Abstract
Ion channels control the sperm ability to fertilize the egg by regulating sperm maturation in the female reproductive tract and by triggering key sperm physiological responses required for successful fertilization such as hyperactivated motility, chemotaxis, and the acrosome reaction. CatSper, a pH-regulated, calcium-selective ion channel, and KSper (Slo3) are core regulators of sperm tail calcium entry and sperm hyperactivated motility. Many other channels had been proposed as regulating sperm activity without direct measurements. With the development of the sperm patch-clamp technique, CatSper and KSper have been confirmed as the primary spermatozoan ion channels. In addition, the voltage-gated proton channel Hv1 has been identified in human sperm tail, and the P2X2 ion channel has been identified in the midpiece of mouse sperm. Mutations and deletions in sperm-specific ion channels affect male fertility in both mice and humans without affecting other physiological functions. The uniqueness of sperm ion channels makes them ideal pharmaceutical targets for contraception. In this review we discuss how ion channels regulate sperm physiology.
Keywords: sperm ion channels, intracellular pH, capacitation, patch clamp, hyperactivation, chemotaxis, male fertility, CatSper, Hv1, KSper, acrosome reaction
INTRODUCTION
In sexual reproduction two haploid gametes (spermatozoon and egg) fuse and restore the original number of chromosomes, resulting in the zygote and the development of a new organism. In many aquatic organisms, mature sperm cells sense the egg and swim toward it in an almost infinite unregulated environment. In contrast, spermatozoa of terrestrial animals are delivered directly into the confined, strictly regulated environment of the female reproductive tract, where they must undergo final maturation before fertilization can occur. Thus, the female reproductive tract has the capacity to select and orient sperm, making it an active recipient of male gametes.
Ion channels control sperm membrane potential, cytoplasmic Ca2+, and intracellular pH (pHi), which in turn regulate motility, the acrosome reaction, and other diverse physiological processes essential for successful fertilization (1–3). The dramatic improvement in our understanding of the sperm ion channels was triggered by the discovery of CatSper (cationic channel of sperm), a novel and complex ion channel that mediates Ca2+ entry in sperm flagellum and is required for sperm hyperactivation and male fertility (4). The interest in functional characterization of the CatSper channel and the mechanisms of its regulation led to the first successful application of the whole-cell patch-clamp technique to mice and then to human spermatozoa (5, 6). The scope of this review is to discuss the role of plasma membrane ion channels in normal sperm physiology, to provide an update of recent important developments in sperm ion channel research, and to discuss sperm channelopathies that cause male infertility.
SPERM MORPHOLOGY
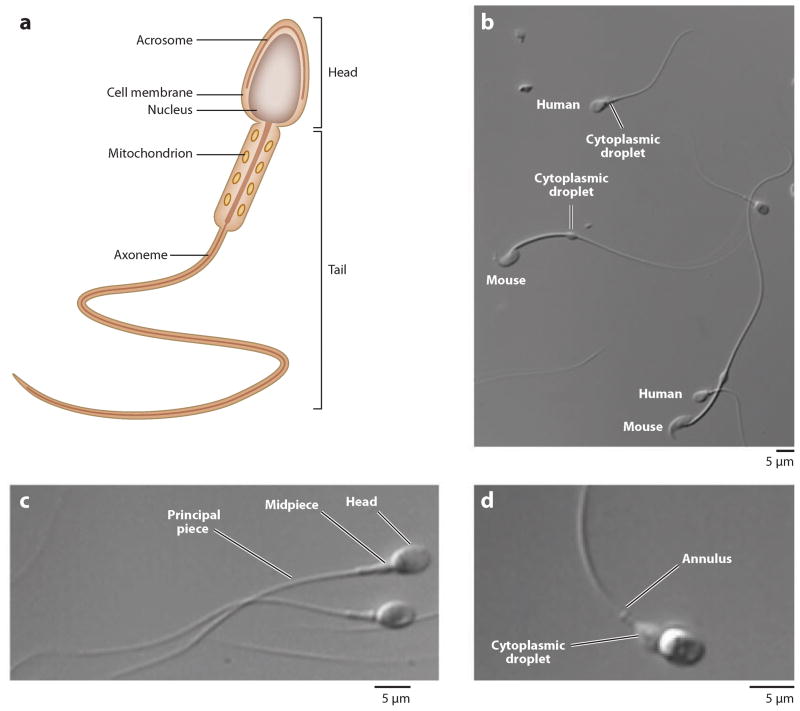
Spermatozoa are terminally differentiated motile cells with a clear cell polarity determined by the two main structural elements: the head, which contains tightly packed DNA, and the motile flagellum, which delivers the genetic material of the sperm head into the egg (Figure 1a). Structurally similar flagella are present in all spermatozoa across the animal and plant kingdoms (Figure 1b). The sperm head consists of the nucleus, the tiny residual nuclear envelope vestiges, and the acrosome (a Golgi-derived vesicle that helps spermatozoa to penetrate egg’s protective vestments). The mammalian flagellum has a central axoneme surrounded by specialized structural components and is composed of three parts: the midpiece, which contains mitochondria wrapped in a spiral pattern around the axoneme; the principal piece, which is primarily responsible for motility; and the endpiece, which contains few structural elements (Figure 1a,c). The midpiece and the principal piece are separated by a ringed septin structure termed the annulus, which prevents diffusion of plasma membrane proteins between these two flagellar domains (Figure 1d ) (7).
Figure 1.
Mammalian spermatozoa. (a) Schematic representation of mammalian sperm. (b) Comparison between human and mouse spermatozoa. (c) Human spermatozoa with head, midpiece, and principal piece as indicated. (d ) Cytoplasmic droplet and annulus are labeled.
The sperm plasma membrane is tightly attached to the underlying cellular structures along the whole sperm body to provide stiffness. Many membrane proteins of the sperm principal piece appear to be anchored to the underlying fibrous sheath to ensure their strict compartmentalization. The fibrous sheath functions as a scaffold for proteins in signaling pathways that regulate sperm maturation, motility, capacitation, hyperactivation, and/or the acrosome reaction. Interestingly, the fibrous sheath also anchors enzymes of the sperm-specific glycolytic pathway that provide ATP for motility (8). The cytoplasmic droplet, the remnant of the precursor cell’s cytoplasm, is the only region of the plasma membrane loosely attached to the intracellular structures (Figure 1d ). The cytoplasmic droplet is likely to serve as a reservoir for adaptation to osmotic changes occurring at ejaculation (9) but in many species is shed from spermatozoa after ejaculation.
The sperm axoneme is composed of microtubules: Nine outer doublet microtubules surround a central pair of singlet microtubules (a 9+2 arrangement) and the associated proteins such as the molecular motor dynein. Axonemal bending is produced by sliding between pairs of outer doublet microtubules (10), and this sliding is powered by ATP hydrolysis by dynein’s heavy chains. This active sliding of microtubules is a linear phenomenon, but the bending and propagation of the wave of motion down the flagellum are not well understood. One theory for the generation of flagellar motion is the geometric clutch hypothesis, whereby dynein engagement alternates between sides of the axoneme as the flagellum bends (11). Sperm axoneme bending is sensitive to intracellular alkalinization (12) and intracellular Ca2+ ([Ca2+]i) (13) so that increasing pHi above 7 stimulates dynein activity and promotes flagellar beating, whereas increasing intracellular Ca2+ enhances asymmetrical flagellum bending (14, 15). As spermatozoa travel through the environment of changing pH, osmolarity and sense extracellular cues such as progesterone and chemoattractants, sperm ion channels, and transporters regulate ion concentrations within the sperm’s cytoplasm to control motility and to trigger physiological responses such as hyperactivation of motility (hyperactivation) and the acrosome reaction.
ACTIVATION OF MOTILITY, CAPACITATION, AND HYPERACTIVATION
Mammalian spermatozoa from all portions of the epididymis have an acidic pHi (~6.8) and are essentially quiescent (16). When spermatozoa are mixed with seminal plasma (pH > 7.0) upon ejaculation, the sperm cytoplasm is alkalinized (17), and sperm become motile. Despite being motile, freshly ejaculated mammalian spermatozoa are unable or poorly able to fertilize the oocyte. To become competent to fertilize the egg, they must undergo capacitation: a phenomenon reported in 1951 by Austin (18) and Chang (19).
Capacitation results in the removal of noncovalently attached glycoproteins acquired in the epididymis, in the removal of adherent seminal plasma proteins, and in the depletion of the membrane cholesterol and other sterols (20, 21). Moreover, during capacitation, intracellular Ca2+, pH, and cyclic adenosine monophosphate (cAMP) increase, and sperm membrane proteins are phosphorylated on tyrosines (22–24). The first steps of capacitation may begin anywhere extracellular pH (pHo) is elevated, such as at the cervical mucus, but the ampulla of Fallopian tubes is critical for the completion of the process. Motility is hyperactivated during capacitation. Hyperactivation is defined by an increase in the angle of the flagellar bend, which results in more asymmetrical (whip-like) movements and more powerful swimming force (14, 15, 25). The second major change is that sperm acquire the ability to undergo the acrosome reaction (18, 19, 26). Capacitation increases the fluidity of the plasma membrane and sensitizes sperm to fertilization cues.
When capacitated spermatazoa encounter the cumulus oophorus (27) or bind the glycoproteins of the egg’s zona pellucida (ZP), there are additional steep increases in sperm pHi and Ca2+, resulting in the acrosome reaction (28–32). The sperm plasma membrane contains specific ion channels and transporters that initiate changes in these ions in the sperm cytoplasm (4–6, 33–39). During the acrosome reaction, hydrolytic enzymes are expelled from the sperm acrosome to facilitate penetration through the egg’s protective vestments (29).
As in all cells, sperm Na+/K+-ATPases establish the high K+ and low Na+ concentration of the sperm cytoplasm (38). As in serum, the extracellular fluids surrounding sperm cells contain 1–2 mM [Ca2+]. Cells maintain a remarkable 20,000-fold gradient from outside the cell to inside the cell, with resting cytosolic Ca2+ concentrations ranging from ~50–100 nM [Ca2+]. In somatic cells, these gradients are maintained by the export of cytoplasmic Ca2+ across the plasma membrane and by the import of Ca2+ into the endoplasmic reticulum and mitochondria. In sperm, these gradients are maintained primarily by a plasma membrane Ca2+-ATPase pump (PMCA4) that extrudes Ca2+ (40–42). Male mice deficient in PMCA4 have impaired sperm motility and are infertile (40, 41). Unlike other cells, spermatozoa do not contain significant amounts of endoplasmic reticulum. How much Ca2+ is stored in sperm mitochondria remains unexplored.
The proton gradient across the sperm plasma membrane is the inverse of the Ca2+ gradient. Serum [H+] (40 nM, pH ~ 7.4) is fourfold lower than the intracellular [H+] of ejaculated spermatozoa (160 nM, pH ~ 6.8) (17, 43, 44). In epididymis, the extracellular fluid (pH 5.5 to 6.8; [H+] from 3160 to 160 nM) is even more acidic (16). Epididymal sperm pHi drops below 6.0 due to the activity of different exchangers, including Na+/H+ and bicarbonate exchangers (45–48). Thus, there is always a concentration gradient in protons between cytoplasm and extracellular fluid. Low epididymal pH (and thus low pHi) appears to be a major factor in rendering spermatozoa quiescent before ejaculation by inhibiting axonemal dynein activity (16, 17, 49). Also, the high viscosity of the cauda epididymal fluid (50, 51) and proteins such as semenogelin (52) inhibits sperm motility. Upon ejaculation, sperm cells are mixed with seminal plasma of much higher pH (~7.4; [H+] = 40 nM), and as sperm pHi rises to ~6.5 ([H+] = 316 nM), sperm become motile for the first time (1, 44, 47). Lactobacilli and other vaginal flora acidify the vagina (pH ~ 4; [H+] = 100 μM); seminal plasma transiently increases female vaginal pH from 4.3 to 7.2 after intercourse (53), alkalinizing the environment and thus enabling spermatozoa to begin swimming. During subsequent transit through the female reproductive tract, pHi increases further but still lags behind pHo. Interestingly, at the peak of fertility in the middle of the menstrual cycle, cervical mucus becomes less viscous, and its pH can reach 9.0, making it less of a barrier to sperm (54). The pH of follicular fluid varies between 7 and 8, depending on the species and the phase of the menstrual cycle (55).
Upon ejaculation, sperm intracellular cAMP is elevated due to HCO3− activation of sperm soluble adenylyl cyclase (sAC) in a pH-independent manner (56). HCO3− concentration is higher in the seminal plasma/female reproductive tract than in the epididymal fluid (57), and HCO3− transporters deliver HCO3− into sperm cells. Moreover, the female oviduct is enriched in CO2, which is converted into HCO3− by sperm extracellular glycosyl phosphatidylinositol–anchored carbonic anhydrase IV (58). Intracellular cAMP induces phosphorylation of axonemal dynein by protein kinase A (PKA) (59–61) to increase flagellar beating and sperm motility (62). sAC is also activated by Ca2+ (63, 64), and extracellular Ca2+ is required for the sAC-dependent increase in the frequency of flagellar beat triggered by HCO3− (65). Not surprisingly, male sAC and PKA knockout mice have impaired sperm motility and are infertile (66–68). PKA and sAC do not seem to be required for initiation of sperm motility but rather increase the frequency of sperm tail beating and improve progressive motility (61, 68, 69).
As mentioned above, the high proton and low Ca2+ concentrations in the sperm cytoplasm suppress sperm motility. Activation of spermatazoa requires alkalinization of sperm cytoplasm by the extrusion of protons and the elevation of intracellular Ca2+ concentration [Ca2+]i. However, because transporters pump ions much more slowly than do channels, changes in intracellular ion concentrations are relatively slow compared with the rapid changes elicited by ion channels. As such, ion channels are primarily responsible for rapid signaling events (70). A more rapid change in sperm motility is achieved by fast diffusion of K+, protons, and Ca2+ down their K+, H+, and Ca2+ concentration gradients across the sperm plasma membrane through selective ion channels. The opening of such channels is controlled by specific cues in the female reproductive tract that regulate the activity of the sperm cells (Figure 2). This regulation is both spatial and temporal in accordance with female anatomy and the phase of the menstrual cycle. Known cues for human spermatozoa are H+ concentration, progesterone, and anandamide released by the cumulus oophorus; glycoproteins of the ZP; and proteins of the oviductal fluid such as serum albumin (31, 32, 71–75). However, there are probably more yet-to-be-discovered factors in the female reproductive tract that directly or indirectly control activity of sperm ion channels that synchronize arrival of the egg and the sperm at the fertilization site.
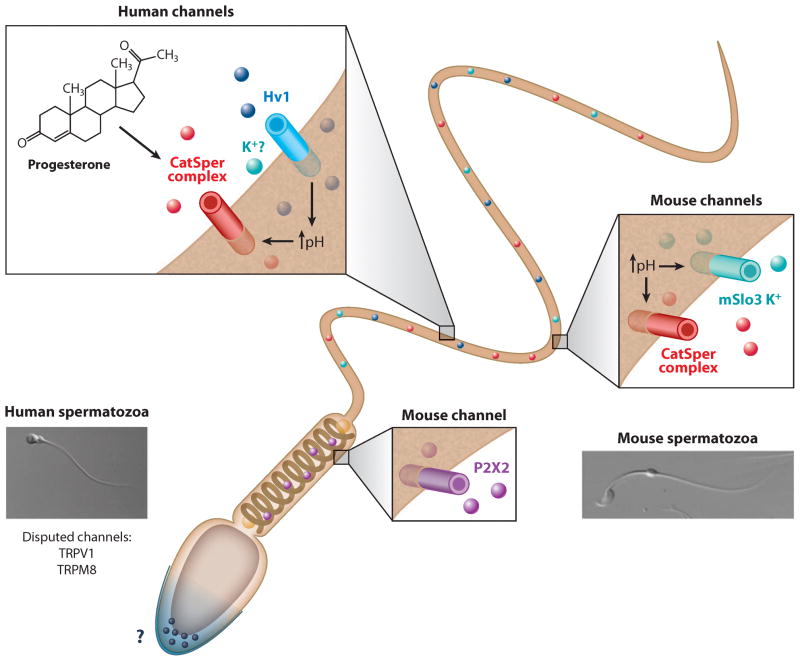
Figure 2.
Sperm ion channel localization and function. Flagellar beating is regulated by at least three ion channels: alkaline-sensitive CatSper (Ca2+ entry), pH-regulated Slo3 (K+ exit), and Hv1 (H+ exit; human sperm only). The upper half of the figure depicts ion channels and their regulation as detected in human sperm (the regulation of the CatSper complex by progesterone and Hv1), whereas the lower half of the figure shows ion channels found in mouse spermatozoa (CatSper complex, Slo3, and P2X2).
SPERM ION CHANNELS
Calcium Channels
Ca2+ is critical to the initiation of cellular motion of all kinds (76). In spermatozoa, however, normal swimming behavior does not require the elevation of Ca2+, a fact that at first surprised physiologists who focus on muscle and nerve. Sperm can swim over a range of [Ca2+]i, even in the absence of a plasma membrane, because they are essentially ciliary dynein ATPase motors. Just as in muscle and nerve, changing Ca2+ triggers changes in the behavior of motor proteins. Nonetheless, the elevation of intracellular Ca2+ is essential for changes in flagellar function that are manifested by capacitation, chemotaxis, and hyperactivated motility. Moreover, Ca2+ is required for initiation of the acrosome reaction.
Increases in [Ca2+]i regulate the sperm’s flagellar waveform and promote its asymmetrical bending (77). Before 2001, the channel responsible for sperm Ca2+ elevation was believed to be a voltage-gated Ca2+ channel (Cav; VGCC) and was perceived as the principal Ca2+ conductance of sperm (78–80). This notion was supported by electrophysiological identification of Cav channels in testicular spermatocytes (immature spermatogenic cells) using the patch-clamp technique (81–83) and by observation of a putative voltage-gated Ca2+ influx into mature sperm cells in response to the application of a high-K+/high-pH extracellular medium (84). Yet, male mice deficient in Cav2.2, Cav2.3, and Cav3.1 were fertile, indicating that these VGCC channels were not essential for sperm physiology or functioned redundantly (85–87). Knockouts of Cav1.2 and Cav2.1 were lethal either at embryonic stages or soon after birth, thus precluding assessment of Ca2+ elevation in sperm (88, 89).
In 2001, the first member of a completely new family of Ca2+-selective ion channel subunits was discovered. Termed CatSper1, it was found to be only in sperm cells and to be required for male fertility (4). Since then, seven CatSper subunits composing the heteromeric CatSper channel have been identified, and at least five of them—CatSper1–4 and CatSperδ—have been shown to be indispensible for proper channel formation and function (Figure 3) (Table 1) (4, 90–96). CatSper’s pore is formed by four α subunits, the products of four distinct genes: Catsper1, Catsper2, Catsper3, and Catsper4. The channel contains three auxiliary subunits—CatSperβ, CatSperγ, and CatSperδ—of unknown stoichiometry (32, 93, 95, 96). All CatSper subunits are sperm-specific proteins and are located in the principal piece of the sperm flagellum. CatSperβ is predicted to have two transmembrane helices connected by a large extracellular loop. CatSperγ and CatSperδ have single predicted transmembrane helices and large extracellular domains. The function of the auxiliary subunits after assembly of the CatSper channel complex is not known.
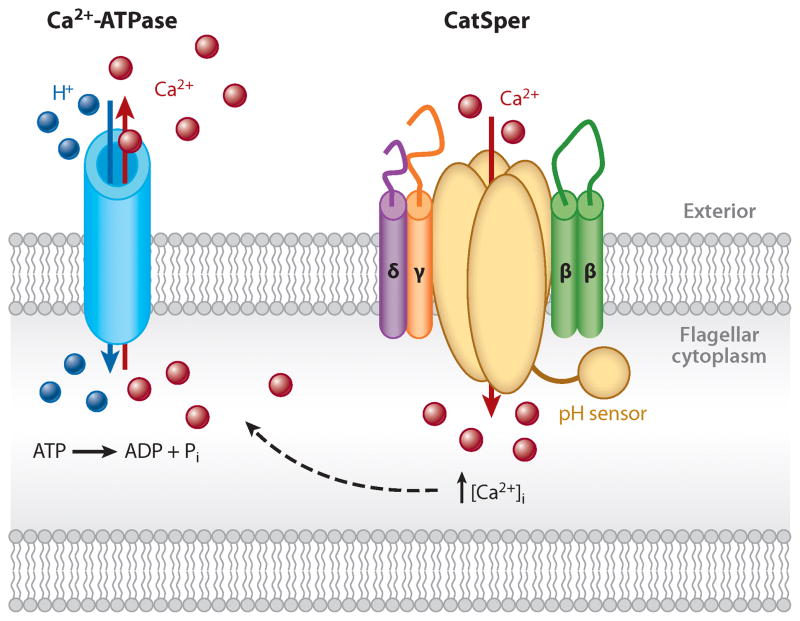
Figure 3.
Regulation of flagellar Ca2+. Ca2+ enters the sperm flagellum via the alkaline-activated CatSper channel and is extruded from the flagellum by a plasma membrane Ca2+-ATPase (42). Ca2+-ATPase pumps hydrolyze ATP to export a cytoplasmic Ca2+ ion and to import extracellular protons. The resulting acidification of flagellar cytoplasm must be prevented by proton extrusion via channels or transporters.
Table 1.
Ion channels of mammalian spermatozoa
| Channel name | Gene name and chromosomal location | Ion selectivity | Subunit(s)/composition | Localization in sperm | Role in sperm physiology, including sperm specificity | Endogenous regulators | Knockout phenotype |
|---|---|---|---|---|---|---|---|
| CatSper |
CatSper1 (11q13.1) CatSper2 (15q15.3), (15q15.3- pseudogene) CatSper3 (5q31.1) CatSper4 (1p36.11) CatSperβ (14q32.11) CatSperγ (19p13.2) CatSperδ (19p13.3); locations shown are for human CatSper |
Ca2+ | Seven subunits total: heteromeric assembly of CatSper1–4 (pore subunits), CatSperβ, -γ, -δ(auxiliary subunits); stoichiometry is unknown | Principal piece | Ca2+ influx into flagella, hyperactivated motility, sperm specific | Progesterone (human sperm only), pHi, egg coat proteins, albumin | Male sterility; sperm from CatSper−/− mice are unable to hyperactivate |
| KSper | Mouse: Slo3 (8A3); human: Slo3 (8p11.23) | K+ | Probable tetramer | Principal piece | Hyperpolarizes sperm membrane, regulates membrane potential; sperm specific | pHi | Male sterility– increased bent hairpin morphology of spermatozoa |
| Hv1 | Human: Hvcn1 (12q24.11) | H+ | Probable homomeric dimer | Principal piece | Extrudes protons from flagellum, alkalinizes cytoplasm; primarily in phagocytic cells (e.g., leukocytes), alveolar type II cells, and spermatozoa (human) | pHi, membrane voltage, removal of zinc, anandamide | IHv1 is not present in murine epididymal sperm; Hvcn1−/− mice are fertile |
| IATP | Human: P2RX2 (12q24.33); mouse: P2rx2 (5) | Na+, K+, Ca2+ | Probable homotrimer | Midpiece | Widespread | P2rx2−/− mice are fertile |
Although interaction of CatSperβ and CatSperγ with the CatSper complex was clearly demonstrated biochemically (93, 95), whether they are required for the functional CatSper channel assembly is not clear. In contrast, CatSperδ not only interacts with the CatSper complex but is essential for functional CatSper (96). Like mice lacking any of the four CatSper α subunits (94), CatSperδ−/− mice have no measurable CatSper current (ICatSper ) and have the identical phenotype of male infertility due to loss of hyperactivated motility (96). Interestingly, CatSperβ, CatSperγ, CatSperδ, CatSper2, CatSper3, and CatSper4 are all undetectable on CatSper1 knockout sperm plasma membranes (92, 93, 95, 96), suggesting that all CatSper subunits are required for proper channel assembly; the absence of a single subunit may lead to degradation of remaining CatSper proteins. Humans with mutations or deletions in CatSper1 and CatSper2 are infertile (97–100). We suspect that loss-of-function mutations in any of the seven known CatSper subunits result in male infertility.
Direct electrophysiological characterization of CatSper1 was achieved with the whole-cell patch-clamp technique applied to mouse spermatozoa (6). Comparison of ion currents recorded from wild-type and CatSper1-deficient spermatozoa confirmed that CatSper1 is required for a highly selective Ca2+ current. Recording from fragments of mouse spermatozoa established that ICatSper originated from the principal piece of the sperm flagellum, corresponding to antibody localization of the CatSper1 protein. ICatSper is weakly voltage dependent (the slope factor of the voltage activation curve k = 30) in comparison to strongly voltage-activated channels (k = 4) (6). Interestingly, the S4 transmembrane helix of CatSper1 contains six positively charged lysine/arginine residues aligned in the same manner as in strongly voltage-sensitive channels. However, CatSper2 has only four such residues, and only two are preserved in CatSper3 and -4. Because the pore of this heteromeric channel is formed by all four CatSpers, the voltage sensitivity of the complete channel is weak (34, 94).
The mouse CatSper channel is gated by changes in pHi: The current is increased approximately sevenfold when pHi is increased from 6.0 to 7.0 (6), corresponding to a (leftward) shift in the G-V curve of −70 mV. The abundance of histidines in the mouse CatSper1 N-terminal domain (51 His in the 250-residue N terminus) is one possible mechanism for this pH sensitivity (4, 34). Intracellular alkalinization by extracellular application of NH4Cl not only causes [Ca2+]i elevation by activating the CatSper channel but also triggers sperm hyperactivation (101).
Another hallmark of capacitation is reduction of sperm membrane cholesterol. Albumin, the main protein of the tubular fluid and an important component of in vitro capacitation media, also causes CatSper-dependent Ca2+ influx into mouse spermatozoa (74), perhaps affecting CatSper gating by modification of the lipid composition of the sperm plasma membrane. Finally, Ca2+ influx into mouse spermatozoa induced by the glycoproteins of the egg’s ZP requires the CatSper channel (31), a property formerly assigned to the putative sperm Cav channels (78, 79). In this regard, the Cav current present in spermatocytes is not detected in mature spermatozoa—all channel-mediated Ca2+ entry in mature spermatozoa is via CatSper.
The subunits of CatSper channel are present in all mammalian genomes and some invertebrate species, such as the sea urchin and the freshwater mold Allomyces macrogynus (102, 103), but not in genomes of birds, amphibians, insects, and worms. The rapid disappearance of CatSper from some intermediate species over millions of years reflects the strong evolutionary pressure on gamete genes (102).
The CatSper channel is present in human sperm (5, 104) and, like mouse CatSper, is weakly voltage dependent but potently activated by intracellular alkalinization. The voltage dependency of human CatSper is slightly steeper (k = 20 compared with k = 30 in mice) than in mouse CatSper. Importantly, the V1/2 (the voltage at which half of the channels are activated) of human CatSper is +85 mV versus +11 mV of mouse CatSper at the same pHi (pHi = 7.5) (6, 104), leading to the question of how human CatSper might be activated at such high membrane potentials.
Progesterone, a major steroid hormone released by the ovaries and the cumulus cells surrounding the egg, induces robust Ca2+ influx into human sperm cells (105, 106), triggers sperm hyperactivation, and initiates the acrosome reaction. These rapid effects are not via the nuclear progesterone receptor (107, 108). Progesterone exerts its effect on Xenopus laevis oocyte maturation and affects neural function without binding nuclear DNA or regulating gene expression. For example, X. laevis oocytes that are arrested in the G2 phase undergo maturation after the addition of extracellular progesterone. This phenomenon can occur even in enucleated cells. Also, progesterone can modulate γ-aminobutyric acid–mediated, glycine-mediated, and 5-hydroxytryptamine-mediated currents in neurons. However, the elusive progesterone receptor associated with humanspermatozoa is probably the best-known example of a nongenomic progesterone receptor (107, 108).
The mystery of progesterone’s short-term responses on sperm was recently solved. Progesterone activates human CatSper at low concentrations [EC50 ≈ 7.7 nM (104)] by shifting the voltage dependency of the human CatSper channel into the physiological range (104). The action of progesterone is rapid (latency <36 ms) and does not depend on intracellular ATP, GDP, cyclic nucleotides, Ca2+, or other soluble intracellular messengers (104, 109). The simplest explanation of these results is that the progesterone-binding site may be located on one of the CatSper subunits or on a currently unidentified protein associated with the CatSper complex. The binding site associated with the CatSper channel for this hormone has not been identified but appears to be accessible from the extracellular space (104).
Prostaglandins are abundant in the seminal plasma (110) and are secreted by the oviduct and cumulus cells surrounding the oocyte (111). Nanomolar concentrations of select prostaglandins, including PGE1, evoke intracellular Ca2+ transients similar in amplitude and waveform to those induced by progesterone (112–114). The relative potency of the human CatSper activators is as follows: progesterone > PGF1 ≥ PGE1 > PGA1 > PGE2 > PGD2 (104). Prostaglandin effects are additive to those of progesterone and thus may be mediated through a different receptor (104, 109). High levels of Zn2+ in seminal plasma (115) are likely to block the CatSper channel and to prevent its activation in the seminal plasma, but once spermatozoa are in the female-dominant environment, Zn2+ should be diluted or chelated (116–118).
In conclusion, the CatSper complex is encoded by at least seven genes, making it the most biochemically complex of all ion channels. This complexity may be required for its assembly, trafficking, and localization to the flagella and for its sensitivity to pHi, progesterone, prostaglandins, and perhaps other proteins. Because orthologs of CatSper subunits present in different species have low identity (50% or less) (32, 93, 102), regulation of the CatSper channel may differ significantly between species. The CatSper channel of murine epididymal sperm cells, for example, is not sensitive to the activators of human CatSper such as progesterone and prostaglandins (104). This difference in CatSper channel regulation and even the absence of CatSper genes in some species highlight the common trend in evolutionary pressure on gamete genes, which applies also to critical genes in sex determination pathways such as SRY and DAX1.
KSper (Slo3): The Principal K+ Channel of Spermatozoa
Incapacitated murine sperm hyperpolarize to approximately −60 mV during capacitation (119), an effect attributed to an increase in K+ permeability. In a series of experiments in which voltage and intracellular and extracellular solutions were controlled, Navarro et al. (120) determined that pHi sets the sperm membrane potential primarily by modifying the K+ conductance. Under direct voltage clamp of mouse epididymal spermatozoa, resting membrane potential hyperpolarized to −45 mV within a few seconds after alkalinization. This hyperpolarization was due to a weakly outwardly rectifying K+ current (IKSper ). IKSper exhibited minimal time and voltage dependence, was relatively K+ selective, and originated from the principal piece of the sperm flagellum. Intracellular alkalinization strongly potentiated IKSper independent of extracellular [K+]. IKSper was not affected by 2 mM membrane-permeant cAMP and cGMP analogs, by increasing extracellular [Ca2+], or by changes in bath osmolarity. Barium, quinine, clofilium, EIPA [a Na+/H+ exchanger (NHE) antagonist], and mibefradil reversibly inhibited IKSper. Thus, IKSper is the only detectable hyperpolarizing current in spermatozoa and largely sets its resting membrane potential. These authors suggested that IKSper is encoded by Slo3 on the basis of its tissue localization and other properties (Table 1). Like IKSper, Xenopus oocyte–expressed Slo3 is also weakly voltage sensitive (~16 mV/e-fold), has relaxed K+ selectivity, and is insensitive to [Ca2+]i and to external tetraethyl ammonium chloride (36, 121–123). This prediction was confirmed by recordings from mice lacking the Slo3 gene (124, 125). However, heterologously expressed mSlo3 had different pH sensitivity, which suggests that Slo3 in spermatozoa may be regulated by other subunits or mechanisms that are absent from heterologous expression systems.
In a carefully done study, genetic deletion of Slo3 abolished all pH-dependent K+ current at physiological membrane potentials in mouse corpus epididymal sperm (125). Slo3−/− mice are infertile and do not exhibit capacitation-dependent membrane hyperpolarization, and Slo3-deficient sperm morphological abnormalities are accentuated by hypotonic challenge. Solutions of lower osmolality (230–310 mOsm kg−1) resulted in an increase in bent and hairpin shapes, whereas spermatozoa kept in a hyperosmolar solution were protected against these changes. Incapacitated Slo3−/− sperm also have modest defects in motility, which may be related to a requirement for osmolar adaptation during spermatogenesis and sperm maturation (125). Only 10% of Slo3−/− sperm were able to fertilize oocytes during in vitro fertilization experiments. In summary, mSlo3 accounts for KSper, the dominant, if not the only, K+-selective channel in mouse epididymal spermatozoa.
The protein responsible for K+ current in human spermatozoa has not been identified but is likely to be the human homolog of Slo3, KCNU1. However, in contrast to the situation in murine sperm cells, human KSper seems to be independent of intracellular alkalinization (P. Lishko & Y. Kirichok, unpublished observation). Murine Slo3 and human Slo3 proteins are 65% identical; mouse Slo3 is more enriched in histidines in the cytoplasmic C terminus. The difference between human and murine sperm K+ current represents another discrepancy in physiology between human and mouse spermatozoa.
The Voltage-Gated Proton Channel Hv1: A Fast Regulator of Intracellular pH in Human Sperm
Intracellular alkalinization is essential for the initiation of motility, capacitation, hyperactivation, and the acrosome reaction. On the basis of experiments with pHi-sensitive fluorescent probes that detected changes in pHi, the NHE (45, 126, 127) and a Na+-dependent Cl−/HCO3− exchanger (46, 47) were proposed to participate in sperm alkalinization. Upon ejaculation, mammalian spermatozoa are exposed to 100–150 mM [Na+] in seminal plasma, a much higher Na+ concentration than the 30 mM [Na+] found in the cauda epididymis. In the female reproductive tract, Na+ levels are similar to those in sera (140–150 mM) (128, 129). Thus, in the exchange of Na+ for H+, spermatozoan pHi should increase. Sperm-specific molecules homologous to known Na+/H+ exchangers (sNHE) (130) are found in the principal piece of sperm flagellum. sNHE knockout mouse spermatozoa have impaired motility, and these males were completely infertile (130). Unfortunately, it has been difficult to demonstrate that sNHE actually functions as an NHE, as no significant difference in pH was found between wild-type and sNHE−/− spermatozoa (130). Complicating matters are the findings that sAC expression levels are significantly reduced in sNHE-deficient spermatozoa and that the sperm motility defect could be rescued by the addition of membrane-permeable cAMP analogs (130, 131).
The proton-selective, voltage-gated ion channel Hv1 (HVCN1) was cloned in 2006. This unusual channel is composed of a voltage sensor domain homologous to the voltage sensor of voltage-gated cation channels (132, 133). In contrast to the conventional ion channel, Hv1 lacks a classical pore region. The permeation pathway seems to be formed by an internal water wire completed by a movement of the charged S4 helix (134). Hv1 molecules dimerize, but each Hv1 subunit can function independently as a voltage-gated proton channel (135–137). The primary function of Hv1 in phagocytes is to allow intracellular protons to flow down their electrochemical gradient as electrons are extruded from cells via NADPH oxidase (NOX); block of Hv1 inhibits the innate immunity function of NOX (138, 139). Hv1 is characterized by strong voltage dependence, activation by high intracellular [H+], unidirectional proton extrusion (Hv1 is physiologically unidirectional), and inhibition by low micromolar concentrations of zinc and potentiation by fatty acids (140).
A voltage-gated proton channel was recorded in human spermatozoa (Table 1) (5). Although it has the electrophysiological and pharmacological properties of Hv1 (5, 141), its function in sperm may not be simply to support NOX. Hv1 is abundantly expressed in human sperm cells within the principal piece of the sperm flagellum, making it ideally positioned to activate pH-dependent proteins of the axoneme and thus to control sperm motility (5, 25, 141). The normal to alkaline pH of the upper female reproductive tract (~7.4) may rapidly alkalinize the acidic intracellular compartments of sperm as they leave the acidic environment of the cauda epididymis and vagina. However, these changes need not be rapid and may easily be accomplished by exchangers, leaving one to wonder about the need for fast H+ adaptation.
Human sperm flagella are long (40 μm), thin (<2 μm), and filled with axonemal structures. Because diffusion is inversely proportional to the area through which a substance diffuses, molecules take many seconds to travel within the extremely narrow flagellum from the mid-piece to the endpiece. Thus, ATP, generated in the mitochondria of the midpiece, is slow to reach the end of the flagellum. Therefore, flagellar movement, especially at the distal parts of the sperm tail, is powered mainly by glycolysis (142–144), which results in cytoplasm acidification. Moreover, axonemal dynein hydrolyzes ATP to produce ADP, Pi, and H+, all of which also contribute to intracellular acidification. The prompt removal of protons is thus vital to dynein function (Figure 4). Sperm Hv1 conducts protons much more rapidly and efficiently than do exchangers or transporters and conducts them unidirectionally to the extracellular space.
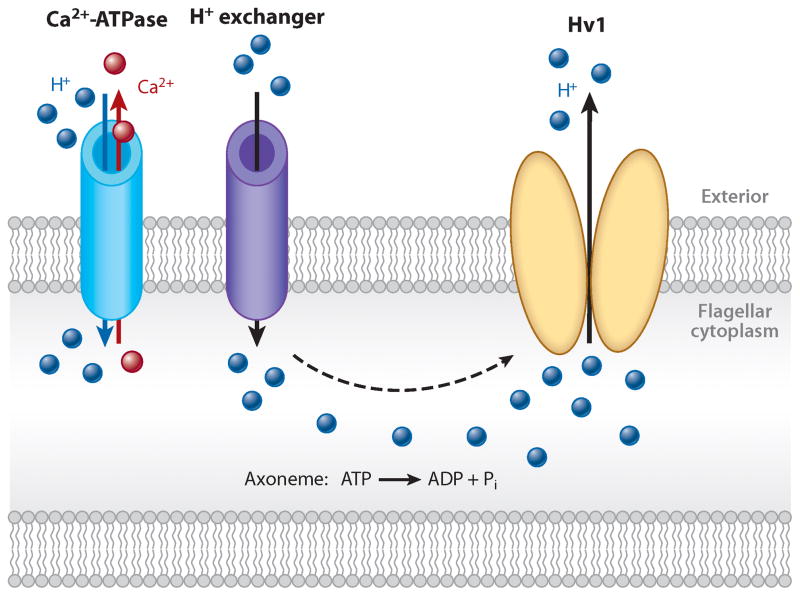
Figure 4.
Regulation of flagellar pH. Protons may accumulate in the sperm flagellum via proton exchange, ATP hydrolysis by axonemal dynein, and active glycolysis. Rapid proton extrusion from the human sperm flagella may be carried out by Hv1 proteins, which form a proton-selective, voltage-gated ion channel restricted to the sperm’s principal piece.
Another possible role assigned to Hv1 is the regulation of intracellular Ca2+ homeostasis. Ca2+ delivered through CatSper is pumped out by a flagellar Ca2+-ATPase that exports a cytoplasmic Ca2+ ion and imports extracellular protons. Its functioning results in decreasing flagellar pHi, potentially inhibiting the CatSper channel. To prevent this scenario and to return the system to the status quo, Hv1 may balance pHi by proton extrusion (Figure 3).
Hv1 is activated by the combination of the pH gradient and membrane depolarization (5, 132, 133, 140). However, because there is always a H+ gradient out of the spermatozoa, the sperm’s membrane potential is an important unknown and changes during sperm travel through the female reproductive tract. Membrane potential is set by Na+/K+-ATPases, which distribute ions over long durations, but is rapidly changed by the opening of ion channels.
Sperm Hv1 can be activated by the removal of extracellular zinc (5, 141). Zinc in humans is highest in seminal plasma (total 2.2 ± 1.1 mM compared with 14 ± 3 μM in serum) (115). Seminal zinc should inhibit Hv1, but as sperm travel through the female reproductive tract, any bound zinc is released through dilution, absorption by the uterine epithelium, and chelation by albumin and other molecules (116–118). Upon arrival at the Fallopian tube, spermatozoa should be essentially free from zinc inhibition. In addition, low micromolar concentrations of the endogenous cannabinoid anandamide strongly potentiate sperm Hv1 (5). The effect of anandamide is not mediated by CB1 or CB2 cannabinoid receptors and is likely due to a direct interaction of anandamide with Hv1 (5). Bulk concentrations of anandamide in the fluids of the male and female reproductive tracts are in the nanomolar range (145). However, because cumulus cells also synthesize and release anandamide, spermatozoa may experience much higher anandamide concentrations during the sperm’s penetration of the cumulus oophorus (75). Finally, Hv1 is activated during in vitro capacitation (5), a time when tyrosine phosphorylation is very active. The mechanism of this potentiation remains unknown, but one hypothesis is that Hv1 is phosphorylated, especially because phosphorylation is the primary mechanism of Hv1 regulation in other tissues (146, 147). Moreover, intracellular alkalinization is considered to be a key factor during capacitation (15), and the coincidence of capacitation and the enhancement of Hv1 activity suggest a strong connection between these two events.
To date, patch-clamp experiments with mouse epididymal spermatozoa have not detected proton currents (5), and Hv1-deficient mice do not exhibit fertility defects (138, 139). Unfortunately, the NHE is electroneutral, and its activity cannot be recorded with patch-clamp techniques. Thus, the identification of all components of H+ exchange in spermatozoa in mammals is an area for future detailed exploration.
In conclusion, sperm Hv1 may play an important role in the regulation of human sperm pHi. By doing so, it could potentially influence almost every aspect of sperm behavior in the female reproductive tract, including initiation of motility, capacitation, hyperactivation, and the acrosome reaction. However, the physiological function of sperm Hv1 remains to be established. To date, the only correlation between human infertility and Hv1 is low levels of sperm HVCN1 mRNA in some infertility patients (148). Studies of genetic infertility in humans may thus help us understand the exact role of Hv1 in male fertility.
The ATP-Gated P2X2 Channel of Mammalian Sperm
To date, transmitter-mediated currents have not been reported in mouse spermatozoa. After screening a number of neurotransmitters and other biological molecules for their ability to induce ion channel currents in the whole spermatazoon, Navarro et al. (37) found a cation-nonselective, Ca2+-permeable current originating from the midpiece of mouse epididymal spermatozoa that is activated by external ATP (IATP ) (Table 1). Various plasma membrane purinergic receptors for ATP (purinergic receptors) were found in sperm by immunocytochemical studies (149), and ATP was reported to mediate an increase in intracellular Ca2+ (74). Navarro et al. (37) show that the behavior of this slowly desensitizing and strongly inwardly rectifying ATP-gated current has biophysical and pharmacological properties that mimic those of the heterologously expressed P2X2 oligomeric cation channel. Moreover, IATP is absent in spermatozoa of mice lacking the P2rx2 gene. Despite the loss of IATP, P2rx2-deficient mice are fertile and have normal sperm morphology, sperm count, motility, and percent of sperm undergoing the acrosome reaction. However, the fertility of P2rx2−/− males declines with frequent mating over days, suggesting that the P2X2 receptor may confer a selection advantage under these conditions, perhaps through energizing mitochondria in the midpiece. ATP reportedly triggers the acrosome reaction in ejaculated bovine and human spermatozoa, reportedly via an uncharacterized sperm ATP-gated Na+ channel (150).
Other Spermatozoan Ion Channels
In addition to the four sperm ion channels reviewed above, less evidence exists for other functional ion channels. Before 2001, the VGCCs were perceived as the principal Ca2+ conductance of sperm (78–80), but patch-clamp recording from mature sperm did not reveal any functional VGCCs and established that the CatSper channel is the principal sperm Ca2+ channel (6). In addition, several stimuli (e.g., increase in pHi, depolarization, bovine serum albumin, and egg coat proteins) that trigger sperm Ca2+ influx previously assigned to VGCCs were later found to do so via the CatSper channel (31, 32). Finally, as discussed above, male mice deficient in Cav2.2, Cav2.3, and Cav3.1, three proteins detected only by antibodies in sperm, are fertile (85–87), indicating that these channels are not essential for sperm physiology or function redundantly (85–87). Our opinion is that VGCCs do not function in mature spermatozoa and do not have a significant role in mature sperm.
Cyclic nucleotide–gated (CNG) channels have also been proposed as mediating sperm Ca2+ influx. Both cAMP and cGMP elicit increases in [Ca2+]i in sperm, as demonstrated in assays in which cell-permeable cAMP or cGMP is applied or when caged cGMP is uncaged (151). Thus, similar to photoreceptors and olfactory neurons, CNG channels may be responsible for the cyclic nucleotide–induced Ca2+ influx in sperm (151). A more recent variation of this model proposes that cyclic nucleotides activate the hyperpolarization-activated and cyclic nucleotide–gated (HCN) channels, resulting in the depolarization and subsequent opening of Ca2+ channels (79, 152). Although CNG and HCN channels may be present in sea urchin spermatozoa, mice and humans deficient in the CNG and HCN channels are fertile and have not been shown to exhibit defects in sperm function despite deficiencies in vision and cardiac function. Furthermore, no CNG or HCN currents in mouse or human sperm have been detected to date. Finally, because CatSper is responsible for the cAMP/cGMP-induced Ca2+ influx into sperm (32), cyclic nucleotides may activate CatSper indirectly, possibly via a PKA-dependent mechanism.
Several of the 28 members of the transient receptor potential (TRP) ion channels were recently proposed to function in mature spermatozoa. These include TRPM8, TRPV1, TRPC2, and others (153–155). For example, the TRPC2 protein was detected in sperm, and an anti-TRPC2 antibody reduced the sustained Ca2+ response elicited by egg coat proteins in mouse sperm and the ZP-induced acrosome reaction (154). However, mice deficient in TRPC2 (as well as in the genes encoding TRPC1–7, TRPV1–4, TRPA1, TRPM1–4, and TRPM8) have no obvious defects in sperm physiology or male fertility. Indeed, in humans, TRPC2 is a pseudogene. Therefore, the contribution of TRPC2, TRPM8, and TRPV1 in sperm physiology, if any, remains to be clarified.
SPERM CHEMOTAXIS
In the search for the egg, spermatozoa of many species are aided by chemotactic factors (73, 152, 156). Chemotaxis was first discovered in invertebrate marine animals such as the sea urchin, starfish, and sea squirt (157–159). Most marine animals produce and release sperm cells and eggs into seawater. To reach the egg in time, sperm cells must navigate a gradient of the chemoattractant(s) released by the egg and swim toward it.
The first putative chemoattractant of sea urchin, Strongylocentrotus purpuratus spermatozoa, a small peptide speract, was isolated from egg coat in 1981 by Hansbrough & Garbers (159). Later, picomolar concentrations of speract were found to activate a K+ channel of S. purpuratus sperm (160). Interestingly, speract binding to the sea urchin spermatozoa also resulted in an increase in pHi (161). Resact, a peptide from the egg coat of another species, Arbacia punctulata, was discovered in 1985 and was clearly shown to attract spermatozoa (162). The current model for sea urchin chemotaxis is built around the actions of resact on spermatozoa from A. punctulata, which suggested that resact activates flagellar guanylyl cyclase (GC) and triggers a signal transduction pathway leading to Ca2+ influx into the sperm flagellum. In short, the sea urchin’s sperm flagellum contains a high density of membrane GC that produces cGMP from GTP in response to resact binding. cGMP is proposed to open K+-selective cyclic nucleotide–gated (KCNG) channels; such opening briefly hyperpolarizes the sperm membrane (163). As a result, HCN channels open and allow Na+ entry into the sperm flagellum. The resulting depolarization opens VGCCs, which conduct Ca2+ into flagellum and change the beating pattern. This sequence of events will require direct confirmation by recording under voltage clamp. Because all the CatSper α genes are present in the sea urchin (102, 103), we suspect that the story of sea urchin chemotaxis is not yet complete.
In contrast to the well-studied chemotaxis of sea urchin spermatozoa, much less is known about the chemotaxis of mammalian sperm. Mammalian spermatozoa are not likely to be engaged in the competitive-race model. Out of millions of spermatozoa delivered into the female reproductive tract, only one of every million succeeds in entering the Fallopian tubes, and <100 are able to reach the ampulla at any given time (73). Spermatozoa may be directed to the oocyte by specific cues or by chemicals released by the cumulus oophorus. Indeed, in 1991 researchers showed that human spermatozoa tend to accumulate in the follicular fluid (164) and that there is a positive correlation between sperm accumulation in the fluid and fertilization rate. Sperm chemotaxis was proposed in frogs, mice, and rabbits (for review see Reference 73). The discovery that human and rabbit spermatozoa are sensitive to picomolar concentrations of female hormone progesterone (165) established progesterone as a potential chemoattractant for human spermatozoa. Progesterone secreted from the cumulus oophorus peaks at midcycle and is present in oviductal and follicular fluid. As mentioned above, picomolar concentrations of progesterone activate CatSper and may regulate directional movement of the spermatozoa (104, 109). Mouse epididymal sperm CatSper is insensitive to progesterone (104), but this hypothesis should also be tested in ejaculated mouse spermatozoa.
Interestingly, Ciona intestinalis (sea squirt) eggs release sperm-attracting and -activating factor (SAAF), a molecule structurally similar to progesterone, and SAAF is a potent chemoattractant for Ciona spermatozoa (166). The molecular target for SAAF is not known, but because the Ciona genome contains CatSper and Hvcn1 genes, the mechanism may be similar to that in human spermatozoa.
CONCLUSIONS
Ion channels of the sperm plasma membrane control the sperm membrane potential, establish intracellular Ca2+ and proton concentrations, direct cell movement, and, most importantly, are required for male fertility. With the ability to patch-clamp sperm, more light will be shed on the molecular identities and physiological regulation of sperm ion channels, resulting in new tools to control the behavior of spermatozoa and to increase or decrease male fertility. Given the enormous evolutionary pressure on genes optimizing gamete performance, there are likely many modifications or fine-tuning of the basic framework discussed above.
SUMMARY POINTS.
Sperm are free-swimming gametes that must adapt to changes in local environments on their journey to the egg. Ion channels of sperm enable sperm to respond and adjust to constantly changing environments and are required for male fertility.
Spermatozoa are compartmentalized cells, and plasma membrane ion channels are found primarily in the flagella. CatSper, KSper, and, in humans, Hv1 are localized in the principal piece of sperm flagellum, where they regulate sperm motility.
Direct recordings of spermatozoan ion currents under voltage clamp are essential for the proper identification of putative ion channel proteins found in sperm by other techniques.
Acknowledgments
This work was supported by grant R01HD068914 (NICHD) to Y.K., by grant 5R01HD47578 to D.R., and by grant U01HD045857 and a grant from the Gates Foundation to D.E.C. The content is solely the responsibility of the authors and does not necessarily represent the official views of NICHD or the NIH.
Glossary
- pHi
intracellular pH
- Acrosome
a cap-like vesicle covering the anterior portion of the head of a spermatozoon. The acrosome contains hydrolytic enzymes for penetrating through the protective vestments of the oocyte
- Acrosome reaction
exocytosis of the acrosomal vesicle upon the spermatozoon’s contact with egg’s protective vestments. Hydrolytic enzymes released by the acrosome digest the zona pellucida and help spermatozoa to reach the egg’s surface
- Cationic channel of sperm (CatSper)
a pH-regulated, calcium-selective ion channel required for sperm hyperactivation
- Hyperactivation
a whip-like, high-amplitude asymmetrical beat of the sperm flagellum that helps spermatozoa overcome the egg’s protective vestments. Hyperactivation is different from the low-amplitude symmetrical beat observed in normal motility
- Fibrous sheath
a unique cytoskeletal structure of two longitudinal columns, connected by closely arrayed semicircular ribs. Fibrous sheath surrounds the outer dense fibers and the axoneme. The fibrous sheath influences the degree of flexibility, the plane of flagellar motion, and the shape of the flagellar beat
- Capacitation
spermatozoa’s acquisition of fertilizing capacity upon exposure to the fluids of the female reproductive tract for several hours. Capacitation results in sperm hyperactivation and the ability to undergo the acrosome reaction
- Epididymis
a part of the male reproductive tract in which spermatozoa continue maturation and are stored
- cAMP
cyclic adenosine monophosphate
- pHo
extracellular pH
- Cumulus oophorus
the mass of cells, derived from granulosa cells of the Graafian follicle, that surrounds the oocyte upon its release during ovulation. To fertilize the oocyte, spermatozoon must first penetrate the cumulus oophorus
- Zona pellucida (ZP)
the dense extracellular matrix surrounding the developing oocyte. The ZP prevents fertilization by multiple spermatozoa (polyspermy), prevents premature implantation, and protects the embryo during its first week of development
- sAC
soluble adenylyl cyclase
- PKA
protein kinase A
- Chemotaxis
directional migration of the spermatozoa toward higher concentrations of chemoattractant released by the egg or cumulus oophorus
- VGCC
voltage-gated calcium channel
- ICatSper
CatSper current
- G-V curve
plot of membrane conductance (G) versus voltage (V) that is used to show the percentage of activated ion channels in relation to membrane potential
- Slo3
a K+-permeant ion channel
- sNHE
sperm Na+/H+ exchanger
- Hv1
the voltage-gated, proton-selective ion channel found usually in phagocytes but also present in human sperm
- CNG channel
cyclic nucleotide–gated channel
- HCN channel
hyperpolarization-activated and cyclic nucleotide–gated channel
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Babcock DF, Rufo GA, Jr, Lardy HA. Potassium-dependent increases in cytosolic pH stimulate metabolism and motility of mammalian sperm. Proc Natl Acad Sci USA. 1983;80:1327–31. doi: 10.1073/pnas.80.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yanagimachi R, Usui N. Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp Cell Res. 1974;89:161–74. doi: 10.1016/0014-4827(74)90199-2. [DOI] [PubMed] [Google Scholar]
- 3.Dan JC. Studies on the acrosome. III Effect of Ca2+ deficiency. Biol Bull. 1954;107:335–49. [Google Scholar]
- 4.Ren D, Navarro B, Perez G, Jackson AC, Hsu S, et al. A sperm ion channel required for sperm motility and male fertility. Nature. 2001;413:603–9. doi: 10.1038/35098027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lishko PV, Botchkina IL, Fedorenko A, Kirichok Y. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 2010;140:327–37. doi: 10.1016/j.cell.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 6.Kirichok Y, Navarro B, Clapham DE. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 2006;439:737–40. doi: 10.1038/nature04417. [DOI] [PubMed] [Google Scholar]
- 7.Kwitny S, Klaus AV, Hunnicutt GR. The annulus of the mouse sperm tail is required to establish a membrane diffusion barrier that is engaged during the late steps of spermiogenesis. Biol Reprod. 2010;82:669–78. doi: 10.1095/biolreprod.109.079566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddy EM, Toshimori K, O’Brien DA. Fibrous sheath of mammalian spermatozoa. Microsc Res Tech. 2003;61:103–15. doi: 10.1002/jemt.10320. [DOI] [PubMed] [Google Scholar]
- 9.Cooper TG. The epididymis, cytoplasmic droplets and male fertility. Asian J Androl. 2011;13:130–38. doi: 10.1038/aja.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Summers KE, Gibbons IR. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci USA. 1971;68:3092–96. doi: 10.1073/pnas.68.12.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindemann CB. Experimental evidence for the geometric clutch hypothesis. Curr Top Dev Biol. 2011;95:1–31. doi: 10.1016/B978-0-12-385065-2.00001-3. [DOI] [PubMed] [Google Scholar]
- 12.Brokaw CJ, Kamiya R. Bending patterns of Chlamydomonas flagella. IV Mutants with defects in inner and outer dynein arms indicate differences in dynein arm function. Cell Motil Cytoskelet. 1987;8:68–75. doi: 10.1002/cm.970080110. [DOI] [PubMed] [Google Scholar]
- 13.Brokaw CJ. Regulation of sperm flagellar motility by calcium and cAMP-dependent phosphorylation. J Cell Biochem. 1987;35:175–84. doi: 10.1002/jcb.240350302. [DOI] [PubMed] [Google Scholar]
- 14.White DR, Aitken RJ. Relationship between calcium, cyclic AMP, ATP, and intracellular pH and the capacity of hamster spermatozoa to express hyperactivated motility. Gamete Res. 1989;22:163–77. doi: 10.1002/mrd.1120220205. [DOI] [PubMed] [Google Scholar]
- 15.Suarez SS. Control of hyperactivation in sperm. Hum Reprod Update. 2008;14:647–57. doi: 10.1093/humupd/dmn029. [DOI] [PubMed] [Google Scholar]
- 16.Acott TS, Carr DW. Inhibition of bovine spermatozoa by caudal epididymal fluid. II Interaction of pH and a quiescence factor. Biol Reprod. 1984;30:926–35. doi: 10.1095/biolreprod30.4.926. [DOI] [PubMed] [Google Scholar]
- 17.Hamamah S, Gatti JL. Role of the ionic environment and internal pH on sperm activity. Hum Reprod. 1998;13(Suppl 4):20–30. doi: 10.1093/humrep/13.suppl_4.20. [DOI] [PubMed] [Google Scholar]
- 18.Austin CR. Observations on the penetration of the sperm in the mammalian egg. Aust J Sci Res B. 1951;4:581–96. doi: 10.1071/bi9510581. [DOI] [PubMed] [Google Scholar]
- 19.Chang MC. Fertilizing capacity of spermatozoa deposited into the fallopian tubes. Nature. 1951;168:697–98. doi: 10.1038/168697b0. [DOI] [PubMed] [Google Scholar]
- 20.Eliasson R. Cholesterol in human semen. Biochem J. 1966;98:242–43. doi: 10.1042/bj0980242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Jonge C. Biological basis for human capacitation. Hum Reprod Update. 2005;11:205–14. doi: 10.1093/humupd/dmi010. [DOI] [PubMed] [Google Scholar]
- 22.Carr DW, Acott TS. Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biol Reprod. 1989;41:907–20. doi: 10.1095/biolreprod41.5.907. [DOI] [PubMed] [Google Scholar]
- 23.Visconti PE, Moore GD, Bailey JL, Leclerc P, Connors SA, et al. Capacitation of mouse spermatozoa. II Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121:1139–50. doi: 10.1242/dev.121.4.1139. [DOI] [PubMed] [Google Scholar]
- 24.Visconti PE, Westbrook VA, Chertihin O, Demarco I, Sleight S, Diekman AB. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J Reprod Immunol. 2002;53:133–50. doi: 10.1016/s0165-0378(01)00103-6. [DOI] [PubMed] [Google Scholar]
- 25.Kirichok Y, Lishko PV. Rediscovering sperm ion channels with the patch-clamp technique. Mol Hum Reprod. 2011;17:478–99. doi: 10.1093/molehr/gar044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahi CA, Yanagimachi R. The effects of temperature, osmolality and hydrogen ion concentration on the activation and acrosome reaction of golden hamster spermatozoa. J Reprod Fertil. 1973;35:55–66. doi: 10.1530/jrf.0.0350055. [DOI] [PubMed] [Google Scholar]
- 27.Jin M, Fujiwara E, Kakiuchi Y, Okabe M, Satouh Y, et al. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc Natl Acad Sci USA. 2011;108:4892–96. doi: 10.1073/pnas.1018202108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florman HM, Tombes RM, First NL, Babcock DF. An adhesion-associated agonist from the zona pellucida activates G protein-promoted elevations of internal Ca2+ and pH that mediate mammalian sperm acrosomal exocytosis. Dev Biol. 1989;135:133–46. doi: 10.1016/0012-1606(89)90164-4. [DOI] [PubMed] [Google Scholar]
- 29.Roldan ER, Murase T, Shi QX. Exocytosis in spermatozoa in response to progesterone and zona pellucida. Science. 1994;266:1578–81. doi: 10.1126/science.7985030. [DOI] [PubMed] [Google Scholar]
- 30.Arnoult C, Zeng Y, Florman HM. ZP3-dependent activation of sperm cation channels regulates acrosomal secretion during mammalian fertilization. J Cell Biol. 1996;134:637–45. doi: 10.1083/jcb.134.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia J, Ren D. Egg coat proteins activate calcium entry into mouse sperm via CATSPER channels. Biol Reprod. 2009;80:1092–98. doi: 10.1095/biolreprod.108.074039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ren D, Xia J. Calcium signaling through CatSper channels in mammalian fertilization. Physiology. 2010;25:165–75. doi: 10.1152/physiol.00049.2009. [DOI] [PubMed] [Google Scholar]
- 33.Lee HC, Garbers DL. Modulation of the voltage-sensitive Na+/H+ exchange in sea urchin spermatozoa through membrane potential changes induced by the egg peptide speract. J Biol Chem. 1986;261:16026–32. [PubMed] [Google Scholar]
- 34.Navarro B, Kirichok Y, Chung JJ, Clapham DE. Ion channels that control fertility in mammalian spermatozoa. Int J Dev Biol. 2008;52:607–13. doi: 10.1387/ijdb.072554bn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez D, Labarca P, Darszon A. Sea urchin sperm cation-selective channels directly modulated by cAMP. FEBS Lett. 2001;503:111–15. doi: 10.1016/s0014-5793(01)02713-2. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber M, Wei A, Yuan A, Gaut J, Saito M, Salkoff L. Slo3, a novel pH-sensitive K+ channel from mammalian spermatocytes. J Biol Chem. 1998;273:3509–16. doi: 10.1074/jbc.273.6.3509. [DOI] [PubMed] [Google Scholar]
- 37.Navarro B, Miki K, Clapham DE. ATP-activated P2·2 current in mouse spermatozoa. Proc Natl Acad Sci USA. 2011;108:14342–47. doi: 10.1073/pnas.1111695108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jimenez T, McDermott JP, Sanchez G, Blanco G. Na,K-ATPase α4 isoform is essential for sperm fertility. Proc Natl Acad Sci USA. 2011;108:644–49. doi: 10.1073/pnas.1016902108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Linares-Hernandez L, Guzman-Grenfell AM, Hicks-Gomez JJ, Gonzalez-Martinez MT. Voltage-dependent calcium influx in human sperm assessed by simultaneous optical detection of intracellular calcium and membrane potential. Biochim Biophys Acta. 1998;1372:1–12. doi: 10.1016/s0005-2736(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 40.Okunade GW, Miller ML, Pyne GJ, Sutliff RL, O’Connor KT, et al. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J Biol Chem. 2004;279:33742–50. doi: 10.1074/jbc.M404628200. [DOI] [PubMed] [Google Scholar]
- 41.Schuh K, Cartwright EJ, Jankevics E, Bundschu K, Liebermann J, et al. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J Biol Chem. 2004;279:28220–26. doi: 10.1074/jbc.M312599200. [DOI] [PubMed] [Google Scholar]
- 42.Wennemuth G, Babcock DF, Hille B. Calcium clearance mechanisms of mouse sperm. J Gen Physiol. 2003;122:115–28. doi: 10.1085/jgp.200308839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Babcock DF, Pfeiffer DR. Independent elevation of cytosolic [Ca2+] and pH of mammalian sperm by voltage-dependent and pH-sensitive mechanisms. J Biol Chem. 1987;262:15041–47. [PubMed] [Google Scholar]
- 44.Hamamah S, Magnoux E, Royere D, Barthelemy C, Dacheux JL, Gatti JL. Internal pH of human spermatozoa: effect of ions, human follicular fluid and progesterone. Mol Hum Reprod. 1996;2:219–24. doi: 10.1093/molehr/2.4.219. [DOI] [PubMed] [Google Scholar]
- 45.Garcia MA, Meizel S. Regulation of intracellular pH in capacitated human spermatozoa by a Na+/H+ exchanger. Mol Reprod Dev. 1999;52:189–95. doi: 10.1002/(SICI)1098-2795(199902)52:2<189::AID-MRD10>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 46.Tajima Y, Okamura N. The enhancing effects of anion channel blockers on sperm activation by bicarbonate. Biochim Biophys Acta. 1990;1034:326–32. doi: 10.1016/0304-4165(90)90059-6. [DOI] [PubMed] [Google Scholar]
- 47.Zeng Y, Oberdorf JA, Florman HM. pH regulation in mouse sperm: identification of Na+-, Cl−-, and HCO3−-dependent and arylaminobenzoate-dependent regulatory mechanisms and characterization of their roles in sperm capacitation. Dev Biol. 1996;173:510–20. doi: 10.1006/dbio.1996.0044. [DOI] [PubMed] [Google Scholar]
- 48.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–47. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carr DW, Acott TS. Inhibition of bovine spermatozoa by caudal epididymal fluid. I Studies of a sperm motility quiescence factor. Biol Reprod. 1984;30:913–25. doi: 10.1095/biolreprod30.4.913. [DOI] [PubMed] [Google Scholar]
- 50.Usselman MC, Cone RA. Rat sperm are mechanically immobilized in the caudal epididymis by “immobilin,” a high molecular weight glycoprotein. Biol Reprod. 1983;29:1241–53. doi: 10.1095/biolreprod29.5.1241. [DOI] [PubMed] [Google Scholar]
- 51.Carr DW, Usselman MC, Acott TS. Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison. Biol Reprod. 1985;33:588–95. doi: 10.1095/biolreprod33.3.588. [DOI] [PubMed] [Google Scholar]
- 52.Mitra A, Richardson RT, O’Rand MG. Analysis of recombinant human semenogelin as an inhibitor of human sperm motility. Biol Reprod. 2010;82:489–96. doi: 10.1095/biolreprod.109.081331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox CA, Meldrum SJ, Watson BW. Continuous measurement by radio-telemetry of vaginal pH during human coitus. J Reprod Fertil. 1973;33:69–75. doi: 10.1530/jrf.0.0330069. [DOI] [PubMed] [Google Scholar]
- 54.Eggert-Kruse W, Kohler A, Rohr G, Runnebaum B. The pH as an important determinant of sperm-mucus interaction. Fertil Steril. 1993;59:617–28. [PubMed] [Google Scholar]
- 55.Maas DH, Storey BT, Mastroianni L., Jr Hydrogen ion and carbon dioxide content of the oviductal fluid of the rhesus monkey (Macaca mulatta) Fertil Steril. 1977;28:981–85. [PubMed] [Google Scholar]
- 56.Chen Y, Cann MJ, Litvin TN, Iourgenko V, Sinclair ML, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–28. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 57.Okamura N, Tajima Y, Soejima A, Masuda H, Sugita Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem. 1985;260:9699–705. [PubMed] [Google Scholar]
- 58.Wandernoth PM, Raubuch M, Mannowetz N, Becker HM, Deitmer JW, et al. Role of carbonic anhydrase IV in the bicarbonate-mediated activation of murine and human sperm. PLoS ONE. 2010;5:e15061. doi: 10.1371/journal.pone.0015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goltz JS, Gardner TK, Kanous KS, Lindemann CB. The interaction of pH and cyclic adenosine 3′,5′-monophosphate on activation of motility in Triton X-100 extracted bull sperm. Biol Reprod. 1988;39:1129–36. doi: 10.1095/biolreprod39.5.1129. [DOI] [PubMed] [Google Scholar]
- 60.Harrison RA. Rapid PKA-catalysed phosphorylation of boar sperm proteins induced by the capacitating agent bicarbonate. Mol Reprod Dev. 2004;67:337–52. doi: 10.1002/mrd.20028. [DOI] [PubMed] [Google Scholar]
- 61.Nolan MA, Babcock DF, Wennemuth G, Brown W, Burton KA, McKnight GS. Sperm-specific protein kinase A catalytic subunit Cα2 orchestrates cAMP signaling for male fertility. Proc Natl Acad Sci USA. 2004;101:13483–88. doi: 10.1073/pnas.0405580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–22. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 63.Jaiswal BS, Conti M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc Natl Acad Sci USA. 2003;100:10676–81. doi: 10.1073/pnas.1831008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Litvin TN, Kamenetsky M, Zarifyan A, Buck J, Levin LR. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J Biol Chem. 2003;278:15922–26. doi: 10.1074/jbc.M212475200. [DOI] [PubMed] [Google Scholar]
- 65.Carlson AE, Hille B, Babcock DF. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev Biol. 2007;312:183–92. doi: 10.1016/j.ydbio.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MA, Robben TJ, et al. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc Natl Acad Sci USA. 2004;101:2993–98. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hess KC, Jones BH, Marquez B, Chen Y, Ord TS, et al. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev Cell. 2005;9:249–59. doi: 10.1016/j.devcel.2005.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xie F, Garcia MA, Carlson AE, Schuh SM, Babcock DF, et al. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev Biol. 2006;296:353–62. doi: 10.1016/j.ydbio.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 69.Wennemuth G, Carlson AE, Harper AJ, Babcock DF. Bicarbonate actions on flagellar and Ca2+-channel responses: initial events in sperm activation. Development. 2003;130:1317–26. doi: 10.1242/dev.00353. [DOI] [PubMed] [Google Scholar]
- 70.Hille B. Elementary properties of pores. In: Hille B, editor. Ionic Channels of Excitable Membranes. 2. Sunderland, MA: Sinauer Assoc; 1992. pp. 291–314. [Google Scholar]
- 71.Fraser LR. The “switching on” of mammalian spermatozoa: molecular events involved in promotion and regulation of capacitation. Mol Reprod Dev. 2010;77:197–208. doi: 10.1002/mrd.21124. [DOI] [PubMed] [Google Scholar]
- 72.Publicover S, Harper CV, Barratt C. [Ca2+]i signalling in sperm—making the most of what you’ve got. Nat Cell Biol. 2007;9:235–42. doi: 10.1038/ncb0307-235. [DOI] [PubMed] [Google Scholar]
- 73.Eisenbach M, Giojalas LC. Sperm guidance in mammals—an unpaved road to the egg. Nat Rev Mol Cell Biol. 2006;7:276–85. doi: 10.1038/nrm1893. [DOI] [PubMed] [Google Scholar]
- 74.Xia J, Ren D. The BSA-induced Ca2+ influx during sperm capacitation is CATSPER channel-dependent. Reprod Biol Endocrinol. 2009;7:119. doi: 10.1186/1477-7827-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El-Talatini MR, Taylor AH, Elson JC, Brown L, Davidson AC, Konje JC. Localisation and function of the endocannabinoid system in the human ovary. PLoS ONE. 2009;4:e4579. doi: 10.1371/journal.pone.0004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clapham DE. Calcium signaling. Cell. 2007;131:1047–58. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 77.Brokaw CJ. Calcium-induced asymmetrical beating of triton-demembranated sea urchin sperm flagella. J Cell Biol. 1979;82:401–11. doi: 10.1083/jcb.82.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Florman HM, Arnoult C, Kazam IG, Li C, O’Toole CM. A perspective on the control of mammalian fertilization by egg-activated ion channels in sperm: a tale of two channels. Biol Reprod. 1998;59:12–16. doi: 10.1095/biolreprod59.1.12. [DOI] [PubMed] [Google Scholar]
- 79.Darszon A, Labarca P, Nishigaki T, Espinosa F. Ion channels in sperm physiology. Physiol Rev. 1999;79:481–510. doi: 10.1152/physrev.1999.79.2.481. [DOI] [PubMed] [Google Scholar]
- 80.Publicover SJ, Barratt CL. Voltage-operated Ca2+ channels and the acrosome reaction: Which channels are present and what do they do? Hum Reprod. 1999;14:873–79. doi: 10.1093/humrep/14.4.873. [DOI] [PubMed] [Google Scholar]
- 81.Hagiwara S, Kawa K. Calcium and potassium currents in spermatogenic cells dissociated from rat seminiferous tubules. J Physiol. 1984;356:135–49. doi: 10.1113/jphysiol.1984.sp015457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arnoult C, Cardullo RA, Lemos JR, Florman HM. Activation of mouse sperm T-type Ca2+ channels by adhesion to the egg zona pellucida. Proc Natl Acad Sci USA. 1996;93:13004–9. doi: 10.1073/pnas.93.23.13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santi CM, Darszon A, Hernandez-Cruz A. A dihydropyridine-sensitive T-type Ca2+ current is the main Ca2+ current carrier in mouse primary spermatocytes. Am J Physiol Cell Physiol. 1996;271:1583–93. doi: 10.1152/ajpcell.1996.271.5.C1583. [DOI] [PubMed] [Google Scholar]
- 84.Wennemuth G, Westenbroek RE, Xu T, Hille B, Babcock DF. CaV 2.2 and CaV 2.3 (N- and R-type) Ca2+ channels in depolarization-evoked entry of Ca2+ into mouse sperm. J Biol Chem. 2000;275:21210–17. doi: 10.1074/jbc.M002068200. [DOI] [PubMed] [Google Scholar]
- 85.Beuckmann CT, Sinton CM, Miyamoto N, Ino M, Yanagisawa M. N-type calcium channel α 1B subunit (CaV 2.2) knock-out mice display hyperactivity and vigilance state differences. J Neurosci. 2003;23:6793–97. doi: 10.1523/JNEUROSCI.23-17-06793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saegusa H, Kurihara T, Zong S, Minowa O, Kazuno A, et al. Altered pain responses in mice lacking α1E subunit of the voltage-dependent Ca2+ channel. Proc Natl Acad Sci USA. 2000;97:6132–37. doi: 10.1073/pnas.100124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim D, Song I, Keum S, Lee T, Jeong MJ, et al. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 88.Jun K, Piedras-Renteria ES, Smith SM, Wheeler DB, Lee SB, et al. Ablation of P/Q-type Ca2+ channel currents, altered synaptic transmission, and progressive ataxia in mice lacking the α1A -subunit. Proc Natl Acad Sci USA. 1999;96:15245–50. doi: 10.1073/pnas.96.26.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seisenberger C, Specht V, Welling A, Platzer J, Pfeifer A, et al. Functional embryonic cardiomyocytes after disruption of the L-type α1C (Cav1.2) calcium channel gene in the mouse. J Biol Chem. 2000;275:39193–99. doi: 10.1074/jbc.M006467200. [DOI] [PubMed] [Google Scholar]
- 90.Lobley A, Pierron V, Reynolds L, Allen L, Michalovich D. Identification of human and mouse CatSper3 and CatSper4 genes: characterisation of a common interaction domain and evidence for expression in testis. Reprod Biol Endocrinol. 2003;1:53. doi: 10.1186/1477-7827-1-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Quill TA, Sugden SA, Rossi KL, Doolittle LK, Hammer RE, Garbers DL. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc Natl Acad Sci USA. 2003;100:14869–74. doi: 10.1073/pnas.2136654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carlson AE, Quill TA, Westenbroek RE, Schuh SM, Hille B, Babcock DF. Identical phenotypes of CatSper1 and CatSper2 null sperm. J Biol Chem. 2005;280:32238–44. doi: 10.1074/jbc.M501430200. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Xia J, Cho KH, Clapham DE, Ren D. CatSperβ, a novel transmembrane protein in the CatSper channel complex. J Biol Chem. 2007;282:18945–52. doi: 10.1074/jbc.M701083200. [DOI] [PubMed] [Google Scholar]
- 94.Qi H, Moran MM, Navarro B, Chong JA, Krapivinsky G, et al. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc Natl Acad Sci USA. 2007;104:1219–23. doi: 10.1073/pnas.0610286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H, Liu J, Cho KH, Ren D. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol Reprod. 2009;81:539–44. doi: 10.1095/biolreprod.109.077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chung JJ, Navarro B, Krapivinsky G, Krapivinsky L, Clapham DE. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun. 2011;2:153. doi: 10.1038/ncomms1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hildebrand MS, Avenarius MR, Fellous M, Zhang Y, Meyer NC, et al. Genetic male infertility and mutation of CATSPER ion channels. Eur J Hum Genet. 2010;18:1178–84. doi: 10.1038/ejhg.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avenarius MR, Hildebrand MS, Zhang Y, Meyer NC, Smith LL, et al. Human male infertility caused by mutations in the CATSPER1 channel protein. Am J Hum Genet. 2009;84:505–10. doi: 10.1016/j.ajhg.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y, Malekpour M, Al-Madani N, Kahrizi K, Zanganeh M, et al. Sensorineural deafness and male infertility: a contiguous gene deletion syndrome. J Med Genet. 2007;44:233–40. doi: 10.1136/jmg.2006.045765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avidan N, Tamary H, Dgany O, Cattan D, Pariente A, et al. CATSPER2, a human autosomal nonsyndromic male infertility gene. Eur J Hum Genet. 2003;11:497–502. doi: 10.1038/sj.ejhg.5200991. [DOI] [PubMed] [Google Scholar]
- 101.Marquez B, Suarez SS. Bovine sperm hyperactivation is promoted by alkaline-stimulated Ca2+ influx. Biol Reprod. 2007;76:660–65. doi: 10.1095/biolreprod.106.055038. [DOI] [PubMed] [Google Scholar]
- 102.Cai X, Clapham DE. Evolutionary genomics reveals lineage-specific gene loss and rapid evolution of a sperm-specific ion channel complex: CatSpers and CatSperβ. PLoS ONE. 2008;3:e3569. doi: 10.1371/journal.pone.0003569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cai X, Clapham DE. Ancestral Ca2+ signaling machinery in early animal and fungal evolution. Mol Biol Evol. 2011 doi: 10.1093/molbev/msr149. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lishko PV, Botchkina IL, Kirichok Y. Progesterone activates the principal Ca2+ channel of human sperm. Nature. 2011;471:387–91. doi: 10.1038/nature09767. [DOI] [PubMed] [Google Scholar]
- 105.Blackmore PF, Beebe SJ, Danforth DR, Alexander N. Progesterone and 17α-hydroxyprogesterone. Novel stimulators of calcium influx in human sperm. J Biol Chem. 1990;265:1376–80. [PubMed] [Google Scholar]
- 106.Thomas P, Meizel S. Phosphatidylinositol 4,5-bisphosphate hydrolysis in human sperm stimulated with follicular fluid or progesterone is dependent upon Ca2+ influx. Biochem J. 1989;264:539–46. doi: 10.1042/bj2640539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 108.Luconi M, Francavilla F, Porazzi I, Macerola B, Forti G, Baldi E. Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids. 2004;69:553–59. doi: 10.1016/j.steroids.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 109.Strünker T, Goodwin N, Brenker C, Kashikar N, Weyand I, et al. The CatSper channel mediates progesterone-induced Ca2+ influx in human sperm. Nature. 2011;471:382–86. doi: 10.1038/nature09769. [DOI] [PubMed] [Google Scholar]
- 110.Mann T, Lutwak-Mann C. Biochemistry of seminal plasma and male accessory fluids: application to andrological problems. In: Mann T, Lutwak-Mann C, editors. Male Reproductive Function and Semen: Themes and Trends in Physiology, Biochemistry and Investigative Andrology. Berlin: Springer-Verlag; 1981. pp. 269–336. [Google Scholar]
- 111.Espey LL, Richards JS. Ovulation. In: Neill DJ, editor. Knobil and Neill’s The Physiology of Reproduction. Vol. 1. St. Louis: Elsevier; 2006. pp. 425–75. [Google Scholar]
- 112.Aitken RJ, Irvine S, Kelly RW. Significance of intracellular calcium and cyclic adenosine 3′,5′-monophosphate in the mechanisms by which prostaglandins influence human sperm function. J Reprod Fertil. 1986;77:451–62. doi: 10.1530/jrf.0.0770451. [DOI] [PubMed] [Google Scholar]
- 113.Schaefer M, Hofmann T, Schultz G, Gudermann T. A new prostaglandin E receptor mediates calcium influx and acrosome reaction in human spermatozoa. Proc Natl Acad Sci USA. 1998;95:3008–13. doi: 10.1073/pnas.95.6.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimizu Y, Yorimitsu A, Maruyama Y, Kubota T, Aso T, Bronson RA. Prostaglandins induce calcium influx in human spermatozoa. Mol Hum Reprod. 1998;4:555–61. doi: 10.1093/molehr/4.6.555. [DOI] [PubMed] [Google Scholar]
- 115.Saaranen M, Suistomaa U, Kantola M, Saarikoski S, Vanha-Perttula T. Lead, magnesium, selenium and zinc in human seminal fluid: comparison with semen parameters and fertility. Hum Reprod. 1987;2:475–79. doi: 10.1093/oxfordjournals.humrep.a136573. [DOI] [PubMed] [Google Scholar]
- 116.Ehrenwald E, Foote RH, Parks JE. Bovine oviductal fluid components and their potential role in sperm cholesterol efflux. Mol Reprod Dev. 1990;25:195–204. doi: 10.1002/mrd.1080250213. [DOI] [PubMed] [Google Scholar]
- 117.Gunn SA, Gould TC. Role of zinc in fertility and fecundity in the rat. Am J Physiol. 1958;193:505–8. doi: 10.1152/ajplegacy.1958.193.3.505. [DOI] [PubMed] [Google Scholar]
- 118.Lu J, Stewart AJ, Sadler PJ, Pinheiro TJ, Blindauer CA. Albumin as a zinc carrier: properties of its high-affinity zinc-binding site. Biochem Soc Trans. 2008;36:1317–21. doi: 10.1042/BST0361317. [DOI] [PubMed] [Google Scholar]
- 119.Muñoz-Garay C, de la Vega-Beltrán JL, Delgado R, Labarca P, Felix R, Darszon A. Inwardly rectifying K+ channels in spermatogenic cells: functional expression and implication in sperm capacitation. Dev Biol. 2001;234:261–74. doi: 10.1006/dbio.2001.0196. [DOI] [PubMed] [Google Scholar]
- 120.Navarro B, Kirichok Y, Clapham DE. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc Natl Acad Sci USA. 2007;104:7688–92. doi: 10.1073/pnas.0702018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xia XM, Zhang X, Lingle CJ. Ligand-dependent activation of Slo family channels is defined by interchangeable cytosolic domains. J Neurosci. 2004;24:5585–91. doi: 10.1523/JNEUROSCI.1296-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tang QY, Zhang Z, Xia XM, Lingle CJ. Block of mouse Slo1 and Slo3 K+ channels by CTX, IbTX, TEA, 4-AP and quinidine. Channels. 2010;4:22–41. doi: 10.4161/chan.4.1.10481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhang X, Zeng X, Lingle CJ. Slo3 K+ channels: voltage and pH dependence of macroscopic currents. J Gen Physiol. 2006;128:317–36. doi: 10.1085/jgp.200609552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Santi CM, Martínez-López P, de la Vega-Beltrán JL, Butler A, Alisio A, et al. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 2010;584:1041–46. doi: 10.1016/j.febslet.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zeng XH, Yang C, Kim ST, Lingle CJ, Xia XM. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc Natl Acad Sci USA. 2011;108:5879–84. doi: 10.1073/pnas.1100240108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woo AL, James PF, Lingrel JB. Roles of the Na,K-ATPase α4 isoform and the Na+/H+ exchanger in sperm motility. Mol Reprod Dev. 2002;62:348–56. doi: 10.1002/mrd.90002. [DOI] [PubMed] [Google Scholar]
- 127.Bibring T, Baxandall J, Harter CC. Sodium-dependent pH regulation in active sea urchin sperm. Dev Biol. 1984;101:425–35. doi: 10.1016/0012-1606(84)90157-x. [DOI] [PubMed] [Google Scholar]
- 128.Borland RM, Biggers JD, Lechene CP, Taymor ML. Elemental composition of fluid in the human Fallopian tube. J Reprod Fertil. 1980;58:479–82. doi: 10.1530/jrf.0.0580479. [DOI] [PubMed] [Google Scholar]
- 129.Mann T. The Biochemistry of Semen and of the Male Reproductive Tract. London/New York: Methuen/Wiley; 1964. [Google Scholar]
- 130.Wang D, King SM, Quill TA, Doolittle LK, Garbers DL. A new sperm-specific Na+/H+ exchanger required for sperm motility and fertility. Nat Cell Biol. 2003;5:1117–22. doi: 10.1038/ncb1072. [DOI] [PubMed] [Google Scholar]
- 131.Wang D, Hu J, Bobulescu IA, Quill TA, McLeroy P, et al. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC) Proc Natl Acad Sci USA. 2007;104:9325–30. doi: 10.1073/pnas.0611296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ramsey IS, Moran MM, Chong JA, Clapham DE. A voltage-gated proton-selective channel lacking the pore domain. Nature. 2006;440:1213–16. doi: 10.1038/nature04700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sasaki M, Takagi M, Okamura Y. A voltage sensor-domain protein is a voltage-gated proton channel. Science. 2006;312:589–92. doi: 10.1126/science.1122352. [DOI] [PubMed] [Google Scholar]
- 134.Ramsey IS, Mokrab Y, Carvacho I, Sands ZA, Sansom MS, Clapham DE. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat Struct Mol Biol. 2010;17:869–75. doi: 10.1038/nsmb.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koch HP, Kurokawa T, Okochi Y, Sasaki M, Okamura Y, Larsson HP. Multimeric nature of voltage-gated proton channels. Proc Natl Acad Sci USA. 2008;105:9111–16. doi: 10.1073/pnas.0801553105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee SY, Letts JA, Mackinnon R. Dimeric subunit stoichiometry of the human voltage-dependent proton channel Hv1. Proc Natl Acad Sci USA. 2008;105:7692–95. doi: 10.1073/pnas.0803277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tombola F, Ulbrich MH, Isacoff EY. The voltage-gated proton channel Hv1 has two pores, each controlled by one voltage sensor. Neuron. 2008;58:546–56. doi: 10.1016/j.neuron.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Okochi Y, Sasaki M, Iwasaki H, Okamura Y. Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun. 2009;382:274–79. doi: 10.1016/j.bbrc.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 139.Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE. Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA. 2009;106:7642–47. doi: 10.1073/pnas.0902761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.DeCoursey TE. Voltage-gated proton channels. Cell Mol Life Sci. 2008;65:2554–73. doi: 10.1007/s00018-008-8056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lishko PV, Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol. 2010;588:4667–72. doi: 10.1113/jphysiol.2010.194142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101:16501–6. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–47. doi: 10.1095/biolreprod.103.026054. [DOI] [PubMed] [Google Scholar]
- 144.Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22:680–95. [PubMed] [Google Scholar]
- 145.Schuel H, Burkman LJ. A tale of two cells: Endocannabinoid-signaling regulates functions of neurons and sperm. Biol Reprod. 2005;73:1078–86. doi: 10.1095/biolreprod.105.043273. [DOI] [PubMed] [Google Scholar]
- 146.Decoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. doi: 10.1152/physrev.00028.2002. [DOI] [PubMed] [Google Scholar]
- 147.Musset B, Capasso M, Cherny VV, Morgan D, Bhamrah M, et al. Identification of Thr29 as a critical phosphorylation site that activates the human proton channel Hvcn1 in leukocytes. J Biol Chem. 2010;285:5117–21. doi: 10.1074/jbc.C109.082727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Platts AE, Dix DJ, Chemes HE, Thompson KE, Goodrich R, et al. Success and failure in human spermatogenesis as revealed by teratozoospermic RNAs. Hum Mol Genet. 2007;16:763–73. doi: 10.1093/hmg/ddm012. [DOI] [PubMed] [Google Scholar]
- 149.Banks FC, Calvert RC, Burnstock G. Changing P2X receptor localization on maturing sperm in the epididymides of mice, hamsters, rats, and humans: a preliminary study. Fertil Steril. 2010;93:1415–20. doi: 10.1016/j.fertnstert.2009.02.061. [DOI] [PubMed] [Google Scholar]
- 150.Foresta C, Rossato M, Chiozzi P, Di Virgilio F. Mechanism of human sperm activation by extra-cellular ATP. Am J Physiol Cell Physiol. 1996;270:1709–14. doi: 10.1152/ajpcell.1996.270.6.C1709. [DOI] [PubMed] [Google Scholar]
- 151.Wiesner B, Weiner J, Middendorff R, Hagen V, Kaupp UB, Weyand I. Cyclic nucleotide-gated channels on the flagellum control Ca2+ entry into sperm. J Cell Biol. 1998;142:473–84. doi: 10.1083/jcb.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kaupp UB, Kashikar ND, Weyand I. Mechanisms of sperm chemotaxis. Annu Rev Physiol. 2008;70:93–117. doi: 10.1146/annurev.physiol.70.113006.100654. [DOI] [PubMed] [Google Scholar]
- 153.Francavilla F, Battista N, Barbonetti A, Vassallo MR, Rapino C, et al. Characterization of the endocannabinoid system in human spermatozoa and involvement of transient receptor potential vanilloid 1 receptor in their fertilizing ability. Endocrinology. 2009;150:4692–700. doi: 10.1210/en.2009-0057. [DOI] [PubMed] [Google Scholar]
- 154.Jungnickel MK, Marrero H, Birnbaumer L, Lemos JR, Florman HM. Trp2 regulates entry of Ca2+ into mouse sperm triggered by egg ZP3. Nat Cell Biol. 2001;3:499–502. doi: 10.1038/35074570. [DOI] [PubMed] [Google Scholar]
- 155.Martínez-López P, Treviño CL, de la Vega-Beltrán JL, De Blas G, Monroy E, et al. TRPM8 in mouse sperm detects temperature changes and may influence the acrosome reaction. J Cell Physiol. 2011;226:1620–31. doi: 10.1002/jcp.22493. [DOI] [PubMed] [Google Scholar]
- 156.Cook SP, Brokaw CJ, Muller CH, Babcock DF. Sperm chemotaxis: Egg peptides control cytosolic calcium to regulate flagellar responses. Dev Biol. 1994;165:10–19. doi: 10.1006/dbio.1994.1229. [DOI] [PubMed] [Google Scholar]
- 157.Miller RL. Chemotaxis of the spermatozoa of Ciona intestinalis. Nature. 1975;254:244–45. doi: 10.1038/254244a0. [DOI] [PubMed] [Google Scholar]
- 158.Matsumoto M, Solzin J, Helbig A, Hagen V, Ueno S, et al. A sperm-activating peptide controls a cGMP-signaling pathway in starfish sperm. Dev Biol. 2003;260:314–24. doi: 10.1016/s0012-1606(03)00236-7. [DOI] [PubMed] [Google Scholar]
- 159.Hansbrough JR, Garbers DL. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981;256:1447–52. [PubMed] [Google Scholar]
- 160.Babcock DF, Bosma MM, Battaglia DE, Darszon A. Early persistent activation of sperm K+ channels by the egg peptide speract. Proc Natl Acad Sci USA. 1992;89:6001–5. doi: 10.1073/pnas.89.13.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cook SP, Babcock DF. Activation of Ca2+ permeability by cAMP is coordinated through the pHi increase induced by speract. J Biol Chem. 1993;268:22408–13. [PubMed] [Google Scholar]
- 162.Ward GE, Brokaw CJ, Garbers DL, Vacquier VD. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985;101:2324–29. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Strunker T, Weyand I, Bonigk W, Van Q, Loogen A, et al. A K+-selective cGMP-gated ion channel controls chemosensation of sperm. Nat Cell Biol. 2006;8:1149–54. doi: 10.1038/ncb1473. [DOI] [PubMed] [Google Scholar]
- 164.Ralt D, Goldenberg M, Fetterolf P, Thompson D, Dor J, et al. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc Natl Acad Sci USA. 1991;88:2840–44. doi: 10.1073/pnas.88.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Teves ME, Barbano F, Guidobaldi HA, Sanchez R, Miska W, Giojalas LC. Progesterone at the picomolar range is a chemoattractant for mammalian spermatozoa. Fertil Steril. 2006;86:745–49. doi: 10.1016/j.fertnstert.2006.02.080. [DOI] [PubMed] [Google Scholar]
- 166.Shiba K, Baba SA, Inoue T, Yoshida M. Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proc Natl Acad Sci USA. 2008;105:19312–17. doi: 10.1073/pnas.0808580105. [DOI] [PMC free article] [PubMed] [Google Scholar]