Abstract
Rrecent studies have highlighted a group of 4-repeat (4R) tauopathies that are characterised neuropathologically by widespread, globular glial inclusions (GGIs). Tau immunohistochemistry reveals 4R immunore-active globular oligodendroglial and astrocytic inclusions and the latter are predominantly negative for Gallyas silver staining. These cases are associated with a range of clinical presentations, which correlate with the severity and distribution of underlying tau pathology and neurodegeneration. Their heterogeneous clinicopathological features combined with their rarity and under-recognition have led to cases characterised by GGIs being described in the literature using various and redundant terminologies. In this report, a group of neuropathologists form a consensus on the terminology and classification of cases with GGIs. After studying microscopic images from previously reported cases with suspected GGIs (n = 22), this panel of neuropathologists with extensive experience in the diagnosis of neurodegenerative diseases and a documented record of previous experience with at least one case with GGIs, agreed that (1) GGIs were present in all the cases reviewed; (2) the morphology of globular astrocytic inclusions was different to tufted astrocytes and finally that (3) the cases represented a number of different neuropathological subtypes. They also agreed that the different morphological subtypes are likely to be part of a spectrum of a distinct disease entity, for which they recommend that the overarching term globular glial tauopathy (GGT) should be used. Type I cases typically present with frontotemporal dementia, which correlates with the fronto-temporal distribution of pathology. Type II cases are characterised by pyramidal features reflecting motor cortex involvement and corticospinal tract degeneration. Type III cases can present with a combination of frontotemporal dementia and motor neuron disease with fronto-temporal cortex, motor cortex and corticospinal tract being severely affected. extrapyramidal features can be present in Type II and III cases and significant degeneration of the white matter is a feature of all GGT subtypes. Improved detection and classification will be necessary for the establishment of neuropathological and clinical diagnostic research criteria in the future.
Introduction
Recent studies have highlighted a group of 4-repeat (4R) tauopathies that are characterised neuropathologically by distinctive and widespread globular glial inclusions (GGIs). Such cases can have a range of clinicopathological presentations, which has resulted in them being described in the literature using various and redundant terminologies. In this paper, we review the historical discovery of cases characterised by GGIs and highlight the difficulties in classifying them in the past. With the aim of harmonising the terminologies that have previously been used to describe such cases, a group of expert neuropathologists form a consensus on their future classification and suggest suitable nomenclature. These recommendations will hopefully improve the detection and correct classification of this relatively rare and under-recognised form of 4R tauopathy.
Historical aspects
In 1998, Molina and colleagues described two types of “glial cytoplasmic inclusions” (GCIs) in a temporal lobe biopsy obtained from a patient with moderate frontotemporal atrophy and a clinical diagnosis of primary progressive aphasia. The first type was described as “sickle or ring-shaped perinuclear inclusions” and the authors acknowledged that these inclusions had some morphological similarities to GCIs observed in multiple system atrophy (MSA). GCIs seen in MSA are consistently negative for phosphorylated-tau [4], although those described by Molina et al. [18] were strongly immunoreactive for phosphorylated tau epitopes. The second type of GCIs were described as “coarsely granular or patchy material in the cell body and proximal portion of the cell processes” and were noted as being reminiscent of the tau-positive glial inclusions of progressive supranuclear palsy (PSP). Images of this particular type of GCI suggested they were morphologically heterogeneous with some of the inclusions being in oligodendroglia whilst others in astrocytes. Nevertheless, the authors noted that characteristic PSP type ‘tufted astrocytes’ were not observed [18]. Although the accurate nosological classification of this case was restricted by its limited brain sampling, Molina et al. [18] concluded that their case could not be “ascribed to one of the nosological entities characterised by glial inclusions, like MSA and PSP”. Despite the GCIs in this case being described as both phospho-tau and Gallyas positive, the relative frequency of the different morphological types and their respective staining properties were not reported.
In 2001, Bigio et al. [3] provided the first detailed pathological, ultrastructural and biochemical assessment of a single case with sporadic frontotemporal dementia (FTD) and severe neurodegeneration in the frontal and temporal lobes associated with “distinctive globular neuronal and glial, tau-positive inclusions in grey and white matter”. The GGIs occurred predominantly in oligodendrocytes and were accompanied by “rare tufted astrocyte-like glial inclusions”. Despite the latter being characteristic of PSP, neuronal loss in subcortical nuclei affected in PSP was only mild. The distribution of pathology, predominance of 4R tau isoforms and susceptibility of oligodendrocytes were noted as being similar to those seen in a familial form of frontotemporal lobar degeneration with MAPT mutation (FTLD–MAPT). This had previously been termed “familial multiple system tauopathy with presenile dementia” (MSTD) and shown to be associated with an exon 10+3 intronic mutation [22, 23]. Due to presumed neuropathological and biochemical similarities with this familial disorder and the absence of MAPT mutations, Bigio et al. [3] classified their case as “sporadic multiple system tauopathy with dementia” (sporadic MSTD). It is of note that in hindsight the morphology of GGIs described by Bigio et al. [3] appears distinct from the coiled body-type inclusions seen in familial MSTD [22, 24].
Globular glial inclusions (GGIs) were subsequently reported in additional single case reports [2, 6, 21]. In one of them “leukoencephalopathy” was emphasised due to the severe white matter damage and oligodendroglial cell loss related to extensive tau pathology in the white matter [21], while another, on the basis of morphological similarities to the case described by Bigio et al. [3], used the term “multiple system tauopathy” [6]. One case report of FTD, described as an “atypical tauopathy”, emphasised prominent white matter involvement with widespread oligodendroglial tau immunoreactivity although described as coiled bodies and not GGIs [10].
In 2006, Josephs et al. [12] described 12 cases in which GGIs were also a morphological feature. In contrast to the above-discussed sporadic MSTD cases presenting as FTD, their cases had clinical evidence of upper motor neuron disease plus extrapyramidal features leading to a number of clinical diagnoses including PSP, primary lateral sclerosis (PLS) and corticobasal syndrome (CBS). Neuropathologically, the cases demonstrated circumscribed cortical atrophy, which was marked in the superior frontal gyrus, mild in the superior parietal lobule and also affected the precentral gyrus. Microscopically there was superficial spongiosis and neuronal loss, which were most severe in the motor cortex, along with damage to the underlying white matter and prominent corticospinal tract degeneration (CST), a distribution of pathology not reported in the sporadic MSTD. GGIs occurred predominantly as “globular oligodendroglial inclusions” (GOIs) and were accompanied by morphological lesions typically found in PSP, such as frequent coiled bodies and fewer tufted astrocytes. It is of note that in contrast to PSP, tufted astrocytes were mostly Gallyas-negative and subcortical nuclei severely affected in PSP were only mildly involved. For these reasons, Josephs et al. [12] classified their cases as “atypical PSP with corticospinal tract degeneration” (PSP–CST).
In 2008, Kovacs et al. [13] reported the first systematic and comprehensive clinicopathological assessment of a series of seven cases with GGIs, which they termed “white matter tauopathy with globular glial inclusions” (WMT–GGI). Clinically, their cases were compatible with the behavioural variant of FTD and were histologically reminiscent of the sporadic MSTD case described by Bigio et al. [3], albeit the density of GGIs particularly in the white matter was much greater [13]. GGIs were predominantly found in oligodendrocytes. In addition, rare coiled bodies were also present. similar to the original sporadic MSTD case [3], “tufted astrocyte-like” inclusions were also identified in the affected grey-matter structures, described as having “dot-like immunopositivity in the proximal segments of branching processes, with a star-like appearance”. Like those described by Josephs et al. [12] these were “non-argyrophilic” (negative with Gallyas silver-staining), although rare PSP-type Gallyas-positive tufted astrocytes were also observed. Biochemical examination, performed in two cases, supported the immunohistochemical observation indicating that this is a predominantly 4R-tauopathy. Kovacs et al. [13] also conducted an exhaustive review of the literature and were the first to suggest that sporadic tauopathies with GGIs were “a distinct neuropathological entity associated with sporadic FTLD”. They also noted that beyond the synucleinopathy MSA, tauopathies with GGIs extend the group of neurodegenerative disorders with a prominent oligodendroglial pathology.
In 2010, Fu et al. [7] published three cases under the term of “sporadic 4R tauopathy with frontotemporal lobar degeneration, parkinsonism and motor neuron disease” (FTLD–P–MND). Tau immunostaining identified numerous “coiled body-like lesions”, which in retrospect were consistent with GOIs. Frequent tau-positive astrocytes also had a globular appearance and similar to some of the cases described above [12, 13], were shown to be mostly Gallyas-negative [7]. The distribution of pathology was similar to that seen in PSP–CST like cases [12] with severe degeneration of the motor cortex and corticospinal tract, but with the added involvement of lower motor neurons [7]. There were also similarities to sporadic MSTD with two of their cases showing significant frontotemporal atrophy and frontal atrophy in the third. Biochemical analysis revealed not only 68 and 64 kDa bands characteristic of 4R-tauopathies, but also a weaker ~33 kDa lower-molecular-weight species [7] that is reported to be more prominent in PSP [27]. Fu et al. [7] acknowledged the overlap with PSP–CST and sporadic MSTD, but concluded that the severity of the tau-positive astrocytic pathology and its lack of Gallyas staining, together with the overall distribution of pathology, were features inconsistent with known neuropathological disorders.
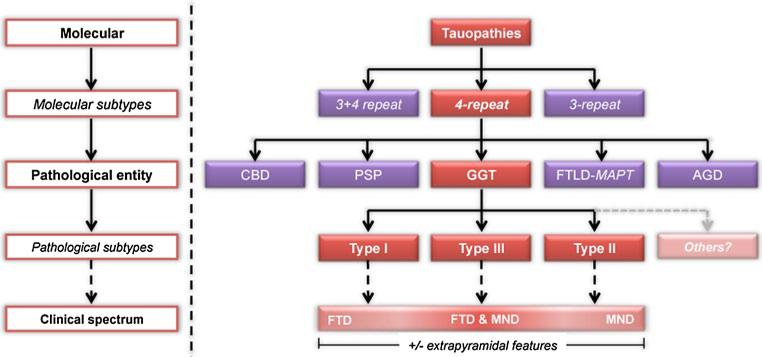
Most recently, Ahmed et al. [1] performed for the first time a comparative clinicopathological and biochemical study of two cases with GGIs representing two subtypes: MSTD and PSP–CST. There were many similarities between their two cases: (1) specific cortical areas and their associated descending white matter tracts were severely affected, (2) neurodegeneration in subcortical nuclei typically affected in PSP was only mild, (3) tau-positive GOIs were prominent and characteristic features, (4) a mixture of large GOIs and typical coiled bodies were present, (5) tau-positive astrocytes were a prominent feature, with many having a globular morphology (termed GAI), (6) tau-positive astrocytic inclusions were mostly Gallyas-negative and (7) biochemical analysis of tau protein revealed not only strong bands at 68 and 64 kDa, but also weaker lower-molecular-weight bands, with a ~35 kDa band. Some of the differences between their two cases were related to the severity and distribution of lesions, which could explain the different clinical presentations. In addition, the GOIs in their sporadic MSTD case were generally larger and more apparently globular than those found in their PSP–CST case. Furthermore, typical coiled bodies were more common in the latter and some even had an intermediate-type morphology as such oligodendroglial inclusions were larger, yet retained an overall coiled body-type appearance. With these similarities and differences in mind, Ahmed et al. [1] suggested that their cases were variants representing the spectrum of a single distinct disease entity; a notion first suggested by Kovacs et al. [13]. As in FTLD–TDP [15] and FTLD–FUS [14], the clinical spectrum (illustrated in Fig. 2) is defined by motor neuron disease at one end and FTD at the other, with or without extrapyramidal features [1]. As GGIs were a consistent and defining feature of sporadic MSTD, PSP–CST and FTLD–P–MND cases Ahmed et al. [1] proposed the collective term globular glial tauopathy (GGT) to encompass these cases.
Fig. 2.
Classification strategy for GGT in relation to other commonly recognised 4R-tauopathies. Abbreviations: CBD corticobasal degeneration, PSP progressive supranuclear palsy, GGT globular glial tauopathies, FTLD-MAPT frontotemporal lobar degeneration with MAPT mutation, AGD argyrophilic grains disease, MND motor neuron disease features, FTD frontotemporal dementia
Difficulties in the classification of cases with GGIs
Since the first report in 1998, at least 30 individual cases have been reported with GGIs being a characteristic feature (Supplementary Table 1) [1–3, 6, 10, 12, 13, 18, 20, 21]. It is of note that the distinctive glial lesions seen in these cases have been described under a number of terms such as GCIs [18], GGIs [3, 13], GOIs [1, 12], coiled bodies [7, 10], tufted astrocytes [3, 12], globular astrocytes [1] or just tau-positive astrocytes [7]. Many of the authors have noted diagnostic difficulties in assigning their respective cases to well-established entities, such as PSP, with which the published cases share some clinical, neuropathological and biochemical features. This also explains why various, novel terms were used to classify such cases, including “sporadic MSTD” [3], a form of “leukoencephalopathy” [21], “atypical tauopathy” [10], WMT–GGI [13], PSP–CST [12] and FTLD–P–MND [7]. Subsequently some confusion has arisen from using terms such as sporadic MSTD, which is morphologically different from the previously described familial MSTD (see also above). Similarly, the term PSP–CST might be diagnostically confusing given that not all cases presented with a PSP syndrome and some of the cardinal neuropathological features of PSP are also missing, such as the significant involvement of subcortical nuclei and the absence of Gallyas-positive tufted astrocytes. Even when the existence of cases with GGIs has been acknowledged in the literature, there have been confusion and redundancy in their classification as demonstrated by the recent FTLD guidelines, which included sporadic MSTD and WMT–GGI as separate FTLD-tau subtypes [17].
The lack of consistent terminology for cases with GGIs and a trend to classify neurodegenerative diseases based on molecular neuropathology prompted Ahmed et al. [1] to propose GGT as an encompassing term for cases with GGIs, followed by a sub-stratification strategy based on the regional distribution and severity of neurodegeneration, hoping that this would pave the way for a formal consensus agreement on the neuropathological terminology of these rare 4R tauopathies.
Consensus classification study
To address some of the problems stated above, the authors of this report (led by G.G.K.) initiated a study to reach a consensus on the classification of this group of 4R-tauopathy. Microscopic images (6–10 per case) of tau immunostained sections and their corresponding publication were provided by the authors of previously reported cases [1–3, 6, 10, 12, 13, 20]. Microscopic images from a total of 22 individual cases with suspected GGIs were sent to participants (authors of this report; n = 16), who were each asked if (1) GOIs were indeed present, (2) coiled body-type inclusions were also present, (3) any astrocytic tau pathology was different to typical PSP tufted astrocytes, (4) the white matter or grey matter was predominately affected by tau pathology, (5) the cases represent a single neuropathological entity and (6) the cases could be separated into distinct subgroups? A majority was defined as 80 % or more of the participants in agreement.
The majority of participants agreed that (1) GOIs were present in every case, including those where GOIs were detected after reevaluation [10, 20]; (2) coiled body inclusions were present in all the PSP–CST and FTLD–P–MND-type cases, whereas they were variably present in the sporadic MSTD cases; (3) the morphology of astrocytic tau inclusions was different from, but may overlap with, tufted astrocytes; (4) the white matter was more affected in the MSTD-type cases, whereas in other cases there was either more grey matter or equal grey vs. white matter involvement; (5) these cases are likely to represent a single disease entity; and (6) the cases could be separated into distinct clinicopathological subgroups. some participants also commented that Gallyas silver staining might be an efficient method to establish differences in astrocytic tau pathology and that the astrocytic and oligodendroglial inclusions in some cases might have a multi-globular morphology with the relative frequency of these glial inclusions differing between cases. Based on this heterogeneity, the majority of participants suggested that at least three subtypes exist, which are described in Table 1.
Table 1.
Description of three subtypes characterised by GGIs
| Subtypes | Regional distribution of oligodendroglial inclusions | GOIs | CBs | GAIs | AHC | Clinical features | Common clinical diagnoses | Previous reports |
|---|---|---|---|---|---|---|---|---|
| Type I | FT | +++ | + | + | - | FLF and/or Dem | FTD, PiD | [1-3, 6, 10, 13, 18, 21] |
| Type II | MTR and/or CST | ++ | ++ | + | ± | FLF, PF and/or EPF | PSP, CBS, MND, PLS | [1, 12] |
| Type III | FT, MTR and/or CST | ++ | ++ | +++ | ++ | FLF, Dem, MND and/or EPF | PSP, CBS, MND | [7, 20] |
GOIs globular oligodendroglial inclusions, CBs coiled body-type inclusions, GAIs globular astrocytic inclusion, AHC anterior horn cell involvement (neuronal loss and tau pathology), FT frontotemporal, FLF frontal-lobe features, Dem dementia, FTD frontotemporal dementia, PiD Pick's disease, MTR motor cortex, CST corticospinal tract, PF pyramidal features, EPF extrapyramidal features, PSP progressive supranuclear palsy, CBS corticobasal syndrome, PLS primary lateral sclerosis, MND motor neuron disease features, - negative/absent, + low severity, ++ moderate severity, +++ high severity
Future classification and nosology
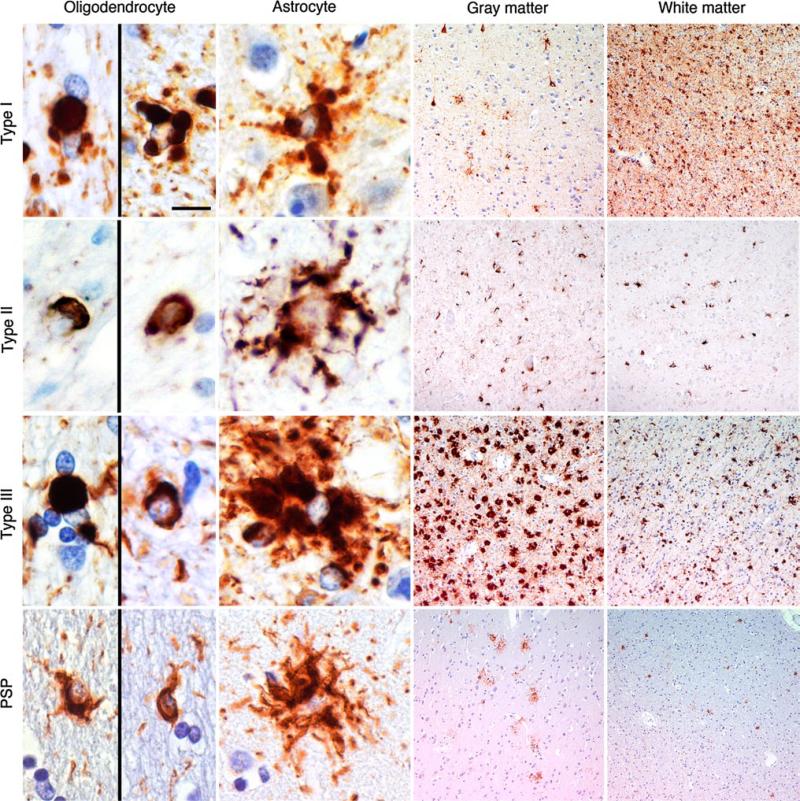
In summary, the neuropathological hallmarks of these cases that allow them to be distinguished from other members of the 4R-tauopathy family are the globular nature of both oligodendroglial and astrocytic inclusions, termed GGIs (Fig. 1). As the severity of oligodendroglial and astrocytic GGI pathologies can be different within a case, the former could be termed GOIs and the latter, GAIs. GOIs are defined as globular structures the size of, or larger than the nucleus of the cell [13] and can be variably accompanied by typical coiled bodies [1]. GAIs are defined as distinct globular or dot-like structures of variable size seen in the cytoplasm or processes of astrocytes. They are mostly non-argyrophilic, indicating a non-fully fibrillar conformation of tau, and might be accompanied by astrocytic inclusions that are morphologically indistinguishable from PSP-type tufted astrocytes; however, like GAIs these tufted astrocyte-like lesions are rarely Gallyas positive. In contrast, GOIs and coiled bodies are consistently Gallyas positive [1, 13].
Fig. 1.
Comparison of oligodendroglial and astrocytic inclusions found in GGT and PSP Comparison of oligodendroglial and astrocytic inclusions and distribution of tau pathology in the grey matter (temporal cortex for cases representing Type I and PSP and motor cortex for cases representing Type II and III) and white matter (underlying white matter of the representative cortical areas) in representative cases of GGT types and PSP. Scale bar represents 10 μm in the images for oligodendrocytes and astrocytes and 100 μm for the overview images of the grey and white matter. Immunostaining is specific for phosphorylated-tau (AT8)
Based on these characteristics and the trend to classify neurodegenerative diseases based on molecular neuropathology [16, 17], in 2011 Ahmed et al. [1] have proposed the term GGT. This terminology could be followed by a sub-stratification strategy (Fig. 2); accordingly cases with predominantly fronto-temporal and without corticospinal involvement (i.e. sporadic MSTD-type cases) would be termed GGT Type I, whereas cases with predominately motor cortex and corticospinal tract degeneration (i.e. PSP–CST type cases) would be classified as GGT Type II. Those with fronto-temporal, motor cortex and corticospinal tract involvement (i.e. those previously termed FTLD–P–MND) would be classified as GGT Type III. This reflects different selective vulnerability, since the distribution of GGIs clearly correlates with the predominant clinical symptoms [1, 7, 12, 13]. The authors of this study support this neuropathology-based classification strategy, which is complemented by the presence and predominance of GOIs and GAIs as well as the presence or absence of upper and lower motor neuron involvement (Table 1) and would allow for phenotypic grouping, account for the heterogeneity within this group and avoid confusion or redundancy in terminology. This study has stopped short of proposing specific neuropathological diagnostic criteria for GGT and its subtypes, but increased recognition and correct classification of these cases, aided by the proposed classification strategy, should provide a big enough sample size to formulate such criteria in the near future. Due to the overlapping neuropathological features of the different GGT subtypes it is likely that future criteria for the subtypes will be based on the regional distribution and severity of lesions and neurodegeneration, in a similar fashion to the way olivopontocerebellar and striatonigral subtypes of MSA are currently distinguished [19]. The previous reports of GGT-type cases [1–3, 6, 10, 12, 13, 20], the majority of which is single case reports, suggest that GGT cases are relatively rare and that sub-typing a rare disorder without fully appreciating its neuropathological heterogeneity might be premature. Nevertheless, the classification strategy that we have proposed (Fig. 2) has the flexibility to incorporate any additional sub-types of GGT that are likely to be discovered in the future.
In comparison with other 4R-tauopathies (Table 2), GGT is most closely associated with PSP owing to similarities in astrocytic inclusion morphology and tau biochemical signature (68, 64 and ~35 kDa bands). In addition to the morphological differences between GOI and typical coiled bodies (Fig. 1), the use of Gallyas silver-staining [8] could prove instrumental as a diagnostic tool to help distinguish GGT from PSP, especially in the evaluation of astrocytic pathology, although occasional Gallyas-positive tufted astrocyte-like inclusions may be noted in GGT cases as well. The neuropathological features of GGT should be easily distinguishable from those of CBD and AGD [5, 25]; however, this is not the case for FTLD–MAPT, which can share neuropathological and biochemical similarities with GGT [9, 11] (Table 2). For these reasons, suspected GGT cases should be routinely sent for MAPT genetic analysis to exclude the diagnosis of FTLD–MAPT. In 25 of the 30 cases reviewed in this paper, information about MAPT genetic analysis was available and in none of the cases was a mutation identified (supplementary Table 1).
Table 2.
Comparison of GGT and other commonly recognised 4R-tauopathies
| Pathological and biochemical features | Has this feature been documented in this particular disorder? |
||||
|---|---|---|---|---|---|
| GGT | PSP | CBD | AGD | FTLD-MAPT | |
| Neuronal tau inclusionsa | Y | Y | Y | Y | Y |
| Oligodendroglial tau inclusionsa | Y | Y | Y | Y | Y |
| Coiled body inclusions b | N | Y | Y | Y | Y |
| Globular oligodendroglial inclusions b | Y | N | N | N | Yd |
| Gallyas-positive oligodendroglial inclusions | Y | Y | Y | Y | Y |
| Astrocytic tau inclusionsa | Y | Y | Y | Y | Y |
| Tufted astrocytes | Yc | Y | N | N | Y |
| Astrocytic plaques | N | N | Y | N | Y |
| Gallyas-positive astrocytic inclusions b | N | Y | Y | Y | Y |
| 4R tau isoforms depositedb | Y | Y | Y | Y | Y |
| 68 and 64 kDa tau bands | Y | Y | Y | Y | Y |
| 35 kDa tau band b | Y | Y | N | -e | Y |
GGT globular glial tauopathies, PSP progressive supranuclear palsy, CBD corticobasal degeneration, AGD argyrophilic grains disease, FTLD-MAPT frontotemporal lobar degeneration with MAPT mutation, 4R 4-repeat
Tau inclusions identified by phospho-tau immunohistochemistry
Predominant
Astrocytic inclusions may be indistinguishable from tufted astrocytes based on morphology alone
Oligodendroglial inclusions with a globular morphology, described as “stout coiled bodies”, have been reported in FTLD-MAPT [11]
Unknown
Conclusion
Presenting with a combination of FTD, MND and/or extrapyramidal features, GGTs belong to the family of the 4R-tauopathies. GGTs are characterised neuropathologically by tau-positive GOIs and GAIs, the latter of which are mostly Gallyas-negative. These characteristic features help GGT to be distinguished from other 4R-tauopathies, in particular PSP. Whether the neuropathological subtypes of GGT are distinct disease entities or part of a spectrum of disease is open to a similar debate that has been on-going for PSP and CBD [5]. The severe involvement of oligodendroglia and resulting white matter changes (myelin pallor, axonal loss and gliosis) suggests that GGT expands the spectrum of primary oligodendrogliopathies. As has been suggested for the oligodendroglial inclusions of MSA [26], GGIs may play a central role in the pathogenesis of GGT. The combination of white matter changes and the deposition of predominantly 4R tau isoforms could be exploited in the future using neuroimaging techniques and CSF protein analysis (respectively), for the detection and diagnosis of GGT during life. The aim of reviewing 30 historical cases classified as GGT was to draw attention to their existence and to help improve the nomenclature used to describe them. Improving the detection and correct classification of GGT cases in the future should increase the number of cases available for retrospective clinicopathological char-acterisation, which will hopefully pave the way for establishing clinical and neuropathological diagnostic research criteria.
Supplementary Material
Acknowledgments
Grant supports: BG—PHS P30 AG010133; CLW and KJH—NIH P30 AG12300, and CLW—Winspear Family Center for Research on the Neuropathology of Alzheimer Disease, and McCune Foundation; EHB—AG13854; GG—Italian Ministry of Health; HT—Research Committee for CNS Degenerative Diseases, the Ministry of Health, Labour and Welfare, Japan; KAJ—NIH R01 AG37491; GGK-DEVELAGE (FP7/2007–2013, No 278486); TR and JLH—the Multiple System Atrophy Trust, The Alzheimer's Research Trust and Parkinson's UK and JLH—the Reta Lila Weston Institute for Neurological Studies. The Queen Square Brain Bank is supported by the Reta Lila Weston Institute for Neurological Studies and the PSP (Europe) Association. Part of this work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health's NIHR Biomedical Research Centres funding scheme and, in part, funded/supported by the National Institute for Health Research (NIHR) Biomedical Research Unit in Dementia based at University College London Hospitals (UCLH), University College London (UCL). Zeshan Ahmed is currently a post-doctoral research fellow at Eli Lilly and Company.
Contributor Information
Zeshan Ahmed, Department of Molecular Neuroscience, Queen Square Brain Bank, Institute of Neurology, University College London, Queen Square, London WC1N 3BG, UK.
Eileen H. Bigio, Department of Pathology, Northwestern Alzheimer Disease Center, Northwestern Feinberg School of Medicine, Chicago, IL, USA
Herbert Budka, Institute of Neurology, Medical University of Vienna, Vienna, Austria; Institute of Neuropathology, University Hospital Zurich, Zurich, Switzerland.
Dennis W. Dickson, Mayo Clinic, Jacksonville, FL, usA
Isidro Ferrer, Institute of Neuropathology, University Hospital Bellvitge, University of Barcelona, CIBERNED, Hospitalet de LLobregat, Spain.
Bernardino Ghetti, Department of Pathology and Laboratory Medicine, Indiana Alzheimer Disease Center, Indiana University School of Medicine, Indianapolis, IN, USA.
Giorgio Giaccone, Fondazione IRCCS Istituto Neurologico Carlo Besta, Milan, Italy.
Kimmo J. Hatanpaa, Department of Pathology, Alzheimer's Disease Center, University of Texas Southwestern Medical Center, Dallas, TX, USA
Janice L. Holton, Department of Molecular Neuroscience, Queen Square Brain Bank, Institute of Neurology, University College London, Queen Square, London WC1N 3BG, UK
Keith A. Josephs, Mayo Clinic, Rochester, MN, USA
James Powers, University of Rochester, School of Medicine and Dentistry, Rochester, NY, USA.
Salvatore Spina, Department of Pathology and Laboratory Medicine, Indiana Alzheimer Disease Center, Indiana University School of Medicine, Indianapolis, IN, USA.
Hitoshi Takahashi, Department of Pathology, Brain Research Institute, Niigata University, Niigata, Japan.
Charles L. White, III, Department of Pathology, Alzheimer's Disease Center, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Tamas Revesz, Department of Molecular Neuroscience, Queen Square Brain Bank, Institute of Neurology, University College London, Queen Square, London WC1N 3BG, UK.
Gabor G. Kovacs, Institute of Neurology, Medical University of Vienna, Vienna, Austria
References
- 1.Ahmed Z, Doherty KM, Silveira-Moriyama L, et al. Globular glial tauopathies (GGT) presenting with motor neuron disease or frontotemporal dementia: an emerging group of 4-repeat tauopathies. Acta Neuropathol. 2011;122:415–428. doi: 10.1007/s00401-011-0857-4. [DOI] [PubMed] [Google Scholar]
- 2.Berry RW, Quinn B, Johnson N, Cochran EJ, Ghoshal N, Binder LI. Pathological glial tau accumulations in neurodegenerative disease: review and case report. Neurochem Int. 2001;39:469–479. doi: 10.1016/s0197-0186(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 3.Bigio EH, Lipton AM, Yen SH, et al. Frontal lobe dementia with novel tauopathy: sporadic multiple system tauopathy with dementia. J Neuropathol Exp Neurol. 2001;60:328–341. doi: 10.1093/jnen/60.4.328. [DOI] [PubMed] [Google Scholar]
- 4.Cairns NJ, Atkinson PF, Hanger DP, Anderton BH, Daniel SE, Lantos PL. Tau protein in the glial cytoplasmic inclusions of multiple system atrophy can be distinguished from abnormal tau in Alzheimer's disease. Neurosci Lett. 1997;230:49–52. doi: 10.1016/s0304-3940(97)00474-6. [DOI] [PubMed] [Google Scholar]
- 5.Dickson DW, Hauw JJ, Agid Y, Litvan I. Progressive supranuclear palsy and corticobasal degeneration. In: Dickson DW, Weller RO, editors. Neurodegeneration: The molecular pathology of dementia and movement disorders. 2nd edn. Wiley-Blackwell; Chichester, West Sussex: 2011. pp. 135–155. [Google Scholar]
- 6.Ferrer I, Hernandez I, Boada M, et al. Primary progressive aphasia as the initial manifestation of corticobasal degeneration and unusual tauopathies. Acta Neuropathol. 2003;106:419–435. doi: 10.1007/s00401-003-0756-4. [DOI] [PubMed] [Google Scholar]
- 7.Fu YJ, Nishihira Y, Kuroda S, et al. Sporadic four-repeat tauopathy with frontotemporal lobar degeneration, Parkinsonism, and motor neuron disease: a distinct clinicopathological and biochemical disease entity. Acta Neuropathol. 2010;120:21–32. doi: 10.1007/s00401-010-0649-2. [DOI] [PubMed] [Google Scholar]
- 8.Gallyas F. Silver staining of Alzheimer's neurofibrillary changes by means of physical development. Acta morphol Acad Sci Hung. 1971;19:1–8. [PubMed] [Google Scholar]
- 9.Ghetti B, Wszolek EK, Boeve BF, Spina S, Goedert M. Frontotemporal dementia and Parkinsonism linked to chromo-some 17. In: Dickson DW, Weller RO, editors. Neurodegeneration: the molecular pathology of dementia and movement disorders. Wiley-Blackwell; Chichester, West Sussex: 2011. pp. 110–134. [Google Scholar]
- 10.Giaccone G, Marcon G, Mangieri M, et al. Atypical tauopathy with massive involvement of the white matter. Neuropathol Appl Neurobiol. 2008;34:468–472. doi: 10.1111/j.1365-2990.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi S, Toyoshima Y, Hasegawa M, et al. Late-onset frontotemporal dementia with a novel exon 1 (Arg5His) tau gene mutation. Ann Neurol. 2002;51:525–530. doi: 10.1002/ana.10163. [DOI] [PubMed] [Google Scholar]
- 12.Josephs KA, Katsuse O, Beccano-Kelly DA, et al. Atypical progressive supranuclear palsy with corticospinal tract degeneration. J Neuropathol Exp Neurol. 2006;65:396–405. doi: 10.1097/01.jnen.0000218446.38158.61. [DOI] [PubMed] [Google Scholar]
- 13.Kovacs GG, Majtenyi K, Spina S, et al. White matter tauopathy with globular glial inclusions: a distinct sporadic frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67:963–975. doi: 10.1097/NEN.0b013e318187a80f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lashley T, rohrer JD, Bandopadhyay R, et al. A comparative clinical, pathological, biochemical and genetic study of fused in sarcoma proteinopathies. Brain. 2011;134:2548–2564. doi: 10.1093/brain/awr160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie IR. The neuropathology of FTD associated With ALS. Alzheimer Dis Assoc Disord. 2007;21:S44–S49. doi: 10.1097/WAD.0b013e31815c3486. [DOI] [PubMed] [Google Scholar]
- 16.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–18. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molina JA, Probst A, Villanueva C, et al. Primary progressive aphasia with glial cytoplasmic inclusions. Eur Neurol. 1998;40:71–77. doi: 10.1159/000007961. [DOI] [PubMed] [Google Scholar]
- 19.Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain. 2004;127:2657–2671. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- 20.Piao YS, Tan CF, Iwanaga K, et al. Sporadic four-repeat tauopathy with frontotemporal degeneration, parkinsonism and motor neuron disease. Acta Neuropathol. 2005;110:600–609. doi: 10.1007/s00401-005-1086-5. [DOI] [PubMed] [Google Scholar]
- 21.Powers JM, Byrne NP, Ito M, et al. A novel leukoencephalopathy associated with tau deposits primarily in white matter glia. Acta Neuropathol. 2003;106:181–187. doi: 10.1007/s00401-003-0719-9. [DOI] [PubMed] [Google Scholar]
- 22.Spillantini MG, Goedert M, Crowther RA, Murrell JR, Farlow MR, Ghetti B. Familial multiple system tauopathy with presenile dementia: a disease with abundant neuronal and glial tau filaments. Proc Natl Acad Sci USA. 1997;94:4113–4118. doi: 10.1073/pnas.94.8.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spillantini MG, Murrell JR, Goedert M, Farlow MR, Klug A, Ghetti B. Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci USA. 1998;95:7737–7741. doi: 10.1073/pnas.95.13.7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spina S, Farlow MR, Unverzagt FW, et al. The tauopathy associated with mutation +3 in intron 10 of Tau: characterization of the MSTD family. Brain. 2008;131:72–89. doi: 10.1093/brain/awm280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tolnay M, Braak H. Argyrophilic grain disease. In: Dickson DW, Weller RO, editors. Neurodegeneration: the molecular pathology of dementia and movement disorders. 2nd edn. Wiley-Blackwell; Chichester, West Sussex: 2011. pp. 165–170. [Google Scholar]
- 26.Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schloss-macher MG. Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- 27.Wray S, Saxton M, Anderton BH, Hanger DP. Direct analysis of tau from PsP brain identifies new phosphorylation sites and a major fragment of N-terminally cleaved tau containing four microtubule-binding repeats. J Neurochem. 2008;105:2343–2352. doi: 10.1111/j.1471-4159.2008.05321.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.