Abstract
The migration of Caenorhabditis elegans gonadal distal tip cells (DTCs) offers an excellent model to study the migration of epithelial tubes in organogenesis. mig-18 mutants cause meandering or wandering migration of DTCs during gonad formation, which is very similar to that observed in animals with mutations in mig-17, which encodes a secreted metalloprotease of the ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family. MIG-18 is a novel secreted protein that is conserved only among nematode species. The mig-17(null) and mig-18 double mutants exhibited phenotypes similar to those in mig-17(null) single mutants. In addition, the mutations in fbl-1/fibulin-1 and let-2/collagen IV that suppress mig-17 mutations also suppressed the mig-18 mutation, suggesting that mig-18 and mig-17 function in a common genetic pathway. The Venus-MIG-18 fusion protein was secreted from muscle cells and localized to the gonadal basement membrane, a tissue distribution reminiscent of that observed for MIG-17. Overexpression of MIG-18 in mig-17 mutants and vice versa partially rescued the relevant DTC migration defects, suggesting that MIG-18 and MIG-17 act cooperatively rather than sequentially. We propose that MIG-18 may be a cofactor of MIG-17/ADAMTS that functions in the regulation of the gonadal basement membrane to achieve proper direction of DTC migration during gonadogenesis.
Keywords: cell migration, ADAMTS, basement membrane, gonadogenesis
THE ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) family of the secreted zinc metalloproteases has important roles in development. Nineteen ADAMTS genes have been identified in the human genome, and mutations in many result in hereditary diseases that are related to disorders of the extracellular matrix (Apte 2009). The functions of ADAMTS-5, -9, and -20 are required for digit formation, and ADAMTS-9 and -20 are needed for closure of the palate in mice (McCulloch et al. 2009; Enomoto et al. 2010). ADAMTS-5 and -15 act in myoblast fusion (Stupka et al. 2013). However, the precise roles of ADAMTS proteases in development still remain elusive.
Among five ADAMTS genes in Caenorhabditis elegans, gon-1 and mig-17 play essential roles in the development of the somatic gonad (Blelloch and Kimble 1999; Nishiwaki et al. 2000). GON-1 is required for active migration of gonadal distal tip cells (DTCs), whereas MIG-17 acts in the directional control of DTC migration. Genetic suppressor analyses of mig-17 mutants identified dominant gain-of-function (gf) mutations in two genes that encode basement membrane proteins, FBL-1C/fibulin-1C and LET-2/α2 subunit of collagen IV (Kubota et al. 2004, 2008). The suppressor fbl-1(gf) mutations result in substitutions of evolutionarily conserved amino acids within the second EGF-like motif of FBL-1C. FBL-1C is recruited to the gonadal basement membrane by MIG-17 activity, where it is likely to be required for directional control of DTC migration (Kubota et al. 2004). The suppression by fbl-1(gf) mutations depends on NID-1/nidogen, a basement membrane protein (Kubota et al. 2008). The two suppressor let-2(gf) mutations result in amino acid changes in the triple helix region and in the C-terminal noncollagenous domain. In contrast to fbl-1(gf) mutations, suppression by let-2(gf) mutations is NID-1 independent (Kubota et al. 2008).
In this study, we analyzed a novel gene, mig-18, mutations in which led to misdirected migration of DTCs similar to that observed in mig-17 mutants. mig-18 was found to encode a small protein that was secreted from muscle cells and localized to the gonadal basement membrane. This tissue distribution of MIG-18 was similar to that observed for MIG-17. The phenotypic analysis of mig-18; mig-17 double mutants revealed that mig-18 did not enhance the phenotype of the mig-17 null allele. Furthermore, fbl-1(gf) and let-2(gf) mutations that suppressed mig-17 also suppressed the DTC migration defects in the mig-18 mutants. These results suggest that MIG-18 acts in the same pathway with MIG-17. Genetic and molecular evidence suggests that MIG-18 functions together with MIG-17/ADAMTS to control directional migration of DTCs.
Materials and Methods
Strains and genetic analysis
Culture, handling, and ethyl methanesulfonate (EMS) mutagenesis of C. elegans were conducted as described (Brenner 1974). The following mutations were used in this work: mig-18(k140), fbl-1(k201, k206), let-2(k196), nid-1(cg118, cg119), unc-25(e156), unc-42(e270), unc-64(e246), and unc-119(e2498) (Brenner 1974; Maduro and Pilgrim 1995; Nishiwaki 1999). mig-18(gm321) and mig-18(tk35) were isolated by genetic screening using EMS and N-ethyl-N-nitrosourea as mutagens, respectively (Brenner 1974; De Stasio and Dorman 2001). mig-18(tm2007) was obtained from the National Bioresource Project for the nematode. The transgenic extrachromosomal arrays containing nid-1::HA (Kubota et al. 2008) were introduced into mig-18(k140)-containing strains by mating.
Microscopy
Gonad migration phenotypes were scored using a Nomarski microscope (Axioplan 2; Zeiss). Analysis of gonadal phenotypes was performed at the young-adult stage as described (Nishiwaki 1999). The patterns of expression of Venus fusion proteins (see below) were analyzed using a confocal laser-scanning microscope (LSM5; Zeiss) equipped with a C-Apochromat 63× (water immersion; numerical aperture 1.2) lens and controlled by PASCAL version 3.2 SP2 software.
Molecular cloning of mig-18
mig-18 was mapped to the right of unc-64 on linkage group III. Single-nucleotide polymorphism mapping (Wicks et al. 2001) placed it to the right of the cosmid clone T03F6. Microinjection rescue experiments using 12 fosmid clones that covered most of the region between T03F6 and the right end of the chromosome identified a fosmid clone, WRM0613bA03, that rescued the mig-18 DTC migration defects. A PCR-amplified fragment of one of the predicted genes contained within this fosmid clone, F11F1.6, rescued mig-18. Genomic sequence analyses revealed nucleotide changes in the coding regions of F11F1.6 in all three mutant alleles of mig-18.
Constructs
To construct mig-18p::SP::Venus::mig-18, the genomic region of mig-18 from −921 to +2022, relative to the adenine of the initiation codon, was amplified by PCR and was cloned into pBluescriptII KS(−) (Invitrogen). The plasmid carrying the Venus gene was kindly provided by Takeshi Ishihara. The Venus gene was amplified by PCR and was inserted downstream of the signal peptide sequence (+69) of mig-18. To remove the signal peptide sequence, the plasmid mig-18p::SP::Venus::mig-18, except for the signal peptide sequence, was amplified by PCR and was self-ligated, producing mig-18p::ΔSP::Venus::mig-18. To construct mig-24p::TM::Venus::mig-18, the SP sequence of mig-18p::SP::Venus::mig-18 plasmid was replaced with the genomic region of mig-22, from +1 to +195 in exon 1, which encodes the type II transmembrane (TM) domain (Suzuki et al. 2006). To construct mig-17p::mig-17::Venus and mig-17p::mig-17::mCherry, the GFP-coding sequence of the mig-17p::mig-17::GFP plasmid (Nishiwaki et al. 2000) was replaced with those of Venus and mCherry from pPD95.79, respectively.
Germline transformation
Germline transformation was carried out as described (Mello et al. 1991). Transgenic strains were made by injecting plasmids into unc-119(e2498) hermaphrodites, and the generated transgenic arrays were transferred to appropriate genetic backgrounds having unc-119(e2498) by mating. mig-18p::SP::Venus::mig-18 plasmid was injected at 5 ng/μl with 25 ng/μl unc-119+ plasmid (pDP#MM016B) (Maduro and Pilgrim 1995) and 100 ng/μl pBluescriptII KS(–) (carrier DNA). mig-24p::mig-22TM::Venus::mig-18 plasmid was injected at 10 ng/μl with 30 ng/μl pDP#MM016B and 130 ng/μl pBluescriptII KS(–). mig-17p::mig-17::Venus plasmid was injected at 100 ng/μl with 25 ng/μl pDP#MM016B and 25 ng/μl pBluescriptII KS(–).
Western blot analysis
Western blot analysis was done as described (Ihara and Nishiwaki 2007).
Quantification of FBL-1C localization
The method for quantification of FBL-1C localization to the basement membrane is shown in supporting information, Figure S1.
Results
mig-18 encodes a novel secreted protein required for directional migration of the DTCs
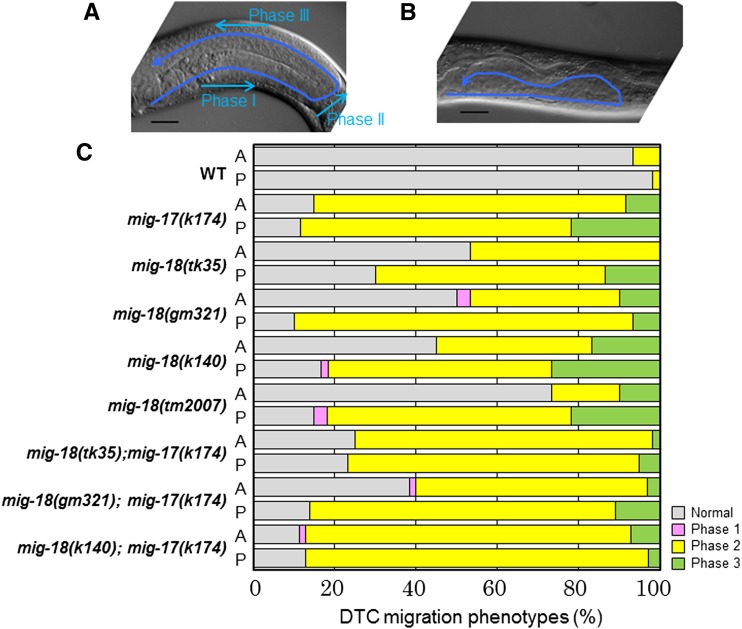
The C. elegans gonad arms extend to the anterior-right and posterior-left areas of the body cavity. The U-shape of the gonad arms reflects the migration paths of the gonadal DTCs during larval development (Figure 1A). The DTCs are generated at the tip of the gonad primordium and migrate in opposite directions along the ventral body-wall muscle (phase I). They turn dorsally and migrate over the lateral hypodermis toward the dorsal body-wall muscle (phase II). Upon reaching the dorsal muscle, the two DTCs turn again and migrate toward the midbody along the dorsal muscle (phase III) (Hedgecock et al. 1987). We isolated three independent mutants in the mig-18 gene by forward genetics screening of gonad morphology. The mig-18 mutants showed defects in phase II and phase III migration, but the phase I migration was essentially normal (Figure 1B). In the mutant animals, the DTCs often partially executed the dorsal migration and moved over the lateral hypodermis, rather than the dorsal muscle, after the second turn (Figure 1, B and C).
Figure 1.
Defective DTC migration of mig-18 mutants. (A and B) Gonad morphology (arrows) of wild-type (A) and mig-18(k140) (B) young-adult hermaphrodites. Posterior gonads are shown. Anterior to the left, dorsal to the top. Bar, 20 μm. The phases of DTC migration are indicated. Both phase II and phase III are defective in B. (C) Percentages of abnormal gonad morphology in the mig-18 and mig-18; mig-17 double mutants. The DTC migration defects were scored based on the earliest defective phase. n = 60 for each experiment.
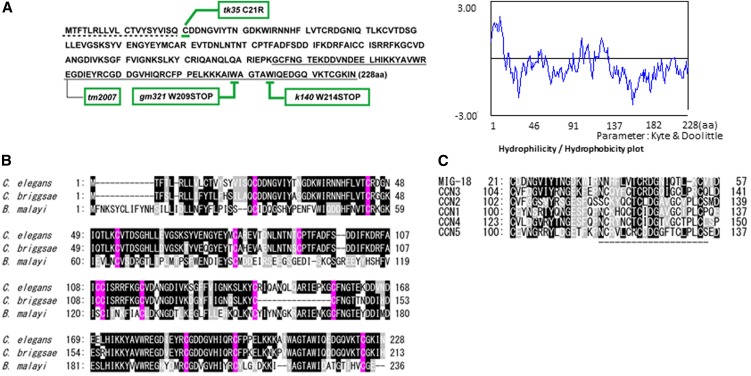
We cloned mig-18 by genetic mapping followed by injection rescue experiments using genomic DNA fragments (data not shown). mig-18 corresponded to the predicted gene F11F1.6 in WormBase. The predicted MIG-18 protein, which consists of 228 amino acids, apparently has homologs only in nematodes (Figure 2, A and B). MIG-18 appeared to be a secreted protein because it has a potential signal peptide for secretion at its N terminus and cysteine motifs that are well conserved among nematode species. Although we could not find orthologs of MIG-18 in other species, we identified a 37-amino-acid stretch that has considerable homologies with the CCN [Cyr61 (cysteine-rich protein 61), CTGF (connective tissue growth factor), and NOV (nephroblastoma overexpressed gene)] family of secreted proteins in mammals (Chen and Lau 2009). Among the CCN proteins, MIG-18 was most similar to CCN3 (Figure 2C). This homologous region contains a 20-amino-acid sequence identified as the binding site for integrin αvβ3 in CCN1 (Chen et al. 2004), although some cysteine residues were not conserved in MIG-18.
Figure 2.
MIG-18 and its homologs. (A) Amino acid sequence of MIG-18 (left) and hydrophilicity/hydrophobicity plot (right). Positions of mig-18 mutations are indicated. Nucleotide changes are tk35, C21R (tgt > cgt); gm321, W209STOP (tgg > tga); and k140, W214STOP (tgg > tag). tm2007 potentially truncates the underlined amino acids due to the 472-bp deletion from g571 with respect to the adenine of the initiation codon, which is within the second intron. The dotted line (left) indicates the potential signal peptide. (B) MIG-18 homologs in nematodes. C. elegans, MIG-18; Caenorhabditis briggsae, CBG21220; Brugia malayi, Bm1_50515. Identical and similar amino acids are shown by black and gray boxes, respectively. Cysteine residues are shown in pink. (C) Homologous regions between MIG-18 and mouse CCN family proteins. Identical and similar amino acids are shown by black and gray boxes, respectively. The region indicated by the dashed line corresponds to the integrin-binding site in human CCN1 (Chen et al. 2004). Analysis of amino acid sequences was done using GENETYX Version 8.
The mutations k140 and gm321 were nonsense mutations that occurred near the C terminus of the gene, whereas tk35 was an amino acid substitution of the cysteine immediately after the signal peptide. tm2007 was a deletion mutation that may truncate the C-terminal 73 amino acids (Figure 2A). The k140 and gm321 alleles were likely to be strong loss-of-function or null alleles, as they displayed similar phenotypes and were not enhanced when in trans to the deficiency eDf2 (Nishiwaki 1999) (data not shown). It was unexpected that the deletion allele tm2007 was weaker than k140 and gm321 especially with regard to the anterior DTC migration. We examined the phenotype of k140/tm2007 heterozygotes. The phenotypic penetrance of k140/tm2007 was 60 ± 8% for anterior and 92 ± 5% for posterior (n = 60) compared to those of k140, 56 ± 4% for anterior and 88 ± 3% for posterior (n = 60), and for tm2007, 27 ± 5% for anterior and 85 ± 7% for posterior (n = 60). Thus, the heterozygotes exhibited the phenotypic penetrance similar to that of k140. We speculate that the tm2007 strain might have a weak recessive suppressor mutation that can weaken the DTC defects of tm2007.
mig-18 acts in the same pathway with mig-17 to control directional migration of DTCs
The phenotypic characteristics of the mig-18 mutants were similar to those of the mig-17 mutants (Nishiwaki et al. 2000). Therefore we generated double mutants between the mig-17(k174) null allele (Ihara and Nishiwaki 2007) and mig-18(k140). The phenotypic penetrance of the double mutants was not stronger than that of mig-17(k174), but it was stronger than that of mig-18 single mutants, especially with respect to effects on anterior DTCs, suggesting that mig-18 acts in the mig-17 pathway.
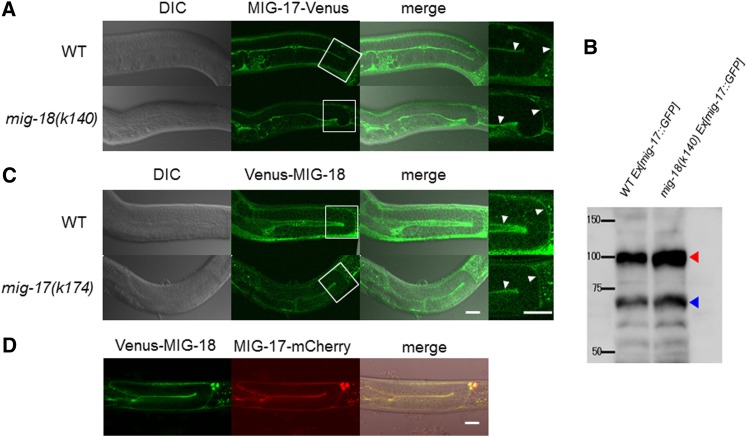
mig-17 encodes a secreted metalloprotease of the ADAMTS family. MIG-17 is produced in the body-wall muscle cells, is secreted into the body cavity, and becomes localized to the surface of the gonad (Nishiwaki et al. 2000). We examined MIG-17-Venus localization in the mig-18 mutants. MIG-17-Venus localized to the gonadal basement membrane in the mig-18 mutants, similar to its localization in wild type (Figure 3A and Figure S2), suggesting that mig-18 is not required for gonadal localization of MIG-17.
Figure 3.
Expression and localization of MIG-17-Venus and Venus-MIG-18. (A and C) DIC (left), confocal (center), and merged (right) images. The enlarged images of the boxed areas in the center panels are shown on the right side. The arrowheads point to the fluorescence from the gonadal basement membrane. (A) Wild-type and mig-18(k140) young adults that express MIG-17-Venus. (B) Western blot analysis of wild-type and mig-18(k140) animals that expressed MIG-17-GFP. Worm extracts (200 μg protein/sample) were analyzed using rabbit anti-GFP (2 mg/ml, Molecular Probes). The red and blue arrowheads indicate pro- and mature forms MIG-17-GFP, respectively. Intensities of the bands were quantified using ImageJ software. (C) Venus-MIG-18 expression in wild-type and mig-17(k174) animals. (D) Colocalization of Venus-MIG-18 and MIG-17-mCherry in the gonadal basement membrane. Confocal images for Venus (left) and mCherry (center) and DIC and confocal merged image (right). The strong dot-like signals on the upper right are of coelomocytes that take up secreted proteins. Posterior gonads are shown. Anterior to the left, dorsal to the top. Bar, 20 μm.
MIG-17 is secreted as a proform, with the prodomain present at its N terminus. This pro-MIG-17 is recruited to the gonadal basement membrane in the prodomain-dependent manner (prodomain targeting) (Ihara and Nishiwaki 2007), where it becomes its mature, active form by proteolytic removal of the prodomain, which is catalyzed by its auto-catalytic activity. Mature MIG-17 then participates in controlling the directed migration of DTCs (Ihara and Nishiwaki 2007). To understand whether MIG-18 functions in the activation of MIG-17, we performed Western blot analysis of MIG-17-GFP. We found that the intensities of the bands for the mature form relative to those of the proform were 0.82 and 0.78 for the wild-type and the mig-18(k140) mutant animals, respectively (Figure 3B). These results suggest that the processing of the prodomain occurred normally in mig-18 mutants and that MIG-18 is not required for this process.
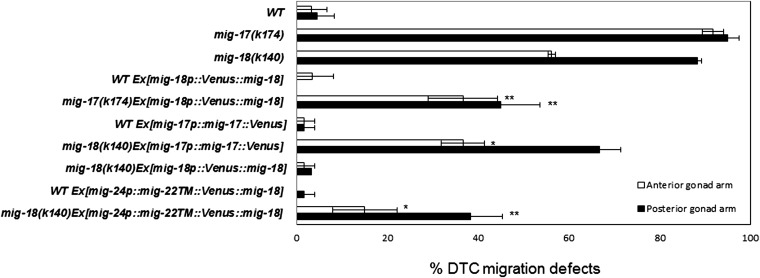
To determine the tissue distribution of MIG-18, we generated MIG-18-Venus, a C-terminal fusion construct. This construct, however, was not functional as it failed to rescue the mig-18 mutant phenotype (data not shown). Thus, we generated a signal peptide (SP)::Venus::mig-18 N-terminal fusion construct that is driven by the mig-18 promoter (mig-18p::SP::Venus::mig-18). Venus-MIG-18 rescued the defective DTC migration in the mig-18 mutants, indicating that the fusion protein is functional (Figure 4). We found that Venus-MIG-18 was expressed in the body-wall muscle cells and the gonadal basement membrane (Figure 3C). Venus-MIG-18 is secreted from the muscle cells because it accumulated in the cytoplasm of the muscle cells and failed to localize to the gonadal basement membrane when its signal peptide was deleted (mig-18p:: ΔSP::Venus::mig-18; Figure S3). Thus MIG-18 and MIG-17 proteins are produced and delivered in the same manner. We asked whether the gonadal localization of Venus-MIG-18 requires MIG-17 activity. Venus-MIG-18 localization was not affected in the mig-17 mutants (Figure 3C and Figure S2), indicating that MIG-17 is not required for gonadal localization of MIG-18.
Figure 4.
Transgenic rescue of DTC migration defects in mig-17 and mig-18 animals. The DTC migration defects of anterior and posterior gonad arms are indicated as percentages. Ex indicates an extrachromosomal array. Data are shown as the mean ± SD (n = 60 for each experiment). P-values for Fisher’s exact test against mig-17(k174) for mig-17 transgenic strains and against mig-18(k140) for mig-18 transgenic strains are indicated: **P < 0.01; *P < 0.05.
We examined whether MIG-18 and MIG-17 colocalize in the gonadal basement membrane. Co-expression of Venus-MIG-18 and MIG-17-mCherry revealed clear colocalization of these proteins in the gonadal basement membrane (Figure 3D).
mig-18 and mig-17 act cooperatively to control DTC migration
Because the transgenic extrachromosomal arrays containing MIG-17-Venus and Venus-MIG-18 are expected to contain multiple copies of these constructs, it is likely that their respective genes are overexpressed as compared with the endogenous genes. We examined whether overexpression of MIG-17-Venus in the mig-18 mutants and whether overexpression of Venus-MIG-18 in the mig-17 mutants could suppress the defective DTC migration of these mutants. Interestingly, we observed partial rescue in both of these experiments (Figure 4). These results suggest that MIG-18 and MIG-17 do not function sequentially but instead act cooperatively to control DTC migration.
The MIG-17-TM-Venus construct can suppress the DTC migration defects of mig-17 mutants when it is expressed in the DTC cell membrane (Ihara and Nishiwaki 2007). Using the type II transmembrane domain of MIG-22 (Suzuki et al. 2006), we generated mig-24p::mig-22TM::Venus::mig-18 and expressed it under the control of the DTC-specific mig-24 promoter (Tamai and Nishiwaki 2007). This membrane-bound construct partially but significantly rescued the DTC migration defects of mig-18(k140) mutant animals (Figure 4). The fluorescence in these animals was detected exclusively in the DTCs (Figure S4). MIG-18 activity at the DTC surface may not be sufficient for its full effect on controlling DTC migration. Alternatively, native MIG-18 activity may be sufficient when it is localized to the DTC surface, but the membrane anchoring of this construct could partially perturb its activity. The observation that mig-18 acts in the same pathway with mig-17, whose function is sufficient at the DTC surface (Ihara and Nishiwaki 2007), supports the latter possibility.
Genetic suppressors of mig-17 also suppress mig-18
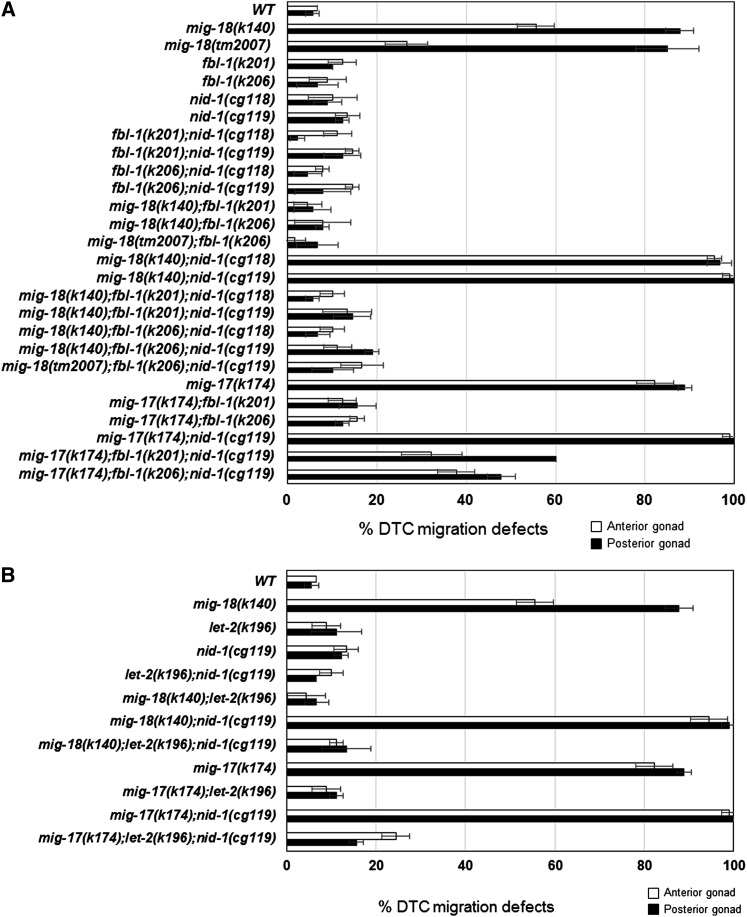
We previously reported that amino acid substitutions in the basement membrane proteins fibulin-1/FBL-1 and the α2 subunit of collagen IV/LET-2 act as dominant gain-of-function suppressors of mig-17 mutants (Kubota et al. 2004, 2008). The suppressor fbl-1(gf) mutants act in a nid-1-dependent manner, whereas the suppressor let-2(gf) mutants act independently of nid-1 (Kubota et al. 2008). If mig-18 acts in the same pathway with mig-17, it is possible that the suppressor mutations fbl-1(gf) and let-2(gf) could suppress mig-18 mutants as well. As expected, the gf mutations fbl-1(k201), fbl-1(k206), and let-2(k196) all suppressed the DTC migration defects of mig-18(k140) mutants (Figure 5, A and B). We introduced nid-1(cg119), a null allele, and nid-1(cg118), a hypomorphic allele that lacks the G2 domain, into mig-18(k140) (Kang and Kramer 2000). Although DTC migration was mostly normal in these nid-1 single mutants, they enhanced the DTC phenotype of mig-18(k140) mutants; a similar enhancement was seen in mig-17(k174) mutants (Figure 5A). let-2(k196) suppressed mig-18(k140) in the nid-1(cg119) mutant background, indicating that the suppression is nid-1 independent, as was observed for the suppression of mig-17 (Figure 5B). Interestingly, however, the suppression activities of fbl-1(k201) and fbl-1(k206) mutants did not depend on nid-1, as they did in the mig-17 mutants (Figure 5A). These observations and the more severe DTC phenotypes of the mig-17 mutants compared to mig-18 mutants suggest that, although MIG-18 and MIG-17 act in the same pathway, MIG-17 could have some functions that are not shared with MIG-18.
Figure 5.
Suppression of mig-18 and mig-17 by fbl-1 and let-2 mutations. The DTC migration defects of anterior and posterior gonad arms are indicated as percentages. (A) Suppression by fbl-1(k201) and fbl-1(k206) alleles. (B) Suppression by the let-2(k196) allele. Data are shown as the mean ± SD (n = 60 for each experiment).
Both MIG-18 and MIG-17 function in FBL-1C accumulation in the gonadal basement membrane
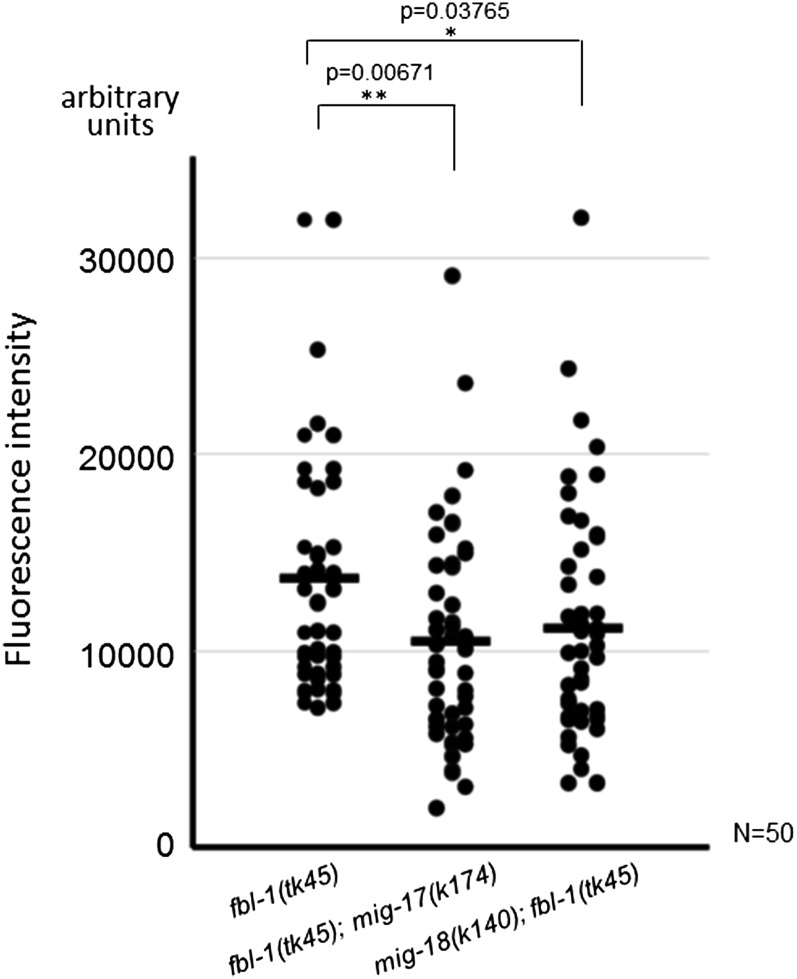
MIG-17 is required for efficient accumulation of FBL-1C/fibulin-1C in the basement membrane (Kubota et al. 2004). Using confocal microscopy, we quantified the levels of FBL-1C-Venus that localized to the gonadal basement membrane. We observed that they were significantly lower in both mig-17 and mig-18 mutants as compared with the amounts observed in the wild type (Figure 6). These results suggest that not only mig-17 but also mig-18 participate in the efficient accumulation of FBL-1C in the gonadal basement membrane.
Figure 6.
Gonadal localization of FBL-1C-Venus in mig-17 and mig-18 mutants. Levels of accumulation of FBL-1C-Venus in the gonadal basement membrane were quantified with confocal microscopy. The fluorescence intensity is given in arbitrary units. Strains are fbl-1(tk45), mig-17(k174); fbl-1(tk45) [which contains unc-42(e270)], and mig-18(k140); fbl-1(tk45), each of which expressed fbl-1C::Venus. Each dot represents the fluorescence intensity of a single animal. The horizontal bars represent mean values. P-values for Fisher’s exact test against fbl-1(tk45) are indicated.
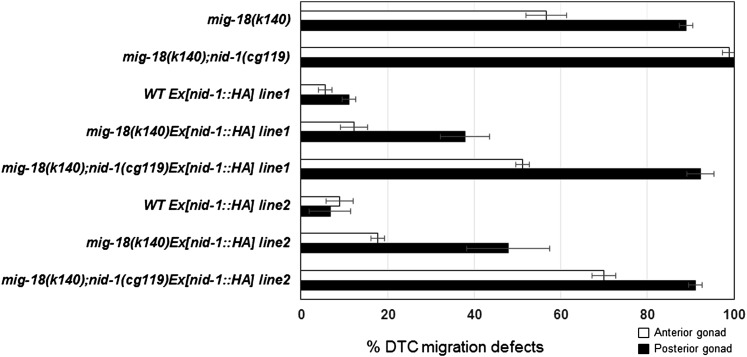
In mig-17 mutants, the localization of NID-1 in the basement membrane is also reduced, and the overexpression of NID-1 can partially rescue the DTC migration defects (Kubota et al. 2008). We found that overexpression of NID-1 also partially rescued the mig-18(k140) mutant and that this effect of NID-1 overexpression was weakened in the nid-1(cg119) null mutant background (Figure 7), suggesting that NID-1 accumulation also plays a role downstream of MIG-18.
Figure 7.
Suppression of mig-18 by overexpression of NID-1-HA. The DTC migration defects of anterior and posterior gonad arms are indicated as percentages. Lines 1 and 2 have independently generated transgenic extrachromosomal arrays that contain nid-1::HA corresponding to those reported previously (Kubota et al. 2008). Data are shown as the mean ± SD (n = 60 for each experiment).
Discussion
In this study, we identified a novel molecule, MIG-18, that is required for the directional control of DTC migration. We found that MIG-18 acts in the MIG-17 pathway, which we characterized previously as being required for DTC migration (Kubota et al. 2008). Although the substrate for MIG-17 is still unclear, MIG-17-dependent proteolysis is required for efficient accumulation of FBL-1C in the gonadal basement membrane. FBL-1C and LET-2 are likely to be activated in the basement membrane by MIG-17 activity, and their activation in turn recruits NID-1 to induce proper DTC migration (Kubota et al. 2008). Because we observed the partial rescue of DTC migration defects both when MIG-18 was overexpressed in mig-17 mutants and when MIG-17 was overexpressed in mig-18 mutants, we suggest that MIG-18 acts cooperatively with MIG-17 rather than upstream or downstream of MIG-17.
It is surprising that overexpression of MIG-18, which appears not to be a protease based on its amino acid sequence, can partially compensate for the loss of the MIG-17 protease in the regulation of DTC migration. One explanation for this might be that MIG-17, when localized to the basement membrane, may also function in DTC migration through its noncatalytic activity by recruiting FBL-1C. This function should, however, be a minor one, as the ability of MIG-17 to control DTC migration strongly depends on its catalytic activity (Ihara and Nishiwaki 2007). Interestingly, there are some reports regarding noncatalytic activities of ADAMTSs. Mammalian ADAMTS10 participates in microfibril biogenesis through its binding to fibrillin-1, in which the catalytic activity of ADAMTS10 appears not to be involved (Kutz et al. 2011). ADAMTS4 binds and colocalizes with fibronectin at the cell surface, where fibronectin can inhibit ADAMTS4 activity as an aggrecanase (Hashimoto et al. 2004). In addition, the Xenopus ADAMTS1 negatively modulates FGF signaling independently of its metalloprotease activity (Suga et al. 2006).
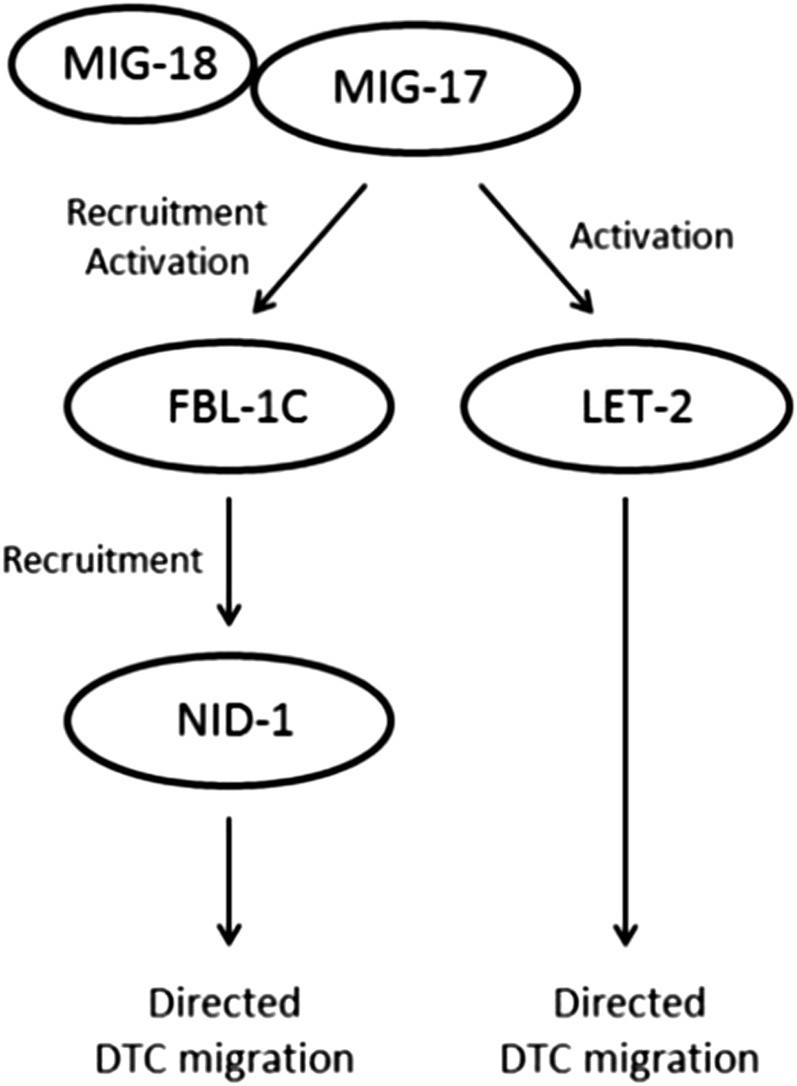
Because MIG-18 and MIG-17 act in the same pathway, we expected that the suppression of mig-18 mutants by the fbl-1 and let-2 gain-of-function mutations would be dependent and independent of nid-1, respectively. However, the fbl-1 mutations suppressed mig-18 in a nid-1-independent manner. This result is different from the suppression of mig-17 mutants by fbl-1(gf), which is dependent on nid-1 (Kubota et al. 2008). However, because the nid-1 dependency of mig-17 suppression was partial (Figure 5A), it is possible that NID-1 may not be a critical component of the MIG-17 pathway for controlling DTC migration. We propose a model in which MIG-18 is a cofactor that enhances the catalytic and noncatalytic activities of mature (i.e., after removal of the prodomain) MIG-17. MIG-18-enhanced MIG-17 recruits FBL-1C efficiently to the gonadal basement membrane and activates FBL-1C and LET-2. The activated FBL-1C recruits NID-1 to the gonadal basement membrane (Figure 8). Because the phenotype of mig-17 mutants is stronger than that of mig-18 mutants, we postulated in this model that MIG-17 has a basal activity even in the absence of MIG-18. In the mig-17(k174); fbl-1(gf) double mutants, the FBL-1C mutant protein weakly localizes to the gonadal basement membrane (Kubota et al. 2004), but it is not activated at all by MIG-17. Thus, the FBL-1C mutant protein requires NID-1 to regulate DTC migration. In contrast, in the mig-18(k140); fbl-1(gf) double mutants, the basal activity of MIG-17 activates the FBL-1C mutant protein, and therefore NID-1 is dispensable in DTC regulation. Although it is not clear why the mig-18 mutation (and also the mig-17 mutation) was partially suppressed by the overexpression of nid-1, it is possible that with overexpression more NID-1 molecules were able to localize to the basement membrane of mig-18 and mig-17 mutants even though FBL-1C was not fully activated.
Figure 8.
Model of MIG-17- and MIG-18-dependent regulation of DTC migration. MIG-18 acts as a cofactor to activate MIG-17. The activated MIG-17 efficiently recruits FBL-1C to the gonadal basement membrane, where it activates FBL-1C and LET-2. The activated FBL-1C recruits NID-1 to control DTC migration.
MIG-18 appears to be a novel protein, but it has partial homologies to CCN proteins found in mammals. Proteins in the CCN family are known as matricellular proteins, which have been defined as a subset of nonstructural proteins in the extracellular matrix that can modulate cell-matrix interactions and cell regulatory functions by different mechanisms (Roberts 2011). In this respect, MIG-18 should also be classified as a matricellular protein. The CCN proteins are involved in the regulation of various cellular functions, such as proliferation, differentiation, survival, adhesion, and migration (Leask and Abraham 2006). CCN6 is highly upregulated in osteoarthritic cartilage and is suggested to act in progressive pseudorheumatoid dysplasia through transcriptional repression of ADAMTS-5 and expression of MMP-10 (Baker et al. 2012). CCN3 is not expressed in advanced melanoma cells, and its expression in aggressive Lu melanoma cells inhibits MMP-2/9 transcription and results in reduced tumor invasion (Fukunaga-Kalabis et al. 2008). CCN3 enhances the migration of chondrosarcoma cells by increasing expression of MMP-13 through integrin receptors (Tzeng et al. 2011). Therefore CCN proteins are involved in the transcriptional regulation of matrix metalloproteinases. However, the functions of CCN proteins in post-translational control of matrix metalloproteinases remain to be determined. Our findings suggest that matricellular proteins may be involved in the post-translational regulation of activities of ADAMTS proteinases during development and during disease pathology.
Supplementary Material
Acknowledgments
We thank Shigehiro Kuraku (RIKEN Center for Developmental Biology) and Hiroki Kaneko (College of Humanities and Sciences, Nihon University) for helpful discussions and Noriko Nakagawa and Asami Sumitani for technical assistance. Some nematode strains used in this work were provided by the Caenorhabditis Genetics Center, which is funded by the National Institutes of Health National Center for Research Resources, and by Shohei Mitani through the National Bioresource Project for the nematode. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas by the Ministry of Education, Culture, Sports, Science and Technology (K.N.) (22111005) and by National Institutes of Health grant NS32057 (G.G.).
Footnotes
Communicating editor: M. Sundaram
Literature Cited
- Apte S. S., 2009. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J. Biol. Chem. 284: 31493–31497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Sharpe P., Culley K., Otero M., Bevan D., et al. , 2012. Dual regulation of metalloproteinase expression in chondrocytes by Wnt-1-inducible signaling pathway protein 3/CCN6. Arthritis Rheum. 64: 2289–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blelloch R., Kimble J., 1999. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature 399: 586–590. [DOI] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Lau L. F., 2009. Functions and mechanisms of action of CCN matricellular proteins. Int. J. Biochem. Cell Biol. 41: 771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Leu S. J., Todorovic V., Lam S. C., Lau L. F., 2004. Identification of a novel integrin alphavbeta3 binding site in CCN1 (CYR61) critical for pro-angiogenic activities in vascular endothelial cells. J. Biol. Chem. 279: 44166–44176. [DOI] [PubMed] [Google Scholar]
- De Stasio E. A., Dorman S., 2001. Optimization of ENU mutagenesis of Caenorhabditis elegans. Mutat. Res. 495: 81–88. [DOI] [PubMed] [Google Scholar]
- Enomoto H., Nelson C. M., Somerville R. P., Mielke K., Dixon L. J., et al. , 2010. Cooperation of two ADAMTS metalloproteases in closure of the mouse palate identifies a requirement for versican proteolysis in regulating palatal mesenchyme proliferation. Development 137: 4029–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga-Kalabis M., Martinez G., Telson S. M., Liu Z. J., Balint K., et al. , 2008. Downregulation of CCN3 expression as a potential mechanism for melanoma progression. Oncogene 27: 2552–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto G., Shimoda M., Okada Y., 2004. ADAMTS4 (aggrecanase-1) interaction with the C-terminal domain of fibronectin inhibits proteolysis of aggrecan. J. Biol. Chem. 279: 32483–32491. [DOI] [PubMed] [Google Scholar]
- Hedgecock E. M., Culotti J. G., Hall D. H., Stern B. D., 1987. Genetics of cell and axon migrations in Caenorhabditis elegans. Development 100: 365–382. [DOI] [PubMed] [Google Scholar]
- Ihara S., Nishiwaki K., 2007. Prodomain-dependent tissue targeting of an ADAMTS protease controls cell migration in Caenorhabditis elegans. EMBO J. 26: 2607–2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S. H., Kramer J. M., 2000. Nidogen is nonessential and not required for normal type IV collagen localization in Caenorhabditis elegans. Mol. Biol. Cell 11: 3911–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y., Kuroki R., Nishiwaki K., 2004. A fibulin-1 homolog interacts with an ADAM protease that controls cell migration in C. elegans. Curr. Biol. 14: 2011–2018. [DOI] [PubMed] [Google Scholar]
- Kubota Y., Ohkura K., Tamai K. K., Nagata K., Nishiwaki K., 2008. MIG-17/ADAMTS controls cell migration by recruiting nidogen to the basement membrane in C. elegans. Proc. Natl. Acad. Sci. USA 105: 20804–20809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutz W. E., Wang L. W., Bader H. L., Majors A. K., Iwata K., et al. , 2011. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J. Biol. Chem. 286: 17156–17167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leask A., Abraham D. J., 2006. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J. Cell Sci. 119: 4803–4810. [DOI] [PubMed] [Google Scholar]
- Maduro M., Pilgrim D., 1995. Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141: 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch D. R., Nelson C. M., Dixon L. J., Silver D. L., Wylie J. D., et al. , 2009. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev. Cell 17: 687–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C. C., Kramer J. M., Stinchcomb D., Ambros V., 1991. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 10: 3959–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., 1999. Mutations affecting symmetrical migration of distal tip cells in Caenorhabditis elegans. Genetics 152: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki K., Hisamoto N., Matsumoto K., 2000. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science 288: 2205–2208. [DOI] [PubMed] [Google Scholar]
- Roberts D. D., 2011. Emerging functions of matricellular proteins. Cell. Mol. Life Sci. 68: 3133–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupka N., Kintakas C., White J. D., Fraser F. W., Hanciu M., et al. , 2013. Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and -15 facilitates myoblast fusion. J. Biol. Chem. 288: 1907–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga A., Hikasa H., Taira M., 2006. Xenopus ADAMTS1 negatively modulates FGF signaling independent of its metalloprotease activity. Dev. Biol. 295: 26–39. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Toyoda H., Sano M., Nishiwaki K., 2006. Chondroitin acts in the guidance of gonadal distal tip cells in C. elegans. Dev. Biol. 300: 635–646. [DOI] [PubMed] [Google Scholar]
- Tamai K. K., Nishiwaki K., 2007. bHLH transcription factors regulate organ morphogenesis via activation of an ADAMTS protease in C. elegans. Dev. Biol. 308: 562–571. [DOI] [PubMed] [Google Scholar]
- Tzeng H. E., Chen J. C., Tsai C. H., Kuo C. C., Hsu H. C., et al. , 2011. CCN3 increases cell motility and MMP-13 expression in human chondrosarcoma through integrin-dependent pathway. J. Cell. Physiol. 226: 3181–3189. [DOI] [PubMed] [Google Scholar]
- Wicks S. R., Yeh R. T., Gish W. R., Waterston R. H., Plasterk R. H., 2001. Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat. Genet. 28: 160–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.