of univalents should have been evident at MI. In fact, univalents were observed in only 4% of MI cells, a frequency that corresponds nicely with the frequency of clearly separated univalents at diplotene (Table 5). Our data suggest, however, that assuming that this category of diplotene cells represents the “true” incidence of recombination failure is similarly misleading; i.e., because a corresponding number of pachytene cells with an SC lacking an MLH1 focus was not observed, we postulate that the majority of autosomal univalents at MI were the result of premature loss of cohesion.
In contrast to the observations at MI, neither aneuploidy nor an increased incidence of unpaired monad chromosomes was detected at MII. This provides further evidence that metaphase checkpoints continue to effectively eliminate cells with errors (Vernet et al. 2011), even in aged males.
Implications for humans
Taken together, our sequential analysis of meiotic stages during the life span of the male mouse provides evidence that advancing paternal age increases the frequency of meiotic errors. By comparison with the female, however, both the incidence of errors and the effect of age are modest (Hunt and Hassold 2002). In addition, because checkpoint stringency is robust in male meiosis by comparison with female meiosis and our data provide no evidence of an age-related change, aberrant chromosome behavior at MI is not an accurate predictor of the frequency of aneuploid gametes in the male.
An obvious and important question is whether the findings from our studies in mice can be extrapolated to humans. Based on the analysis of mature sperm, the incidence of aneuploidy appears considerably higher in humans, with estimates of aneuploid sperm in the 0–0.4% range in inbred male mice (Mroz et al. 1999) and in the 1–7% range in humans (e.g., Martin et al. 1991; Hassold 1998; Shi and Martin 2000). Although the incidence of errors is higher in men, our data suggest that it is unlikely that this is simply a reflection of a higher endogenous error rate. That is, unless cell cycle checkpoint stringency is also less efficient, meiotic errors should be efficiently eliminated. Importantly, however, evidence from both species suggests that perturbations in the testicular environment (e.g., impaired spermatogenesis as in XXY mice and infertile men) and environmental exposures can significantly elevate the incidence of disomic sperm (Mroz et al. 1999; Dakouane et al. 2005; Templado et al. 2013). Thus, the higher incidence of chromosomally abnormal sperm reported in human studies may be indicative of poorer fertility in general. The error rate and the effects of age are modest in the human male and, consequently, are of less concern than aging in the human female. Nevertheless, our observations clearly demonstrate adverse effects of age on meiotic chromosome behavior in male mice. Recent trends in human reproduction that include fathering children at later ages, coupled with concerns about declining fertility, further underscore the relevance of understanding both the cause(s) of meiotic errors in men and the impact of age on human spermatogenesis.
Supplementary Material
Acknowledgments
The authors acknowledge the technical assistance of Crystal Lawson, Libby Pascucci, Renee Wilson, Molly Jennings, and Emily Nagler. This work was supported by National Institutes of Health grants ES013527 (to P.A.H.) and HD21341 (to T.J.H.) and National Institute of General Medical Sciences grant T32GM083864 to the School of Molecular Biosciences, Washington State University.
Footnotes
Communicating editor: J. C. Schimenti
Literature Cited
- Baker S. M., Plug A. W., Prolla T. A., Bronner C. E., Harris A. C., et al. , 1996. Involvement of mouse Mlh1 in DNA mismatch repair and meiotic crossing over. Nat. Genet. 13: 336–342. [DOI] [PubMed] [Google Scholar]
- Barlow A. L., Benson F. E., West S. C., Hulten M. A., 1997. Distribution of the Rad51 recombinase in human and mouse spermatocytes. EMBO J. 16: 5207–5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz K. M., Sowash A. R., Koenig L. R., Urcuyo D., Khan F., et al. , 2012. Disruption of CHTF18 causes defective meiotic recombination in male mice. PLoS Genet. 8: e1002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne P. S., Mahadevaiah S. K., Turner J. M., 2009. The consequences of asynapsis for mammalian meiosis. Nat. Rev. Genet. 10: 207–216. [DOI] [PubMed] [Google Scholar]
- Chiang T., Duncan F. E., Schindler K., Schultz R. M., Lampson M. A., 2010. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 20: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole F. L., Kauppi L., Lange J., Roig I., Wang R., et al. , 2012. Homeostatic control of recombination is implemented progressively in mouse meiosis. Nat. Cell Biol. 14: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakouane M., Bicchieray L., Bergere M., Albert M., Vialard F., et al. , 2005. A histomorphometric and cytogenetic study of testis from men 29–102 years old. Fertil. Steril. 83: 923–928. [DOI] [PubMed] [Google Scholar]
- Drumond A. L., Meistrich M. L., Chiarini-Garcia H., 2011. Spermatogonial morphology and kinetics during testis development in mice: a high-resolution light microscopy approach. Reproduction 142: 145–155. [DOI] [PubMed] [Google Scholar]
- Evans E. P., Breckon G., Ford C. E., 1964. An air-drying method for meiotic preparations from mammalian testes. Cytogenetics 3: 289–294. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Harvey J. F., Morton N. E., Jacobs P. A., 1995. Trisomy 18: studies of the parent and cell division of origin and the effect of aberrant recombination on nondisjunction. Am. J. Hum. Genet. 56: 669–675. [PMC free article] [PubMed] [Google Scholar]
- Flurkey, K., J. M. Currer, and D. E. Harrison, 2007 The mouse in aging research, pp. 637–672 in The Mouse in Biomedical Research, edited by J. G. Fox. American College Laboratory Animal Medicine (Elsevier), Burlington, MA. [Google Scholar]
- Fonseka K. G., Griffin D. K., 2011. Is there a paternal age effect for aneuploidy? Cytogenet. Genome Res. 133: 280–291. [DOI] [PubMed] [Google Scholar]
- Goriely A., Wilkie A. O., 2012. Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am. J. Hum. Genet. 90: 175–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. K., Abruzzo M. A., Millie E. A., Sheean L. A., Feingold E., et al. , 1995. Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum. Mol. Genet. 4: 2227–2232. [DOI] [PubMed] [Google Scholar]
- Gruhn J. R., Rubio C., Broman K. W., Hunt P. A., Hassold T. J., 2014. Cytological studies of human meiosis: sex-specific differences in recombination originate at, or prior to, establishment of double-strand breaks. PLoS One (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T. J., 1998. Nondisjunction in the human male, pp. 383–406 in Meiosis and Gametogenesis, edited by Handel M. A. Academic Press, San Diego. [Google Scholar]
- Hassold T., Hunt P., 2001. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2: 280–291. [DOI] [PubMed] [Google Scholar]
- Hassold T., Pettay D., Robinson A., Uchida I., 1992. Molecular studies of parental origin and mosaicism in 45,X conceptuses. Hum. Genet. 89: 647–652. [DOI] [PubMed] [Google Scholar]
- Hassold T., Merrill M., Adkins K., Freeman S., Sherman S., 1995. Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am. J. Hum. Genet. 57: 867–874. [PMC free article] [PubMed] [Google Scholar]
- Holloway J. K., Booth J., Edelmann W., Mcgowan C. H., Cohen P. E., 2008. MUS81 generates a subset of MLH1–MLH3-independent crossovers in mammalian meiosis. PLoS Genet. 4: e1000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook, E. B., and J. L. Hammerton, 1977 The frequency of chromosome abnormalities detected in consecutive newborn studies, pp. 63–79 in Population Cytogenetics Studies in Humans, edited by E. B. P. Hook. Academic Press, New York. [Google Scholar]
- Hunt P. A., Hassold T. J., 2002. Sex matters in meiosis. Science 296: 2181–2183. [DOI] [PubMed] [Google Scholar]
- Johnson L., Zane R. S., Petty C. S., Neaves W. B., 1984. Quantification of the human Sertoli cell population: its distribution, relation to germ cell numbers, and age-related decline. Biol. Reprod. 31: 785–795. [DOI] [PubMed] [Google Scholar]
- Johnson L., Nguyen H. B., Petty C. S., Neaves W. B., 1987. Quantification of human spermatogenesis: germ cell degeneration during spermatocytogenesis and meiosis in testes from younger and older adult men. Biol. Reprod. 37: 739–747. [DOI] [PubMed] [Google Scholar]
- Johnson L., Grumbles J. S., Bagheri A., Petty C. S., 1990. Increased germ cell degeneration during postprophase of meiosis is related to increased serum follicle-stimulating hormone concentrations and reduced daily sperm production in aged men. Biol. Reprod. 42: 281–287. [DOI] [PubMed] [Google Scholar]
- Kauppi L., Barchi M., Baudat F., Romanienko P. J., Keeney S., et al. , 2011. Distinct properties of the XY pseudoautosomal region crucial for male meiosis. Science 331: 916–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong A., Barnard J., Gudbjartsson D. F., Thorleifsson G., Jonsdottir G., et al. , 2004. Recombination rate and reproductive success in humans. Nat. Genet. 36: 1203–1206. [DOI] [PubMed] [Google Scholar]
- Lenzi M. L., Smith J., Snowden T., Kim M., Fishel R., et al. , 2005. Extreme heterogeneity in the molecular events leading to the establishment of chiasmata during meiosis in human oocytes. Am. J. Hum. Genet. 76: 112–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister L. M., Kouznetsova A., Hyslop L. A., Kalleas D., Pace S. L., et al. , 2010. Age-related meiotic segregation errors in mammalian oocytes are preceded by depletion of cohesin and Sgo2. Curr. Biol. 20: 1511–1521. [DOI] [PubMed] [Google Scholar]
- Liu L., Keefe D. L., 2008. Defective cohesin is associated with age-dependent misaligned chromosomes in oocytes. Reprod. Biomed. Online 16: 103–112. [DOI] [PubMed] [Google Scholar]
- Lu S., Zong C., Fan W., Yang M., Li J., et al. , 2012. Probing meiotic recombination and aneuploidy of single sperm cells by whole-genome sequencing. Science 338: 1627–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn A., Koehler K. E., Judis L., Chan E. R., Cherry J. P., et al. , 2002. Covariation of synaptonemal complex length and mammalian meiotic exchange rates. Science 296: 2222–2225. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Ko E., Rademaker A., 1991. Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am. J. Med. Genet. 39: 321–331. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Spriggs E., Ko E., Rademaker A. W., 1995. The relationship between paternal age, sex ratios, and aneuploidy frequencies in human sperm, as assessed by multicolor FISH. Am. J. Hum. Genet. 57: 1395–1399. [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Kolas N. K., Tarsounas M., Marcon E., Cohen P. E., et al. , 2002. The time course and chromosomal localization of recombination-related proteins at meiosis in the mouse are compatible with models that can resolve the early DNA-DNA interactions without reciprocal recombination. J. Cell Sci. 115: 1611–1622. [DOI] [PubMed] [Google Scholar]
- Mroz K., Hassold T. J., Hunt P. A., 1999. Meiotic aneuploidy in the XXY mouse: evidence that a compromised testicular environment increases the incidence of meiotic errors. Hum. Reprod. 14: 1151–1156. [DOI] [PubMed] [Google Scholar]
- Nagaoka S. I., Hassold T. J., Hunt P. A., 2012. Human aneuploidy: mechanisms and new insights into an age-old problem. Nat. Rev. Genet. 13: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver T. R., Feingold E., Yu K., Cheung V., Tinker S., et al. , 2008. New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet. 4: e1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Bonet M., Turek P. J., Sun F., Ko E., Martin R. H., 2005. Temporal progression of recombination in human males. Mol. Hum. Reprod. 11: 517–522. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy P. J., Sheffield J. W., 1991. Effect of temperature and the role of testicular descent on post-natal testicular androgen production in the mouse. J. Reprod. Fertil. 91: 357–364. [DOI] [PubMed] [Google Scholar]
- Paul C., Robaire B., 2013. Ageing of the male germ line. Nat. Rev. Urol. 10: 227–234. [DOI] [PubMed] [Google Scholar]
- Peters A. H., Plug A. W., Van Vugt M. J., De Boer P., 1997. A drying-down technique for the spreading of mammalian meiocytes from the male and female germline. Chromosome Res. 5: 66–68. [DOI] [PubMed] [Google Scholar]
- Ray M. A., Johnston N. A., Verhulst S., Trammell R. A., Toth L. A., 2010. Identification of markers for imminent death in mice used in longevity and aging research. J. Am. Assoc. Lab. Anim. Sci. 49: 282–288. [PMC free article] [PubMed] [Google Scholar]
- Robbins W. A., Baulch J. E., Moore D., Weier H. U., Blakey D., et al. , 1995. Three-probe fluorescence in situ hybridization to assess chromosome X, Y, and 8 aneuploidy in sperm of 14 men from two healthy groups: evidence for a paternal age effect on sperm aneuploidy. Reprod. Fertil. Dev. 7: 799–809. [DOI] [PubMed] [Google Scholar]
- Rousseaux S., Hazzouri M., Pelletier R., Monteil M., Usson Y., et al. , 1998. Disomy rates for chromosomes 14 and 21 studied by fluorescent in-situ hybridization in spermatozoa from three men over 60 years of age. Mol. Hum. Reprod. 4: 695–699. [DOI] [PubMed] [Google Scholar]
- Royo H., Polikiewicz G., Mahadevaiah S. K., Prosser H., Mitchell M., et al. , 2010. Evidence that meiotic sex chromosome inactivation is essential for male fertility. Curr. Biol. 20: 2117–2123. [DOI] [PubMed] [Google Scholar]
- Ryu B. Y., Orwig K. E., Oatley J. M., Avarbock M. R., Brinster R. L., 2006. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24: 1505–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. M., 1994. Regulation of spermatogenesis, pp. 1363–1434 in The Physiology of Reproduction, edited by E. Knobil and J. D. Neill Raven Press, New York. [Google Scholar]
- Sharpe R. M., McKinnell C., Kivlin C., Fisher J. S., 2003. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125: 769–784. [DOI] [PubMed] [Google Scholar]
- Shi Q., Martin R. H., 2000. Aneuploidy in human sperm: a review of the frequency and distribution of aneuploidy, effects of donor age and lifestyle factors. Cytogenet. Cell Genet. 90: 219–226. [DOI] [PubMed] [Google Scholar]
- Sloter E., Nath J., Eskenazi B., Wyrobek A. J., 2004. Effects of male age on the frequencies of germinal and heritable chromosomal abnormalities in humans and rodents. Fertil. Steril. 81: 925–943. [DOI] [PubMed] [Google Scholar]
- Solari A. J., 1989. Sex chromosome pairing and fertility in the heterogametic sex of mammals and birds, pp. 77–107 in Fertility and Chromosome Pairing: Recent Studies in Plants and Animals, edited by Gillies C. B. CRC Press, Boca Raton, FL. [Google Scholar]
- Speed R. M., 1977. The effects of ageing on the meiotic chromosomes of male and female mice. Chromosoma 64: 241–254. [DOI] [PubMed] [Google Scholar]
- Templado C., Uroz L., Estop A., 2013. New insights on the origin and relevance of aneuploidy in human spermatozoa. Mol. Hum. Reprod. 19: 634–643. [DOI] [PubMed] [Google Scholar]
- Vernet N., Mahadevaiah S. K., Ojarikre O. A., Longepied G., Prosser H. M., et al. , 2011. The Y-encoded gene zfy2 acts to remove cells with unpaired chromosomes at the first meiotic metaphase in male mice. Curr. Biol. 21: 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S., Sukeno M., Nakagawa T., Ohbo K., Nagamatsu G., et al. , 2006. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development 133: 1495–1505. [DOI] [PubMed] [Google Scholar]
- Zaragoza M. V., Jacobs P. A., James R. S., Rogan P., Sherman S., et al. , 1994. Nondisjunction of human acrocentric chromosomes: studies of 432 trisomic fetuses and liveborns. Hum. Genet. 94: 411–417. [DOI] [PubMed] [Google Scholar]
- Zhang X., Ebata K. T., Robaire B., Nagano M. C., 2006. Aging of male germ line stem cells in mice. Biol. Reprod. 74: 119–124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.