Abstract
In the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation, the Scientific/Educational Session on Autologous Transplantation addressed the role of novel agents and immunomodulatory strategies in management of relapse after autologous hematopoietic stem cell transplantation (AHSCT). Concepts were illustrated through in-depth discussion of multiple myeloma, with broader discussion of areas relevant for relapse of other malignancies as well as in the setting of allogeneic transplantation. Dr. Hari provided an overview of the epidemiology of relapse after AHSCT in multiple myeloma, addressing clinical patterns, management implications, and treatment options at relapse, highlighting the implications of novel therapeutic agents in initial, maintenance and relapse treatment. Dr. Avigan discussed current concepts in tumor vaccine design, including whole-cell and antigen-specific strategies, use of an AHSCT platform to reverse tumor-associated immunosuppression and tolerance, and combining vaccines with immunomodulatory agents to promote establishment of durable antitumor immunity. Dr. Hsu reviewed the immunogenetics of natural killer (NK) cells and general NK biology, the clinical importance of autologous NK activity (e.g., lymphoma and neuroblastoma), as well as the impact of existing therapies on promotion of NK-cell activity (e.g., immunomodulatory drugs, monoclonal antibodies) and strategies for enhancing autologous and allogeneic NK-cell effects through NK-cell gene profiling.
INTRODUCTION
Gains in understanding the biologic effects of antitumor therapy on the immune system yield important insights into the mechanisms of tumor control and relapse after both allogeneic and autologous hematopoietic stem cell transplantation (SCT). In the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation, the Scientific/Educational Session on Autologous Transplantation: Relapse Prevention Using Novel Agents and Immunomodulatory Strategies discussed parallels between autologous and allogeneic SCT platforms with respect to relapse biology, prevention and treatment. Discussion of multiple myeloma (MM) relapse after autologous transplantation illustrated the evolution of disease characteristics following SCT, including the impact of the novel, immunomodulatory agents that are now standard therapies for MM before and after SCT. Immunomodulatory relapse interventions were discussed, including use of vaccine-based tumor targeting and exploitation of NK cell biology to achieve optimal treatment outcomes.
I. RELAPSE AFTER AUTOLOGOUS HEMATOPOIETIC CELL TRANSPLANTATION: THE MULTIPLE MYELOMA PARADIGM
MM epitomizes many of the challenges posed by relapse after SCT, with disease being the major cause of treatment failure and rapid evolution of diagnostic and therapeutic tools yielding tectonic shifts in the clinical landscape. The following discussion of MM relapse after autologous transplant (AHSCT) provides a paradigm for considering new approaches to prevention and treatment of post-transplant relapse – approaches which could extend to AlloSCT as well.
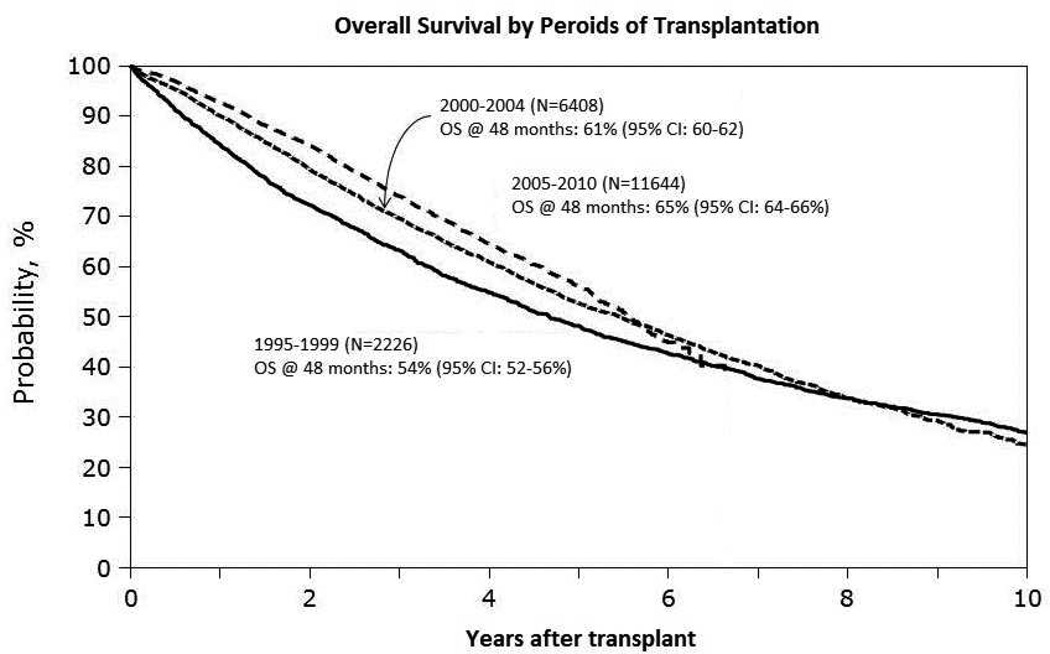
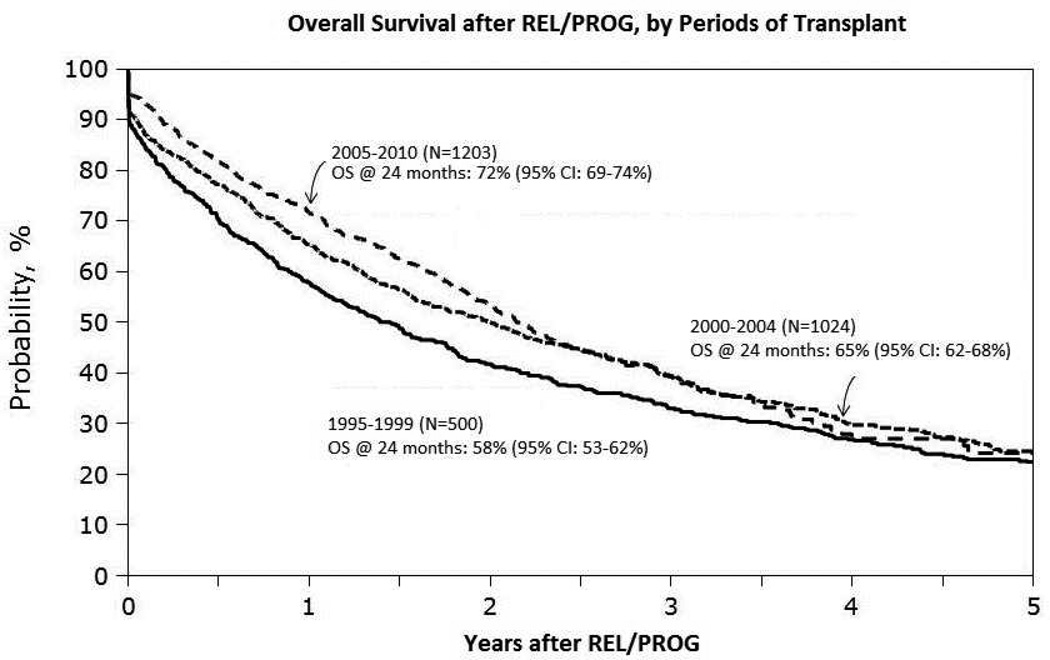
Major advances in MM drug therapy have led to superior induction regimens, better autologous hematopoietic stem cell transplantation (AHSCT) outcomes and improved relapse survival after relapse (1). Many patients now receive upfront AHSCT with sensitive disease in complete or very good partial remission (CR or VGPR) and achieve higher rates of post-transplant CR leading to superior progression-free survival (PFS). Novel agents – immunomodulatory drugs (thalidomide derivatives, IMiDs) and proteasome inhibitors (PI) have also improved survival following post-transplant progression (2). Analysis of the Center for International Blood and Marrow Transplant Research (CIBMTR) registry data on more than 20,000 recipients of upfront AHSCT for MM demonstrated improved five-year overall survival (OS) over time, including major gains in post-transplant relapse survival (3) (Figures 1 & 2).
FIGURE 1.
CIBMTR Analysis of Survival Trends over Time after AHSCT for MM. A. Kaplan-Meier estimates of OS after AHSCT for patients who received AHSCT between 1995–1999, 2000–2004 and 2005–2010. B. OS following myeloma relapse/progression after AHSCT as reported to the CIBMTR for patients who relapsed between 1995–1999, 2000–2004 and 2005–2010 (3).
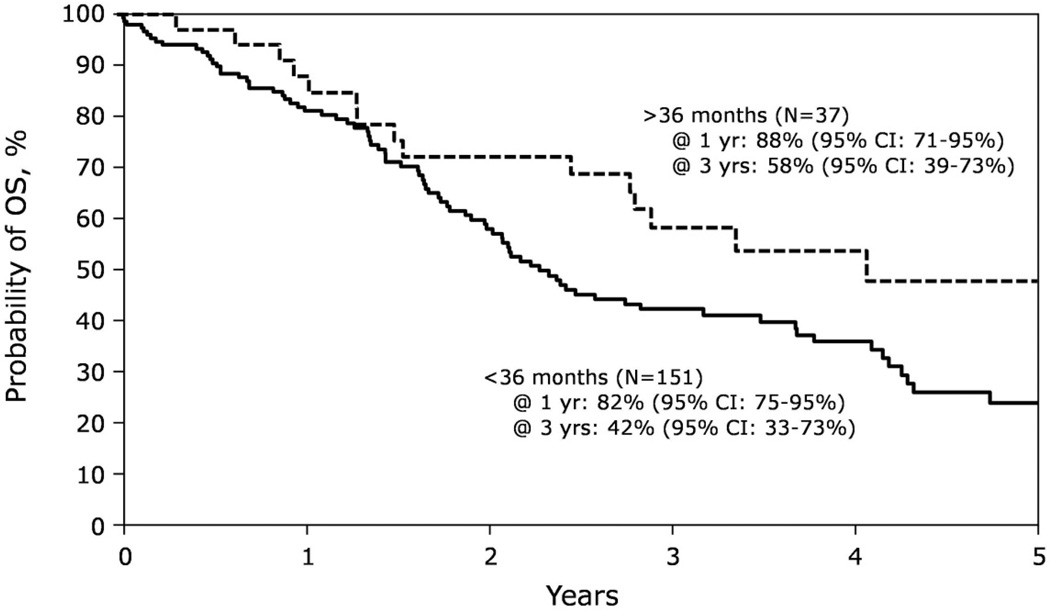
FIGURE 2.
Salvage Second-AHSCT for Relapsed MM. Kaplan-Meier estimates of OS after salvage second-AHSCT for multiple myeloma relapse, stratified by time to relapse after first AHSCT (<36 months vs. >36 months), as reported to the CIBMTR (30).
Diagnosing Relapse
Defining relapse of MM post-transplant is complicated; persistence or recurrence of a myeloma clone detected biochemically does not reliably predict clinical progression after either AHSCT or allogeneic SCT (4). Even absent CR, a significant proportion of patients treated with AHSCT will achieve prolonged periods of an “MGUS-like” state. Additionally, biochemical monitoring may miss progression after AHSCT; clinical relapse frequently involves a non-secretory component and aggressive relapse often presents as extramedullary disease (EMD), with discordance between imaging, e.g., MRI, PET and biochemical disease parameters (5). These distinctions have been addressed in few published studies. While current guidelines distinguish biochemical versus clinical progression, most prior studies utilized biochemical criteria (6), limiting interpretation of relapse presentation, incidence and outcome of AHSCT relapse by reliance upon traditional criteria.
Clinical Behavior of Relapsed MM
Relapsed myeloma differs from newly diagnosed disease in clinical behavior and biologic correlates (7). OS from first relapse is generally shorter than the progression-free interval following transplant, although distinct clinical patterns are evident: relapse after early AHSCT (≤ three prior regimens) with expected sensitivity to IMiD or PI therapy; relapse or transplant-refractory disease resistant to prior IMiD/PI therapy (“double-refractory disease”); and primary-refractory disease. Retrospective data show outcomes of patients with AHSCT relapse have improved over time, coincident with the introduction of novel agents (8). The presentation of clinical relapse is similarly variable and influenced by monitoring methods and frequency. In a Spanish registry study, two-thirds of AHSCT recipients developed “classical” MM relapse, with increase in M-spike and organ impairment; EMD and secondary plasma cell leukemia (sPCL) signified relapse in 14% and 2%, respectively, whereas 18% presented initially with isolated biochemical relapse (9).
In patients relapsing in the era preceding novel-agents (1985–98), Kumar et al., reported a median OS of 17 months, with 84% dying by 5 years (8). Coincident with the introduction of IMiD and PI therapies, survival has improved for patients with first relapse after 2000, compared to those diagnosed with initial relapse before that year (23.9 v 11.8 months, respectively) (1). A recent international survey suggested a response rate of 58% to first-salvage treatment after AHSCT, with progression-free survival (PFS) and OS of 13 and 25 months, respectively (with diminishing response rates to successive salvage regimens) (10). Consistent with the contribution of modern agents, the addition of bortezomib to thalidomide/dexamethasone therapy appears to improve outcomes (median OS of three years) (11), although the benefit does not seem to extend to those with double-refractory MM (12). Current practice and ongoing investigations integrate novel agents into upfront MM therapy and AHSCT consolidation and/or maintenance; the impact of these changes on AHSCT outcomes and survival after relapse remains to be defined. However, the poor outcomes seen in the double-refractory setting suggest that effective therapy for relapse in patients transplanted in the current era may be difficult to achieve with available agents.
Long-Term Survivorship
Even in the pre-novel agent era, a minority had prolonged CR or non-progressive PR (MGUS-like clonal persistence). A 12-year disease-free interval from AHSCT has been suggested as the threshold after which clinical progression is rare (13), and is often used to define the “cure fraction” or “operationally cured” for MM therapy. In their Total Therapy 1 tandem-AHSCT trial (1989–1998), Barlogie et al. showed 33% and 17% OS at ten and 15 years, respectively, with 15% and 7% EFS and 18% and 12% maintaining CR (14). CIBMTR data suggest that at ten years 25 – 29% of AHSCT recipients are alive and 10 – 15% are progression-free (15).
Long-term PFS may reflect immune-mediated disease control (98). Superior immune reconstitution has been observed in AHSCT recipients with long-term disease-free survival (DFS) (16, 17), who demonstrate increased numbers of CD8+ T cells, NK cells, dendritic cells and normal plasma cells. Further, regulatory T cells (“Tregs”) were found to be reduced, potentially favoring the action of cytotoxic cells, recovery of B-cell production and homing of normal plasma cells into the BM (18).
Predicting Relapse Risk
With adoption of increasingly stringent response criteria, a clear association has emerged between complete response and EFS and OS after AHSCT. As many recipients with clinical CR will ultimately relapse, MRD monitoring with increasingly sensitive PCR-based and multi-parameter flow cytometry (MFC) methods of detecting clonal plasma cell populations is being evaluated to identify risk of relapse (19). PCR-based methods are highly sensitive (10−4 – 10−6), but informative sequences cannot readily identify the myeloma clone for a substantial minority of patients. While initial studies of PCR methods found molecular evidence of myeloma in nearly all patients with clinical CR, retrospective studies of patients who had achieved immunofixation-defined CR after AlloSCT identified molecular CR (mCR) in half (20), more mCR following treatment with AlloSCT than AHSCT (21) and that mCR predicted less relapse and longer relapse-free survival (22). While somewhat less sensitive than PCR (10−4), MFC can distinguish clonal from normal bone marrow plasma cells in nearly all patients with MM (23); MFC detection of MRD at Day 100 after AHSCT has been shown to predict relapse and survival (24, 25). While both PCR and MFC assessment of MRD demonstrate that deeper levels of remission after therapy for MM correlate with superior PFS, no level of remission that equates to a cure has been defined to date and MRD monitoring remains available only in the research setting. Nonetheless, MRD techniques validated in the AHSCT setting (26) may identify patients whose relapse risk warrants additional maintenance or immune-based intervention post-transplant.
Recent advances in gene sequencing technology have opened exciting new avenues of cancer investigation with potential implications for defining relapse risk (98). Analyses of the genomic changes that underlie disease progression demonstrate distinct patterns of clonal dynamics (26, 27). In patients with high-risk MM, for example, disease is characterized by clonal heterogeneity at diagnosis and by genomic instability, likely determinants of evolution toward increasingly aggressive disease. How to integrate target genomic complexity therapeutically should be a high priority for future research.
Maintenance and Consolidation Strategies to Prevent Relapse
Efforts to improve outcomes after AHSCT include consolidation (short-course therapy applied to eradicate residual disease) or maintenance (prolonged lower-intensity therapy applied to prevent relapse). While improving PFS, prevention strategies have not consistently resulted in OS benefits; further, the effect of these strategies on “operational cure” rates are undetermined (Table 1). While there appears little doubt that deeper remissions yield PFS benefit, many issues regarding preventive interventions remain unresolved: the optimal regimen, schedule and duration of preventive therapy; in whom preventive therapy is unnecessary or, perhaps, harmful; the potential for emergence of resistant clones; and how preventive therapy compares with early retreatment in terms of overall survival and quality of life.
Table 1.
Maintenance / Consolidation Strategies After AHSCT – Recent Studies
| Study | Agent | Comment | Median PFS / TTP in Superior arm |
|---|---|---|---|
| CALGB 100104/CTN McCarthy P et al., N Engl J Med. 2012; 10;366(19):1770. |
Maintenance: Lenalidomide vs. Placebo |
Favored lenalidomide: TTP 46 mo vs. 27 mo. Overall mortality: 15 vs. 23% 3-Yr OS: 88% vs. 80% |
Lenalidomide TTP 46 mo |
| IFM Study Attal M et al., N Engl J Med. 2012; 10;366(19):1782. |
Consolidation/Maintenance: Lenalidomide x 2mo → Lenalidomide vs. Placebo |
PFS favored lenalidomide: 41 mo vs. 23 mo. No survival benefit: >70% in both groups alive at 4 years |
Lenalidomide PFS 41 mo |
| HOVON 65-GMMG-HD4 Sonneveld et al., J Clin Oncol. 2012; 20;30(24):2946. |
Induction/Maintenance: Bortezomib-based Induction/Maintenance vs. Conventional ChemoRx Induction and Thalidomide Maintenance |
Role of maintenance was difficult to separate. Bortezomib arm superior (PFS 35mo vs. 28mo; OS benefit in multivariate models). |
Bortezomib induction/maintenance PFS 35 mo |
| MRC Myeloma IX Trial Morgan G et al., Blood. 2012; 5;119(1):7. |
Maintenance: Thalidomide vs. None after intensive or non-intensive upfront therapy |
52% stopped thalidomide. PFS superior for thalidomide but OS similar. Patients with high-risk FISH had worse OS on thalidomide. |
Thalidomide/AHSCT PFS 30 mo |
Salvage Treatment of Relapse
Treatment options for MM relapse post-AHSCT are often limited by host and/or disease-related factors. Efficacy of bortezomib and lenalidomide in relapse has been demonstrated in phase 3 trials and is reflected by improved post-relapse survival since the introduction of novel agents (1). Novel-agent based combinations (extensively reviewed (28)) appear to improve efficacy and can achieve clinically meaningful responses, as exemplified by results of the recently reported phase 3 comparison of thalidomide/dexamethasone (TD) with or without bortezomib (VTD) for progression or relapse after AHSCT.
Patients relapsing within 12 to 18 months of AHSCT are at very high risk for rapid progression and early mortality. Even when remission can be reinduced, post-remission strategies to prevent subsequent relapse have not been established. Late relapse after AHSCT (beyond 36 months) has a variable prognosis, perhaps influenced by employment of maintenance therapy. Patients with biochemical MM progression while receiving lower-dose maintenance have many treatment options, including use of ”treatment” doses of the maintenance agent. In contrast, patients with late clinical relapse and rapid MM progression may require combination approaches to reinduce remission, with second-AHSCT offering potentially meaningful clinical benefit.
Outcomes after relapse following current multiagent induction-AHSCT-consolidation-maintenance protocols have yet to be determined. From the University of Arkansas’s “total therapy” approach (TT), comparison between successive iterations suggests an overall benefit to upfront use of the entire therapeutic arsenal, yet it may render subsequent relapse more difficult to treat (29). Several new therapeutic targets have been identified in MM, and small-molecule inhibitors as well as novel monoclonal antibodies are in clinical development for relapse treatment (Table 2). Broadly, second generation PIs and IMiDs show promising single-agent activity, and histone deacetylase inhibitors, phosphatidylinositol 3-kinase pathway inhibitors and cyclin-dependent kinase inhibitors are being tested in combination with novel agents.
Table 2.
Newer Agents in Trials for Relapsed MM
| Drug Compounds | ||
| Agent | Therapeutic Class | Comment |
|---|---|---|
| Carfilzomib | Irreversible PI, intravenous | Accelerated approval by FDA; lower risk of neuropathy |
| Ixazomib (MLN9708) | Reversible PI, oral | Phase 1 and 2 studies |
| Oprozomib (ONX 0912) | Irreversible PI, oral | Phase 1 and 2 studies |
| Marizomib (NPI-0052) | Irreversible PI, intravenous | Phase 1 and 2 studies |
| Pomalidomide | Novel IMiD, oral | Accelerated approval by FDA |
| Vorinostat | Histone deacetylase (HDAC) inhibitor, oral |
Phase 2 and 3 studies, with novel agents |
| Panabinostat (LBH589) | HDAC inhibitor, oral | Phase 2 and 3 studies, with novel agents |
| Rocilinostat (ACY-1215) | HDAC6-selective inhibitor, oral |
Phase 1 and 2 studies, with novel agents |
| AT7519 | Cyclin-dependent kinase (CDK) inhibitor, intravenous |
Phase 1/2 study, with bortezomib |
| Monoclonal Antibodies | ||
| Antibody | Target | Comment |
| Elotuzumab | CS1 | Phase 3, with lenalidomide |
| Daratumumab | CD38 | Phase 2 ongoing |
| SAR650984 | CD38 | Phase 1 studies ongoing, with lenalidomide |
| BT062 | CD138 | Phase 1/2a (DM-4 conjugate), with lenalidomide and dexamethasone, ongoing |
| Lorvotuzumab | CD56 | Phase 1 (DM-1 conjugate), with lenalidomide and dexamethasone, ongoing |
| Milatuzumab | CD74 | Phase 1/2 study (doxorubicin conjugate) ongoing |
Salvage second AHSCT is increasingly performed, with significantly greater clinical benefit for those with late MM relapse (30)(Figure 2), and significantly better than salvage AlloSCT after AHSCT relapse (31). In spite of a long-demonstrated graft-versus-myeloma effect, TRM has significantly limited the therapeutic benefit of AlloSCT for MM; with employment of reduced-intensity conditioning regimens, treatment-related morbidity and mortality are modestly reduced but increased relapse negates any survival benefit over AHSCT (32). Several randomized trials evaluating the tandem ASCT-alloHCT approach to reduce the risk of relapse in the upfront transplant setting with discordant results (33–36). A recent meta-analysis of the published AHSCT-AlloSCT vs. AHSCT studies concluded that while CR rates are higher for alloHCT, neither EFS nor OS benefit could not be consistently demonstrated (37). Thus while AlloSCT may be potentially curative, GVHD and TRM remain prohibitive.
II. THE PROMISE OF CANCER VACCINES AS POSTTRANSPLANT IMMUNOTHERAPY
The potential promise of cellular immunotherapy in targeting hematological malignancies is highlighted by the observation that allogeneic SCT is uniquely curative for a subset of patients with relapsed or high-risk disease with disease responses to donor lymphocyte infusion (DLI) suggesting that lymphocytes mediate the therapeutic effect (38–40). However, the lack of specificity of donor lymphocytes limits response rates and results in considerable morbidity and mortality from graft-versus-host disease (GVHD), driving efforts to develop a safer and more effective means of harnessing cell-mediated anti-tumor immune responses. A promising alternative immunotherapeutic strategy is the use of cancer vaccines to educate immune effector cells in an effort to establish antitumor immunity. The design of an effective tumor vaccine is dependent on optimizing antigen presentation, reversing critical elements of tumor mediated immune suppression, and the prevention of re-establishment of tumor tolerance.
Tumor Vaccines
Tumor cells bear unique antigens that differentiate them from normal tissues and serve as potential targets for immune effector cells. Candidate antigens for acute myeloid leukemia (AML) have included WT1 (41, 42), proteinase 3 (PR1 peptide) (43, 44), RHAMM (45), Aurora-A (46), PRAME (47), and MUC1 (48). These antigens may be shared by the primitive stem cell population allowing for the potential targeting of this critical source of disease relapse. In multiple myeloma, a variety of tumor associated antigens have been identified that may be selectively targeted by host immunity. These include the cancer testis antigens, MUC1, HM1.24, CYP1B1, SP17, PRAME, Wilm’s Tumor 1 (WT-1), and heat shock protein gp96 (49–52). Despite the presence of antigenic targets, tumor cells evade immune recognition through the presentation of antigen in the absence of co-stimulation and in the context of inhibitory factors that promote tolerance and immune escape (53). In contradistinction, cancer vaccines are engineered to enhance the presentation of tumor-associated antigens resulting in antigen specific T cell activation and expansion. A recent follow-up of a phase 1 clinical trial evaluating a WT1 peptide-based vaccine reported three of eight AML patients treated with MRD had remained in remission for at least eight years (54).
In order to enhance effective presentation of tumor-associated antigens in vivo, dendritic cell vaccine strategies have been evaluated. Dendritic cells (DCs) are potent antigen-presenting cells that can be generated in large numbers ex vivo; tumor antigens and peptides can be loaded onto ex-vivo generated DCs and used as a tumor vaccine (55). Clinical studies have demonstrated successful expansion of antigen-specific T cells following administration of peptide-loaded DC vaccines, with potential implications for prevention and treatment of relapse (54, 56–59).
Vaccine strategies designed to induce T cell responses directed against an individual antigen are potentially limited by tumor escape through the down-regulation of the target antigen. Alternatively, the use of whole tumor cells as a source of antigen may elicit a broader, i.e., polyclonal T-cell response. One novel approach induces differentiation of DCs from myeloid leukemia blasts ex vivo in an effort to create a potent antigen-presenting cell that expresses a panel of autologous leukemia-derived antigens (60, 61). Potential concerns include the functional capacity of engineered antigen-presenting cells in vivo as well as loss of antigens characteristic of the primitive clone in the process of differentiation. The GVAX vaccine approach uses tumor cells genetically engineered to express GM-CSF resulting in recruitment of native DCs to the site of vaccination and their subsequent processing and presentation of antigens. In a phase 1 study, vaccination of patients with high-risk AML was well tolerated and associated with immune response and an attendant low risk of relapse (62). We have developed a vaccine model in which patient-derived AML or multiple myeloma cells are fused with autologous DCs such that a broad array of tumor antigens are presented in the context of DC mediated costimulation, resulting in the expansion of both tumor-specific helper and cytotoxic T lymphocyte (CTL) populations (63, 64). In a phase 1 study, vaccination of patients with DC/myeloma fusions was associated with the expansion of myeloma-specific T cells and resulted in disease stabilization in a majority of patients with advanced disease (65).
Vaccination following AHSCT
A major limitation to developing an effective immunologic and clinical response to vaccination involves the immunosuppressed milieu characteristic of patients with advanced malignancy. As such, AHSCT offers a unique platform for examining the efficacy of tumor vaccine therapy. High-dose chemotherapy results in tumor cytoreduction as well as immunomodulatory effects of depleting regulatory T cells and disruption of tumor tolerance. Preclinical models have demonstrated transient enhanced response to tumor vaccines in the early period of immune reconstitution following high dose chemotherapy (66). In a phase 2 clinical trial, administration of GVAX in conjunction with AHSCT in AML patients induced antitumor immune responses in the majority of patients, and a reduction in residual disease in a subset (67).
In study of patients with multiple myeloma undergoing therapy with AHSCT in conjunction with DC/myeloma fusions, vaccination resulted in the expansion of myeloma-specific T cells and conversion from partial to complete responses in a subset of patients (68). Of note, the posttransplant period was associated with significant reduction in circulating regulatory T cells. In another study, vaccination with an idiotype-based vaccine following AHSCT was associated with improved outcomes as compared to a historical cohort of patients treated with transplantation alone (69). Response to vaccination in the posttransplant period may be augmented by the administration of lymphocytes activated and expanded by CD3/CD28 costimulation ex vivo (70–72).
Immunomodulation to Enhance Response to Vaccination
Another approach to increase vaccine potency is the adjunctive use of immunomodulatory agents, which may promote expansion of memory T cell populations critical to durable responses. Tumor-associated factors that inhibit DC maturation and activation, e.g., VEGF and IDO, are potential therapeutic targets to enhance vaccine activity. Ipilumumab blocks signaling via the negative T-cell regulatory checkpoint, CTLA-4, and has recently received FDA approval as an immunotherapy for advanced stage melanoma (73–75). Its use is now being explored as a potential vaccine adjuvant (76). The PDL1-PD-1 pathway is a critical mediator of tolerance in the setting of chronic viral infection and malignancy, by inhibiting both DC-mediated stimulation of T cells and CTL-mediated killing of tumor targets (77, 78). PD-1 and PDL-1 directed antibodies are currently being studied as immunotherapeutic agents. Preclinical studies have demonstrated that vaccination with DC/MM fusions in conjunction with PD-1 blockade results in suppression of vaccine-induced expansion of regulatory T cells, increased CTL expansion and enhanced myeloma-specific CTL responses (79). Clinical studies are exploring whether PD-1 blockade can facilitate establishment of durable vaccine-mediated immune responses are underway.
In summary, tumor vaccines hold promise as means of eliminating residual disease and preventing disease recurrence following AHSCT. Novel immunomodulatory agents and immune checkpoint blockade may have the potential to augment vaccine-induced memory T cell responses in vivo. While results of preclinical and early-phase clinical studies show promise, evaluating vaccine efficacy in the setting of minimal disease is a challenge that ultimately will require evaluation in the context of randomized clinical trials with survival endpoints.
III. NK CELL IMMUNOGENETICS: IMPLICATIONS FOR RELAPSE RISK AND PREVENTION
The role of innate immunity in tumor recognition and response has been recognized for decades, with the name bestowed to natural killer (NK) cells based on their ability to kill tumor targets. The clinical importance of this innate surveillance system for diseased cells (i.e., infected, transformed, and injured cells) was first documented in patients lacking functional NK activity, whose recurrent viral infections and lymphoproliferative disorders would lead to early demise (80, 81). Since the identification of the NK cell, investigators have sought to capture their antitumor effects for the treatment of cancer. Their efforts, however, were initially slowed by deficiencies in understanding the molecular mechanisms controlling NK activity, specifically the receptor-ligand relationships dually responsible for conferring NK effector potential against nonself targets while simultaneously preventing NK reactivity to autologous cells. This report will review the NK cell surface receptors and ligands important for NK education, highlighting the clinical situations in which specific NK subsets are most effective, and emphasizing pharmacologic and genetic approaches to exploit NK antitumor effects.
NK Receptors and Ligands
The NK cell is studded with an array of inhibitory and activating receptors, whose signals integrate to dictate NK response. Receptors in humans include the inhibitory and activating forms of the killer Ig-like receptors (KIR), the CD94/NKG2A heterodimer, the natural cytotoxicity receptors (NKp46, NKp44, and NKp30), NKG2D, the signaling lymphocytic activation molecule (SLAM) receptors, DNAM1, and the Fcγ receptor IIIA (CD16). While seemingly complex, the receptor-ligand relationships can be generally distilled into two types: 1) recognition of self-determinants, such as self-HLA, resulting in NK tolerance to normal autologous cells and ignorance to tumor cells with upregulated HLA; and 2) detection of “stress-induced self molecules” normally expressed at low levels on normal cells but upregulated on infected or transformed cells, resulting in NK activation and tumor toxicity. In humans, stress-induced molecules include the NKp30 ligand B7H6 and the NKG2D ligands MICA, MICB, and ULBP family members. Fine-tuning the response is the adaptor molecule SAP, which is critical for the activating properties of the SLAM receptor, particularly in its response to hematopoietic target cells. In addition, tumor targets can avoid NK toxicity by expressing class I molecules and the inhibitory molecule 4Ig-B7H3. A separate category of NK-tumor interaction occurs through CD16, whose binding of therapeutic monoclonal antibodies triggers ADCC.
Diversity of the Functional NK Repertoire
Thus, NK-tumor interactions can involve simultaneous mechanisms of NK activation and inhibition signals. The degree of the NK cell’s response, however, is highly dependent on its “education” or licensed capacity for killing, where the molecular interactions most closely linked to education are between the KIR receptors and their HLA class I ligands, HLA-C1, HLA-C2, and HLA-Bw4. Not only is interaction between the inhibitory receptor and its self-HLA ligand important for signaling tolerance to self, it is also required for the NK cell to achieve functional competence (82), where high-affinity KIR-HLA allotype combinations not only lead to higher functional capacity, but also greater potency of inhibition (83). Expression of receptors occurs stochastically, leading to a repertoire that is diverse in number and abundance of receptors on the cell surface. Functionally translated, this phenotypic diversity results in a continuum of response to target cells. In addition, KIR and HLA regions segregate independently, leading to frequent genetic combinations where individuals lack one or more class I ligands for autologous KIR (missing ligand). In these individuals, unlicensed NK cells expressing inhibitory KIR for nonself HLA represent a significant proportion of the NK repertoire and are not sensitive to class I expression on the target cell.
Harnessing Autologous NK Effects
An understanding of KIR-HLA protein interactions and their perturbation in various disease states is useful in designing effective strategies to exploit autologous and allogeneic NK activity against cancer. Robust NK reconstitution has been associated with improved outcomes for AML, non-Hodgkin lymphoma, and multiple myeloma following autologous hematopoietic cell transplantation (HCT) (84, 85) indicating that NK proliferation agents, such as IL-15, may enhance antitumor effects. Patients undergoing autologous HCT for various solid tumors experience an improved outcome if the patient is missing one or more KIR ligands (86). Among these tumors, high-risk neuroblastoma is particularly informative, as its treatment typically includes anti-GD2 monoclonal antibody therapy, whose primary mechanism of action is through NK-mediated ADCC. Unlicensed NK cells expressing KIR for nonself HLA are highly capable of ADCC with anti-GD2 antibody, while licensed NK cells expressing KIR for self-HLA are inhibited by induced class I expression on the tumor surface (87). Because NK cells mediate ADCC for other monoclonal antibodies in clinical use, including the anti-CD20 antibody Rituximab, it will be important to establish whether KIR-HLA genotypes predictive of missing ligand are similarly advantageous. Two novel pharmacologic approaches hold promise in this setting: monoclonal antibody activation of CD137 to enhance ADCC (88); and anti-KIR antibodies to interfere with class I-mediated inhibition of licensed NK cells (89).
New options in the treatment of multiple myeloma enlist higher NK activity as mechanisms of their efficacy (90). Proteasome inhibitors decrease class I expression and upregulate NKG2D ligand expression on myeloma cells; and immunomodulatory drugs such as lenalidomide enhance NK function through induced T-cell production of IL-2, downregulation of PD-L1, increased natural cytotoxicity and heightened ADCC. Promising new approaches include the anti-PD1 antibody and the anti-inhibitory KIR antibody to overcome inhibitory signaling from the myeloma target, as well as the anti-CD1 monoclonal antibody, which targets CD1-expressing myeloma cells and recruits NK cells for ADCC activity.
Capturing Allogeneic NK Effects
The first clinical setting to harness the potential of the allogeneic NK cell was HLA-mismatched HCT, where alloreactive donor NK cells stimulated by missing self demonstrated potent effects against AML (91). Since then, it has become clear that donor NK alloreactivity can occur in the HLA-matched setting as well. Cytokine-enhanced activity of unlicensed NK cells (92) likely contribute to reduced AML relapse among patients lacking any KIR ligand (93), and not just ligands present in the donor. Donor activating KIR are also associated with reduced relapse(94) and decreased mortality (95). Activating KIR, however, are not all similar. KIR2DS1, the only activating KIR for which a ligand is known, is important in NK education and tolerance, and KIR2DS1-positive cells from HLA-C2 homozygous individuals have reduced function compared to KIR2DS1-positive cells from HLA-C1 individuals (96). Translated to the clinical setting, KIR2DS1-positive donors are associated with lower relapse in AML patients, but only if the donor is not HLA-C2 homozygous (97). The possibility of improving transplant outcomes by incorporating KIR gene and allele typing into donor selection algorithms is now real, and prospective trials demonstrating the effectiveness of donor KIR-HLA gene profiling in donor selection will be an important step in broadening the frontiers of transplantation.
IV. FUTURE DIRECTIONS & RESEARCH PRIORITIES
A major achievement of the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation was the establishment of an international consortium of investigators with the objective of conducting collaborative research in the field of relapse after HSCT. This session highlighted the far-reaching effects of novel agents and immunomodulatory interventions on the clinical manifestations of relapse and the implications for possible relapse prevention and treatment trials. The discussions identified opportunities to define and therapeutically exploit areas of common as well as distinct biology between the allogeneic and autologous SCT platforms – and the potential to improve treatment outcomes in both.
ACKNOWLEDGMENTS
Xiaobo Zhong, Center for International Blood and Marrow Transplant Research, Milwaukee, WI, provided statistical assistance on multiple myeloma survival data. This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and National Heart, Lung and Blood Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 3.Costa LJ, Zhong X, Zhang M-J, et al. Trends in Utilization and Outcomes of Autologous Hematopoietic Cell Transplantation (AHCT) in the Upfront Management of Patients with Multiple Myeloma: A CIBMTR Analysis. ASH Annual Meeting Abstracts. 2012;120:596. [Google Scholar]
- 4.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 5.Bartel TB, Haessler J, Brown TL, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–2076. doi: 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laubach JP, Mitsiades CS, Mahindra A, et al. Management of relapsed and relapsed/refractory multiple myeloma. J Natl Compr Canc Netw. 2011;9:1209–1216. doi: 10.6004/jnccn.2011.0098. [DOI] [PubMed] [Google Scholar]
- 8.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 9.Alegre A, Granda A, Martinez-Chamorro C, et al. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87:609–614. [PubMed] [Google Scholar]
- 10.Durie BG, Moreau P, Sonneveld P, et al. Regional differences in the treatment approaches for relapsed multiple myeloma: An IMF study. ASCO Meeting Abstracts. 2012;30:8095. [Google Scholar]
- 11.Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 Randomized Phase III Trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 12.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Lopez J, Blade J, Mateos MV, et al. Long-term prognostic significance of response in multiple myeloma after stem cell transplantation. Blood. 2011;118:529–534. doi: 10.1182/blood-2011-01-332320. [DOI] [PubMed] [Google Scholar]
- 14.Barlogie B, Zangari M, Bolejack V, et al. Superior 12-year survival after at least 4-year continuous remission with tandem transplantations for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:469–474. doi: 10.3816/CLM.2006.n.027. [DOI] [PubMed] [Google Scholar]
- 15.Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 16.Hiwase DK, Hiwase S, Bailey M, Bollard G, Schwarer AP. Higher infused lymphocyte dose predicts higher lymphocyte recovery, which in turn, predicts superior overall survival following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008;14:116–124. doi: 10.1016/j.bbmt.2007.08.051. [DOI] [PubMed] [Google Scholar]
- 17.Pessoa de Magalhaes RJ, Vidriales MB, Paiva B, et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica. 2012;98:79–86. doi: 10.3324/haematol.2012.067272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart AJ, Jagasia MH, Kim AS, Mosse CA, Savani BN, Kassim A. Minimal residual disease in myeloma: are we there yet? Biol Blood Marrow Transplant. 2012;18:1790–1799. doi: 10.1016/j.bbmt.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Corradini P, Voena C, Tarella C, et al. Molecular and clinical remissions in multiple myeloma: role of autologous and allogeneic transplantation of hematopoietic cells. J Clin Oncol. 1999;17:208–215. doi: 10.1200/JCO.1999.17.1.208. [DOI] [PubMed] [Google Scholar]
- 20.Martinelli G, Terragna C, Zamagni E, et al. Molecular remission after allogeneic or autologous transplantation of hematopoietic stem cells for multiple myeloma. J Clin Oncol. 2000;18:2273–2281. doi: 10.1200/JCO.2000.18.11.2273. [DOI] [PubMed] [Google Scholar]
- 21.Corradini P, Cavo M, Lokhorst H, et al. Molecular remission after myeloablative allogeneic stem cell transplantation predicts a better relapse-free survival in patients with multiple myeloma. Blood. 2003;102:1927–1929. doi: 10.1182/blood-2003-01-0189. [DOI] [PubMed] [Google Scholar]
- 22.Rawstron AC, Davies FE, DasGupta R, et al. Flow cytometric disease monitoring in multiple myeloma: the relationship between normal and neoplastic plasma cells predicts outcome after transplantation. Blood. 2002;100:3095–3100. doi: 10.1182/blood-2001-12-0297. [DOI] [PubMed] [Google Scholar]
- 23.Paiva B, Gutierrez NC, Rosinol L, et al. High-risk cytogenetics and persistent minimal residual disease by multiparameter flow cytometry predict unsustained complete response after autologous stem cell transplantation in multiple myeloma. Blood. 2012;119:687–691. doi: 10.1182/blood-2011-07-370460. [DOI] [PubMed] [Google Scholar]
- 24.Rawstron AC, Child JA, de Tute RM, et al. Minimal Residual Disease Assessed by Multiparameter Flow Cytometry in Multiple Myeloma: Impact on Outcome in the Medical Research Council Myeloma IX Study. J Clin Oncol. 2013;31:2540–2547. doi: 10.1200/JCO.2012.46.2119. [DOI] [PubMed] [Google Scholar]
- 25.Paiva B, Martinez-Lopez J, Vidriales MB, et al. Comparison of immunofixation, serum free light chain, and immunophenotyping for response evaluation and prognostication in multiple myeloma. J Clin Oncol. 2011;29:1627–1633. doi: 10.1200/JCO.2010.33.1967. [DOI] [PubMed] [Google Scholar]
- 26.Egan JB, Shi CX, Tembe W, et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood. 2012;120:1060–1066. doi: 10.1182/blood-2012-01-405977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120:1067–1076. doi: 10.1182/blood-2012-01-405985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venner CP, Connors JM, Sutherland HJ, et al. Novel agents improve survival of transplant patients with multiple myeloma including those with high-risk disease defined by early relapse (<12 months) Leuk Lymphoma. 2011;52:34–41. doi: 10.3109/10428194.2010.531409. [DOI] [PubMed] [Google Scholar]
- 29.Usmani SZ, Crowley J, Hoering A, et al. Improvement in long-term outcomes with successive Total Therapy trials for multiple myeloma: are patients now being cured? Leukemia. 2012;27:226–232. doi: 10.1038/leu.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michaelis LC, Saad A, Zhong X, et al. Salvage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. 2013;19:760–766. doi: 10.1016/j.bbmt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freytes CO, Vesole DH, Zhong X, et al. Second Transplants in Relapsed Multiple Myeloma (MM): Autologous (AHCT) Versus Non-Myeloablative/Reduced Intensity (NST/RIC) Allogeneic Transplantation (AlloHCT) ASH Annual Meeting Abstracts. 2011;118:824. [Google Scholar]
- 32.Koehne G, Giralt S. Allogeneic hematopoietic stem cell transplantation for multiple myeloma: curative but not the standard of care. Curr Opin Oncol. 2012;24:720–726. doi: 10.1097/CCO.0b013e328358f619. [DOI] [PubMed] [Google Scholar]
- 33.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 34.Giaccone L, Storer B, Patriarca F, et al. Long-term follow-up of a comparison of nonmyeloablative allografting with autografting for newly diagnosed myeloma. Blood. 2011;117:6721–6727. doi: 10.1182/blood-2011-03-339945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krishnan A, Pasquini MC, Logan B, et al. Autologous haemopoietic stem-cell transplantation followed by allogeneic or autologous haemopoietic stem-cell transplantation in patients with multiple myeloma (BMT CTN 0102): a phase 3 biological assignment trial. Lancet Oncol. 2011;12:1195–1203. doi: 10.1016/S1470-2045(11)70243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gahrton G, Iacobelli S, Bjorkstrand B, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–5063. doi: 10.1182/blood-2012-11-469452. [DOI] [PubMed] [Google Scholar]
- 37.Armeson KE, Hill EG, Costa LJ. Tandem autologous vs autologous plus reduced intensity allogeneic transplantation in the upfront management of multiple myeloma: meta-analysis of trials with biological assignment. Bone Marrow Transplant. 2013;48:562–567. doi: 10.1038/bmt.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tricot G, Vesole DH, Jagannath S, Hilton J, Munshi N, Barlogie B. Graft-versus-myeloma effect: proof of principle. Blood. 1996;87:1196–1198. [PubMed] [Google Scholar]
- 39.Zeiser R, Bertz H, Spyridonidis A, Houet L, Finke J. Donor lymphocyte infusions for multiple myeloma: clinical results and novel perspectives. Bone Marrow Transplant. 2004;34:923–928. doi: 10.1038/sj.bmt.1704670. [DOI] [PubMed] [Google Scholar]
- 40.Kolb HJ, Schattenberg A, Goldman JM, et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood. 1995;86:2041–2050. [PubMed] [Google Scholar]
- 41.Ohminami H, Yasukawa M, Fujita S. HLA class I-restricted lysis of leukemia cells by a CD8(+) cytotoxic T-lymphocyte clone specific for WT1 peptide. Blood. 2000;95:286–293. [PubMed] [Google Scholar]
- 42.Gaiger A, Reese V, Disis ML, Cheever MA. Immunity to WT1 in the animal model and in patients with acute myeloid leukemia. Blood. 2000;96:1480–1489. [PubMed] [Google Scholar]
- 43.Scheibenbogen C, Letsch A, Thiel E, et al. CD8 T-cell responses to Wilms tumor gene product WT1 and proteinase 3 in patients with acute myeloid leukemia. Blood. 2002;100:2132–2137. doi: 10.1182/blood-2002-01-0163. [DOI] [PubMed] [Google Scholar]
- 44.Molldrem J, Dermime S, Parker K, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 45.Greiner J, Li L, Ringhoffer M, et al. Identification and characterization of epitopes of the receptor for hyaluronic acid-mediated motility (RHAMM/CD168) recognized by CD8+ T cells of HLA-A2-positive patients with acute myeloid leukemia. Blood. 2005;106:938–945. doi: 10.1182/blood-2004-12-4787. [DOI] [PubMed] [Google Scholar]
- 46.Ochi T, Fujiwara H, Suemori K, et al. Aurora-A kinase: a novel target of cellular immunotherapy for leukemia. Blood. 2009;113:66–74. doi: 10.1182/blood-2008-06-164889. [DOI] [PubMed] [Google Scholar]
- 47.Rezvani K, Yong AS, Tawab A, et al. Ex vivo characterization of polyclonal memory CD8+ T-cell responses to PRAME-specific peptides in patients with acute lymphoblastic leukemia and acute and chronic myeloid leukemia. Blood. 2009;113:2245–2255. doi: 10.1182/blood-2008-03-144071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brossart P, Schneider A, Dill P, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;61:6846–6850. [PubMed] [Google Scholar]
- 49.Hundemer M, Schmidt S, Condomines M, et al. Identification of a new HLA-A2-restricted T-cell epitope within HM1.24 as immunotherapy target for multiple myeloma. Exp Hematol. 2006;34:486–496. doi: 10.1016/j.exphem.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim SH, Wang Z, Chiriva-Internati M, Xue Y. Sperm protein 17 is a novel cancer-testis antigen in multiple myeloma. Blood. 2001;97:1508–1510. doi: 10.1182/blood.v97.5.1508. [DOI] [PubMed] [Google Scholar]
- 51.Szmania S, Tricot G, van Rhee F. NY-ESO-1 immunotherapy for multiple myeloma. Leuk Lymphoma. 2006;47:2037–2048. doi: 10.1080/10428190600742292. [DOI] [PubMed] [Google Scholar]
- 52.Atanackovic D, Arfsten J, Cao Y, et al. Cancer-testis antigens are commonly expressed in multiple myeloma and induce systemic immunity following allogeneic stem cell transplantation. Blood. 2007;109:1103–1112. doi: 10.1182/blood-2006-04-014480. [DOI] [PubMed] [Google Scholar]
- 53.Avigan D. Dendritic cells: development, function and potential use for cancer immunotherapy. Blood Rev. 1999;13:51–64. doi: 10.1016/s0268-960x(99)90023-1. [DOI] [PubMed] [Google Scholar]
- 54.Tsuboi A, Oka Y, Kyo T, et al. Long-term WT1 peptide vaccination for patients with acute myeloid leukemia with minimal residual disease. Leukemia. 26:1410–1413. doi: 10.1038/leu.2011.343. [DOI] [PubMed] [Google Scholar]
- 55.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mailander V, Scheibenbogen C, Thiel E, Letsch A, Blau IW, Keilholz U. Complete remission in a patient with recurrent acute myeloid leukemia induced by vaccination with WT1 peptide in the absence of hematological or renal toxicity. Leukemia. 2004;18:165–166. doi: 10.1038/sj.leu.2403186. [DOI] [PubMed] [Google Scholar]
- 57.Rezvani K, Yong AS, Mielke S, et al. Leukemia-associated antigen-specific T-cell responses following combined PR1 and WT1 peptide vaccination in patients with myeloid malignancies. Blood. 2008;111:236–242. doi: 10.1182/blood-2007-08-108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Keilholz U, Letsch A, Busse A, et al. A clinical and immunologic phase 2 trial of Wilms tumor gene product 1 (WT1) peptide vaccination in patients with AML and MDS. Blood. 2009;113:6541–6548. doi: 10.1182/blood-2009-02-202598. [DOI] [PubMed] [Google Scholar]
- 59.Schmitt M, Schmitt A, Rojewski MT, et al. RHAMM-R3 peptide vaccination in patients with acute myeloid leukemia, myelodysplastic syndrome, and multiple myeloma elicits immunologic and clinical responses. Blood. 2008;111:1357–1365. doi: 10.1182/blood-2007-07-099366. [DOI] [PubMed] [Google Scholar]
- 60.Roddie H, Klammer M, Thomas C, et al. Phase I/II study of vaccination with dendritic-like leukaemia cells for the immunotherapy of acute myeloid leukaemia. Br J Haematol. 2006;133:152–157. doi: 10.1111/j.1365-2141.2006.05997.x. [DOI] [PubMed] [Google Scholar]
- 61.Li L, Giannopoulos K, Reinhardt P, et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int J Oncol. 2006;28:855–861. [PubMed] [Google Scholar]
- 62.Ho VT, Vanneman M, Kim H, et al. Biologic activity of irradiated, autologous, GM-CSF-secreting leukemia cell vaccines early after allogeneic stem cell transplantation. Proc Natl Acad Sci U S A. 2009;106:15825–15830. doi: 10.1073/pnas.0908358106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasir B, Borges V, Wu Z, et al. Fusion of dendritic cells with multiple myeloma cells results in maturation and enhanced antigen presentation. Br J Haematol. 2005;129:687–700. doi: 10.1111/j.1365-2141.2005.05507.x. [DOI] [PubMed] [Google Scholar]
- 64.Rosenblatt J, Wu Z, Vasir B, et al. Generation of tumor-specific T lymphocytes using dendritic cell/tumor fusions and anti-CD3/CD28. J Immunother. 2010;33:155–166. doi: 10.1097/CJI.0b013e3181bed253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosenblatt J, Vasir B, Uhl L, et al. Vaccination with dendritic cell/tumor fusion cells results in cellular and humoral antitumor immune responses in patients with multiple myeloma. Blood. 117:393–402. doi: 10.1182/blood-2010-04-277137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Borrello I, Sotomayor EM, Rattis FM, Cooke SK, Gu L, Levitsky HI. Sustaining the graft-versus-tumor effect through posttransplant immunization with granulocyte-macrophage colony-stimulating factor (GM-CSF)-producing tumor vaccines. Blood. 2000;95:3011–3019. [PubMed] [Google Scholar]
- 67.Borrello IM, Levitsky HI, Stock W, et al. Granulocyte-macrophage colony-stimulating factor (GM-CSF)-secreting cellular immunotherapy in combination with autologous stem cell transplantation (ASCT) as postremission therapy for acute myeloid leukemia (AML) Blood. 2009;114:1736–1745. doi: 10.1182/blood-2009-02-205278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenblatt JAI, Vasir B, Katz T, Uhl L, Wu Z, Somaiya P, Mills H, Joyce RM, Levine JD, Tzachanis D, Boussiotis V, Glotzbecker B, Francoeur K, Dombagoda D, Tsumer M, Bisharat L, Giallombardo N, Conway K, Fitzgerald D, Barhad R, Richardson P, Anderson K, Munshi N, Rowe J, Kufe D, Avigan D. Dendritic Cell Tumor Fusion Vaccination in Conjunction with Autologous Transplantation for Multiple Myeloma. ASH Annual Meeting Abstracts. 2009;114:783. 2009. [Google Scholar]
- 69.Lacy MQ, Mandrekar S, Dispenzieri A, et al. Idiotype-pulsed antigen-presenting cells following autologous transplantation for multiple myeloma may be associated with prolonged survival. Am J Hematol. 2009;84:799–802. doi: 10.1002/ajh.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2010;117:788–797. doi: 10.1182/blood-2010-08-299396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stadtmauer EA, Vogl DT, Luning Prak E, et al. Transfer of influenza vaccine-primed costimulated autologous T cells after stem cell transplantation for multiple myeloma leads to reconstitution of influenza immunity: results of a randomized clinical trial. Blood. 2010;117:63–71. doi: 10.1182/blood-2010-07-296822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005;11:1230–1237. doi: 10.1038/nm1310. [DOI] [PubMed] [Google Scholar]
- 73.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ozao-Choy J, Carvajal RD, Hamid O. Ipilimumab for metastatic melanoma. Drugs Today (Barc) 2012;48:381–393. doi: 10.1358/dot.2012.48.6.1811777. [DOI] [PubMed] [Google Scholar]
- 75.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karan D, Van Veldhuizen P. Combination immunotherapy with prostate GVAX and ipilimumab: safety and toxicity. Immunotherapy. 4:577–580. doi: 10.2217/imt.12.53. [DOI] [PubMed] [Google Scholar]
- 77.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 79.Rosenblatt J, Glotzbecker B, Mills H, et al. PD-1 blockade by CT-011, anti-PD-1 antibody, enhances ex vivo T-cell responses to autologous dendritic cell/myeloma fusion vaccine. J Immunother. 2011;34:409–418. doi: 10.1097/CJI.0b013e31821ca6ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rezaei N, Mahmoudi E, Aghamohammadi A, Das R, Nichols KE. X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol. 2011;152:13–30. doi: 10.1111/j.1365-2141.2010.08442.x. [DOI] [PubMed] [Google Scholar]
- 81.Biron CA, Byron KS, Sullivan JL. Severe herpesvirus infections in an adolescent without natural killer cells. N Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- 82.Kim S, Poursine-Laurent J, Truscott SM, et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 83.Yawata M, Yawata N, Draghi M, Little A, Partheniou F, Parham P. Roles for HLA and KIR polymorphisms in natural killer cell repertoire selection and modulation of effector function. J Exp Med. 2006;203:633–645. doi: 10.1084/jem.20051884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porrata L, Litzow M, Tefferi A, et al. Early lymphocyte recovery is a predictive factor for prolonged survival after autologous hematopoietic stem cell transplantation for acute myelogenous leukemia. Leukemia. 2002;16:1311–1318. doi: 10.1038/sj.leu.2402503. [DOI] [PubMed] [Google Scholar]
- 85.Porrata L, Gertz M, Inwards D, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001;98:579–585. doi: 10.1182/blood.v98.3.579. [DOI] [PubMed] [Google Scholar]
- 86.Leung W, Handgretinger R, Iyengar R, Turner V, Holladay MS, Hale GA. Inhibitory KIR-HLA receptor-ligand mismatch in autologous haematopoietic stem cell transplantation for solid tumour and lymphoma. Br J Cancer. 2007;97:539–542. doi: 10.1038/sj.bjc.6603913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarek N, Le Luduec JB, Gallagher MM, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. J Clin Invest. 2012;122:3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kohrt HE, Houot R, Goldstein MJ, et al. CD137 stimulation enhances the antilymphoma activity of anti-CD20 antibodies. Blood. 2011;117:2423–2432. doi: 10.1182/blood-2010-08-301945. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 89.Romagne F, Andre P, Spee P, et al. Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–2677. doi: 10.1182/blood-2009-02-206532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Godfrey J, Benson DM., Jr. The role of natural killer cells in immunity against multiple myeloma. Leuk Lymphoma. 2012;53:1666–1676. doi: 10.3109/10428194.2012.676175. [DOI] [PubMed] [Google Scholar]
- 91.Ruggeri L, Capanni M, Casucci M, et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood. 1999;94:333–339. [PubMed] [Google Scholar]
- 92.Yu J, Venstrom JM, Liu XR, et al. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113:3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsu K, Gooley T, Malkki M, et al. KIR ligands and prediction of relapse after unrelated donor hematopoietic cell transplantation for hematologic malignancy. Biol Blood Mar Transpl. 2006;12:828–836. doi: 10.1016/j.bbmt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 94.Cooley S, Trachtenberg E, Bergemann T, et al. Donors with group B haplotypes improve relapse-free survival after unrelated hemaotpoietic transplantation for acute myeloid leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venstrom J, Gooley T, Spellman S, et al. Donor activating KIR3DS1 is associated with decreased acute GvHD in unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2010:115. doi: 10.1182/blood-2009-08-236943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chewning J, Gudme C, Hsu K, Selvakumar A, Dupont B. KIR2DS1-positive NK cells mediate alloresponse against the C2 HLA-KIR ligand group in vitro. J Immunol. 2007;179:854–868. doi: 10.4049/jimmunol.179.2.854. [DOI] [PubMed] [Google Scholar]
- 97.Venstrom JM, Pittari G, Gooley TA, et al. HLA-C-Dependent Prevention of Leukemia Relapse by Donor Activating KIR2DS1. New England Journal of Medicine. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gress RE, Miller JS, Battiwalla M, et al. Proceedings from the National Cancer Institute’s Second International Workshop on the Biology, Prevention, and Treatment of Relapse after Hematopoietic Stem Cell Transplantation: Part I. Biology of relapse after transplantation. Biol Blood Marrow Transplant. 2013;19:1537–1545. doi: 10.1016/j.bbmt.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]