Summary
T cells are activated by antigen (Ag) bearing dendritic cells (DCs) in lymph nodes in 3 phases. The duration of the initial phase of transient, serial DC-T cell interactions is inversely correlated with Ag dose. The second phase, characterized by stable DC-T cell contacts, is believed to be necessary for full-fledged T cell activation. Here we have shown that this is not the case. CD8+ T cells interacting with DCs presenting low-dose, short-lived Ag did not transition to phase 2, while higher Ag dose yielded phase 2 transition. Both antigenic constellations promoted T cell proliferation and effector differentiation, but yielded different transcriptome signatures at 12h and 24h. T cells that experienced phase 2 developed long-lived memory, whereas conditions without stable contacts yielded immunological amnesia. Thus, T cells make fate decisions within hours after Ag exposure resulting in long-term memory or abortive effector responses, correlating with T cell-DCs interaction kinetics.
Introduction
When a naive T cell (TN) exits the thymus, it embarks on a single-minded mission: to find and eliminate pathogens to which it can respond. T cells rely on T cell receptors (TCR), which recognize peptides in major histocompatibility complexes (pMHC) on antigen (Ag) presenting cells (APCs)(Germain and Stefanova, 1999). In theory, there are billions of Ags for CD8+ T cells, which recognize MHC class I molecules complexed with non-covalently bound peptides. In practice, for a given MHC allele, peptide number is limited by specific residues that determine if and how long a peptide can be presented (Townsend and Bodmer, 1989). Nonetheless, the diversity of potential T cell Ags is enormous and requires a large repertoire of T cells, each with its own randomly assembled TCR. This need for TCR diversity is balanced by the metabolic cost of T cell generation, so the frequency of TN cells that express a ‘cognate’ TCR specific for any individual pMHC complex is only 1 in 105-107 (Blattman et al., 2002; Casrouge et al., 2000).
Ag-specific TN cells must quickly assess whether an Ag is present, whether it poses a threat and, if so, what response will be appropriate(Lanzavecchia and Sallusto, 2000). This information is provided to TN cells by dendritic cells (DCs) in lymph nodes (LNs), which constantly recruit TN cells from the blood and receive Ag-carrying DCs via afferent lymphatics from nearby tissues(von Andrian and Mempel, 2003). TN cells migrate rapidly (>10μm/min) within the LN cortex to query local DCs for the presence of cognate Ag. A single DC can be contacted by ~5,000 T cells/hr(Miller et al., 2004a) and this high scanning efficiency is necessary, in particular for CD8+ TN cells, because antigenic peptides in MHC class I can dissociate quickly(Zinkernagel and Doherty, 1974). This challenge becomes particularly relevant when TN cells must respond to transient, non-replicating Ags, such as recombinant vaccines.
As TN cells encounter Ag-presenting DCs they must decide whether or not to respond. For full activation, TN cells require multiple signals, including TCR recognition of cognate pMHCs, costimulation by B7 family members, and cytokines(Henrickson and von Andrian, 2007). This generates rapidly proliferating effector cells (TEff) that migrate to inflamed tissues where they produce cytokines (esp. interferon-γ [IFN-γ]) and kill APCs. Upon Ag clearance, most TEff cells apoptose, but in many settings a few Ag-experienced T cells persist as long-lived memory cells that respond more quickly and efficiently to cognate Ag than TN cells(Williams and Bevan, 2007).
CD8+ T cells can be ‘programmed’ by short-term access to Ag presenting DCs to allow differentiation of TEff and memory cells, indicating that CD8+ TN cells can make early fate decisions(Williams and Bevan, 2007). However, while specific T cell markers have been correlated with memory differentiation(Joshi et al., 2007; Kaech et al., 2002; Sarkar et al., 2008; Wherry et al., 2007), most of these markers appear only on day 4 or later after Ag encounter. To date, reliable standardized in vivo models that can be ‘tuned’ to either induce or fail to induce T cell memory have been missing.
Here, we used multi-photon intravital microcopy (MP-IVM) in mouse popliteal LNs (popLNs) to analyze how and when interactions between CD8+ TN cells and Ag-presenting DCs influence effector and memory differentiation. This study was informed by earlier findings that CD8+ T cells are primed in LNs in 3 phases(Mempel et al., 2004). Phase 1 can last up to ~8h and is characterized by transient T cell interactions with Ag-pulsed DCs. T cells integrate the antigenic stimuli from each encounter until the cumulative signal triggers phase 2 when T cells form a long-lasting contact with a single DC(Mempel et al., 2004). The higher the concentration of cognate pMHCs per DC, the faster T cells reach phase 2 and the shorter is phase 1(Henrickson et al., 2008). Phase 2 lasts ~12 hours and is accompanied by upregulation of activation markers and cytokine production. Phase 3 begins ~1d after T cell entry into the LN when T cells return to short interactions and proliferate vigorously. Sequential phases of transient and stable DC-T cell contacts have been independently observed in many systems(Hugues et al., 2004; Mempel et al., 2004; Miller et al., 2004b; Skokos et al., 2007; Stoll et al., 2002), however, it has been unclear if the stable contacts that define phase 2 are necessary for full-fledged T cell activation, as is widely assumed, or if they simply correlate with T cell differentiation to TEff and memory cells. It has also been uncertain whether and how early interaction dynamics influence TN cell fate, particularly the acquisition of longevity and self-renewal capacity needed for immunologic memory.
To address this question, we adapted an experimental strategy whereby Ag-specific CD8+ TN cells are allowed to encounter in LNs a finite number of DCs that were pulsed with either a low or a high dose of a naturally occurring viral Ag(Henrickson et al., 2008). This enabled us to ask how T cells make fate decisions in vivo while Ag dose and persistence were precisely controlled. Our approach may not mimic a typical infection where the kinetics of Ag availability are more complex, however, similar conditions might be encountered when T cells respond to non-replicating vaccine Ags. We found that only DCs pulsed with the high Ag dose supported phase 2-like tight interactions with cognate T cells, but DCs pulsed with either Ag dose induced T cell proliferation and TEff cell differentiation. The differential interactive behavior induced by high- versus low-dose Ag-pulsed DCs was paralleled by distinct transcriptional programs in activated T cells. Moreover, only T cells that interacted with DCs presenting high dose Ag gave rise to sustained immunological memory, suggesting that information exchange in phase 2 is critical for long-term protection and avoiding immunological amnesia.
RESULTS
MP-IVM studies of low dose peptide-pulsed DCs interacting with Ag specific T cells
To control the dose and availability of Ag in mouse LNs, we used a protocol that delivers Ag by footpad injection of peptide pulsed DCs, so Ag availability was limited to a few 100 DCs that migrated to the draining popLN. TN cells were injected intravenously (iv) 18h later, allowing a few 1000 transferred cells to access the popLN before further entry was blocked (Henrickson et al., 2008; Mempel et al., 2004). This protocol ensures that all measured responses were due to locally confined T cell-APC interactions (Fig. 1A).
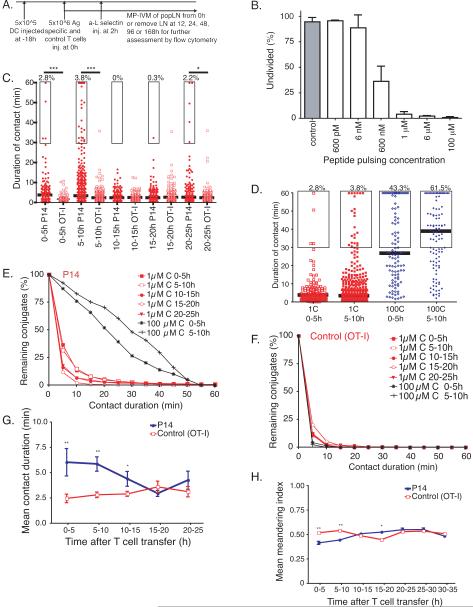
Figure 1. P14 T cells maintain brief interactions with 1μM C-peptide pulsed DCs.
(A) Experimental paradigm for study of P14 Tcra−/− (P14) and control (OT-I) T cell interactions with peptide pulsed DCs. Unless otherwise mentioned, this will be the protocol for experiments throughout the paper. (B) Percentage of antigen specific (P14) T cells at 48h that remain unproliferated after exposure in vivo to DCs pulsed with various concentrations of C-peptide (bars are mean +/−SD). (C-G) P14 and control (OT-I Rag1−/−) T cell interactions with DCs pulsed with 1μM or 100μM C-peptide pulsed DCs (1C or 100C, respectively) were visualized in popLNs by MP-IVM at the indicated time points after T cell injection iv. (C) Duration of P14 T cell-1C-peptide pulsed DC (1C DC) contacts at various timepoints after T cell transfer was assessed in 3D reconstructed videos (bar at median, *** = p<0.0001, ** = p =.0013 by Mann-Whitney). (D) Duration of T cell-DC contacts with 1C DC or 100C DC at various timepoints after T cell transfer was assessed in 3D reconstructed videos. P14 and control T cell interactions with 1C vs. 100C DCs were visualized in popliteal LNs by MP-IVM from 0-10h after T cell injection (bar at median, box surrounds durations of 30-60min, percentage of events above box). (E-F) Cumulative distribution plots of interaction durations for P14 (E) and control (F) T cells interacting with 1C or 100C. (G) The bootstrap corrected means of the interaction durations between P14 T cells (blue) control T cells (red) with DCs. (1C: N=2-4 experiments per timepoint; mean ± 95% CI, ** = p<0.0004 and p = 0.0008). (H) The bootstrap corrected means of the meandering indices (MI) of P14 and control T cells when interacting with 1C or 100C DCs. Cell centroids in 3D were measured by semi-automated cell tracking and the MI was calculated by dividing the displacement for each cell track by the total path length for that cell track. (n=2-4 expt per timept; mean ± 95% C.I., ** = p<4×10−4, * = p=0.017). Figure 1, see also Movies S1-6.
For Ag pulsing, splenic DCs were incubated in vitro for 1h with varied doses of gp33-41 (KAVYNFATC, “C-peptide”), an immunodominant peptide of lymphocytic choriomeningitis virus (LCMV) that complexes with MHC H-2Db and activates CD8+ TN from TCR transgenic P14 mice (Pircher et al., 1989). During DC pulsing, endogenous peptides in surface-expressed H-2Db are replaced by C-peptide; the fraction of C-peptide loaded H2-Db complexes on DCs depends on the peptide concentration in the pulsing buffer and decreases over time as non-covalently bound C-peptide dissociates (Henrickson et al., 2008).
Pulsing conditions were chosen after measuring the ability of DCs pulsed with different C-peptide concentrations to yield TN proliferation in vivo. A pulsing concentration of 1μM was the lowest at which pulsed DCs reproducibly induced proliferation of >95% of P14 cells (Fig. 1B). MP-IVM was performed with DCs pulsed with either the threshold dose (1μM) of C-peptide (1C DC) or with a higher dose, 100μM (100C DC), which provided maximal initial loading of DC-expressed H2-Db. Pulsed DCs were washed, labeled fluorescently and injected into a footpad. 18h later, fluorescent P14 TN cells and differentially labeled control OT-I TN cells (bearing an irrelevant TCR) were co-injected iv; after 2h, further T cell homing to LNs was blocked with anti-L-selectin to synchronize intranodal T cell dwell-time and permit exact kinetic studies of T cell activation (Fig. 1A). At different times thereafter, mice were anesthetized and the popLN draining the injected footpad was prepared for MP-IVM.
Earlier studies show that P14 TN cells that encounter DCs pulsed with an intermediate dose (10μM; 10C) of C-peptide engage in 3 interactive phases (Mempel et al., 2004). Also, work with an altered peptide ligand for P14 T cells, KAVYNFATM (M-peptide), which has a higher affinity and longer half-life in H2-Db than C-peptide (Achour et al., 2002; Boulter et al., 2007; Henrickson et al., 2008), shows that M-peptide concentration is reciprocally correlated with the duration of the first phase during which T cell undergo brief serial contacts with DCs (Henrickson et al., 2008). T cells that encounter DCs bearing a higher amount of M-peptide require fewer interactions to initiate phase 2, which is defined as the period during which the median T cell-DC contact duration is ≥30min (Mempel et al., 2004). Any M-peptide dose that supports P14 cell priming also induces phase 2-like contacts, while phase 2 is absent when DCs are pulsed with a M-peptide dose just below the activation threshold (Henrickson et al., 2008). This correlation of interactive behavior and proliferative response seems consistent with the idea that phase 2-like DC-T cell conjugates are a prerequisite for T cell activation (Fooksman et al., 2009).
P14 T cell interactions differed when exposed to 1C DCs (Fig. 1C-H), which failed to promote phase 2 interactions (Table 1, Videos 1-5). At most, ~3.8% of contacts lasted ≥30min at any time from 5-10h post T cell transfer when phase 2 is usually observed (Henrickson et al., 2008; Mempel et al., 2004). In contrast, phase 2-like tight conjugates were prevalent when 100C DCs or 10μM M-peptide pulsed DCs (10M DCs) were used (Fig. 1D-F, Video 6). Of note, although the mean contact duration of P14 cells with 1C DCs never exceeded ~7min, these cognate interactions were still subtly but significantly prolonged when compared to non-cognate interactions of control OT-I cells using either Mann-Whitney test (Fig. 1C) or bootstrap corrected mean (Fig. 1G).
Table 1.
Summary of the duration of interactions of P14 and control CD8+T cells with both 100C DCs and 1C DCs from MP-IVM studies in popLN of recipient B6 mice from 0-25h after T cell transfer. For each 5h bin, the number of total DC-T cell interactions, the number of interactions with a duration of greater than 30min, the definition of a stable DC-T cell contact, and the percentage of interactions greater than 30min.
| Time after T cell transfer and T cell specificity | Number of interactions assessed | Number of interactions ≥30min duration | % of total |
|---|---|---|---|
| 0-5h P14, 1C | 142 | 4 | 2.8 |
| 0-5h control, 1C | 93 | 0 | 0 |
| 5-10h P14, 1C | 613 | 23 | 3.8 |
| 5-10h control, 1C | 405 | 1 | 0.25 |
| 10-15h P14, 1C | 301 | 0 | 0 |
| 10-15h control, 1C | 210 | 0 | 0 |
| 15-20h P14, 1C | 355 | 1 | 0.28 |
| 15-20h control, 1C | 187 | 1 | 0.53 |
| 20-25h P14, 1C | 233 | 5 | 2.2 |
| 20-25h control, 1C | 230 | 1 | 0.43 |
| 0-5 P14, 100C | 104 | 45 | 43.3 |
| 0-5 control, 100C | 62 | 0 | 0 |
| 5-10 P14, 100C | 109 | 67 | 61.5 |
| 5-10 control, 100C | 55 | 0 | 0 |
We also measured the meandering index (MI), the ratio of a migrating cell's linear displacement to the total path length (Henrickson et al., 2008) using automated custom-designed cell tracking software, which avoids potential observer bias. Free-moving TN cells in Ag-free LNs have a median MI of ~0.5, whereas phase 2-like DC-T cell interactions confine T cells and reduce the MI to <0.3 (Henrickson et al., 2008)). The median MI of P14 cells encountering 1C DCs during the first 10h after T cell transfer was slightly lower than that of control OT-I cells, but always remained >0.4 (Fig. 1H). Thus, 1C DCs subtly confined P14 TN cells, but did not support phase 2-like interactions (Fig. 1B-F).
Proliferation after TN cell exposure to high versus low Ag constellations
Next, we examined the kinetics and magnitude of P14 cell proliferation. CFSE-labeled P14 TN cells (CD45.2+) were injected iv into congenic recipients (CD45.1+) 18h after footpad injection of DCs, and CFSE dilution of transferred P14 T cells was monitored (Fig. 2A). Consistent with our dose-finding experiments (Fig. 1B), both 1C DC and 100C DC induced rapid P14 cell proliferation with nearly identical kinetics during the first 48h (Fig. 2B), suggesting that CD8+ TN cells can be activated in vivo without sustained contacts with Ag presenting DCs. However, a strong inflammatory challenge usually induces temporary trapping of T cells in LNs (Shiow et al., 2006). Conceivably, the weak stimulus from 1C DCs might not have exerted this effect, so unstimulated P14 cells could have exited the LN. Thus, it remained theoretically possible that the proliferated cells did not arise from precursors that engaged in transient interactions and instead were progeny of the small fraction (<4%) that underwent stable contacts with 1C DC (Fig. 1C).
Figure 2. High and low dose antigen both lead to the majority of transferred T cells participating in effector response.
Standard protocol (Figure 1A), with CD45.1 recipients who received 1C or 100C DCs in the right footpad and control DCs in the left footpad (to serve as internal controls). 2h after T cell injection, anti-L-selectin antibody (Ab) and FTY-720 (or vehicle alone), were injected to prevent further T cell entry and exit, respectively. At 4h, 24h and 96h recipients were sacrificed and quantitative flow cytometry with beads was used to enumerate the number of remaining transferred cells. (A) Representative flow cytometry plots. (B) Summary of percentage of transferred cells that have proliferated at indicated timepoints (vehicle treated recipients). C) The number of total undivided, transferred cells at indicated timepoints and conditions (N= 4 expt, mean +/− SD, 3-7 mice per condition, except 100C at 4h with 2).
To account for potential premature exit of undivided TN cells from LNs, we injected mice at 2h post T cell transfer with anti-L-selectin (to block further homing) and FTY-720, a sphingosine-1-phosphate (S1P) receptor agonist that prevents T cell egress from LNs (Mandala et al., 2002). By simultaneously blocking T cell entry and exit we could accurately monitor the entire LN-resident P14 T cell population regardless of Ag constellation. Thus, we quantified the number of transferred CFSEbright P14 cells that had not proliferated in LNs containing 1C, 100C or control peptide pulsed DCs (Fig. 2C).
4h after T cell transfer, similar numbers of undivided P14 cells were recovered from popLNs in all conditions, indicating that early T cell recruitment and retention were not substantially affected by our experimental manipulations (Fig. 2C). On days 2 and 4, nearly all P14 T cells in both FTY-720 treated and control recipients of 1C or 100C DCs had divided at least once, whereas Ag-free LNs contained mostly undivided cells (Fig. 2C). Thus, essentially all P14 cells that encountered 1C DCs proliferated even though their interactions with DCs were almost exclusively brief and dynamic.
Ag dose effects on effector burst kinetics and TN cell apoptosis
While our proliferation studies provided proof for efficient T cell stimulation by both 1C and 100C DCs, cell division alone may not necessarily predict the ensuing effector burst. Indeed, during the first 48h, the absolute number of P14 T cells that had divided at least once was similar in LNs draining 1C DCs and 100C DCs (Fig. 3A), however, by 96h LNs that received 100C DCs contained substantially more P14 cells than those that received 1C DCs (p<0.03). When expressed as percentage of LN-resident lymphocytes (Fig. 3B), the frequency of P14 TEff cells at 48h was twice as high after treatment with 1C DCs (mean±SEM: 1.94±0.3%) than with 100C DCs (0.96±0.16%). Although this difference did not reach statistical significance (p = 0.08), the degree of CFSE dilution at 48h was similar in P14 cells that had encountered 1C and 100C DCs, indicating that the early proliferative advantage in the 1C setting was not due to accelerated division (Fig. 2A).
Figure 3. Higher dose antigen eventually yields larger effector pool after a larger early apoptotic loss.
Standard protocol (Figure 1a), with CD45.1 recipients who received 1C or 100C DCs in the right footpad and control peptide pulsed DCs in the left footpad (to serve as internal controls). 2h after T cell injection, anti-L-selectin Ab and FTY-720 (or vehicle alone), were injected to prevent further T cell entry and exit, respectively. (A-B; E-F) At 4h, 48h, 96h or 7d recipients were sacrificed and quantitative flow cytometry, with beads, was used to enumerate the number of remaining transferred cells in the popLN. This is presented as (A) the absolute number of recovered CD45.2+ cells recovered at 4h, 48h and 96h in the LN (vehicle treated; mean ±SEM) and (F) vehicle or FTY treated recipient (mean±SEM). Number of CD.45.2 (transferred and progeny of transferred) cells, as a percentage of total LN cells at each timepoint, either additionally treated with vehicle (B) or (E) FTY-720 (A-B; E-F: N=4 expt, 3-7 mice per condition, except 100C at 4h with 2; B and E: mean±SD). (C-D) Percentage of apoptotic transferred CFSE labeled P14 T cells at 24h after T cell transfer (Annexin V+, 7-AAD+), with 1C DC, 100C DC or DCs pulsed with control peptide, (C) representative flow cytometry and (D) summary of percentage of cells which are apoptotic at 24h, (N=3-6 expt and 3-8 mice per condition, bar at median). (G) The percentage at d7 of transferred CD4- B220- cells in the LN, CD8+ T cell negative selected spleen and bone marrow that represents recovered transferred cells. On left, representative flow cytometry and on right, summary of flow cytometry data. (N=2 expt, 4 mice per condition).
The apparent delay in P14 TEff burst induced by 100C DCs was caused by Ag-induced T cell apoptosis preceding the onset of proliferation; ~half the P14 cells exposed to 100C DCs were apoptotic at 24h, while much less apoptosis was seen with 1C DCs (Fig. 3C-D). This curtailing of the TEff response by high dose Ag likely accounted for the lower P14 cell frequency among LN T cells at 48h, however, the 100C stimulated cells continued to expand until 96h, whereas the P14 cells exposed to 1C DC peaked at 48h and then declined (Fig. 3B). When 1C DC recipients were treated with FTY-720, the number of P14 cells continued to climb in LNs until d4 (Fig. 3E-F), indicating that 1C and 100C DC induced differential egress from LNs after d2.
CD8+ TEff function after low and high dose Ag exposure
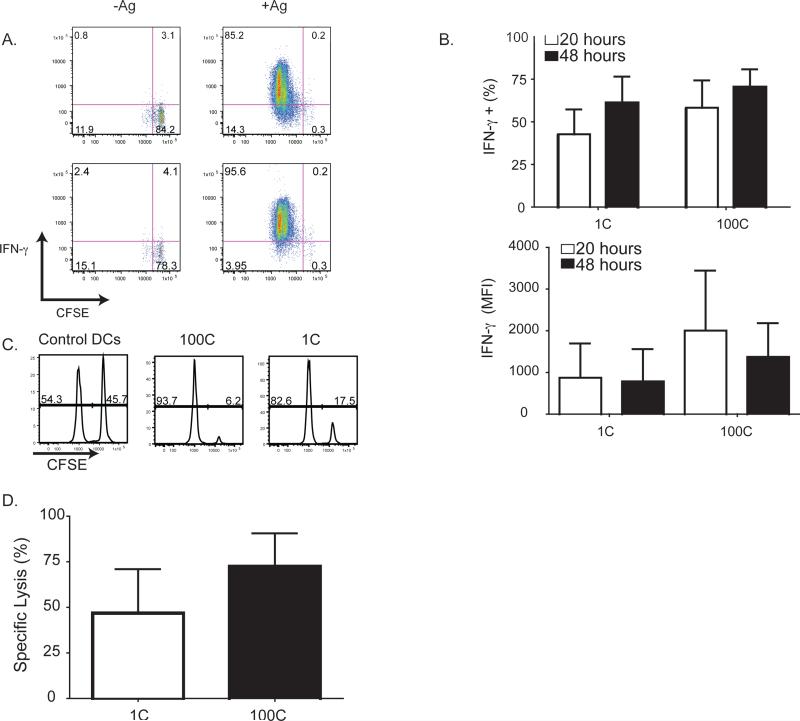
Having determined that 1C and 100C DCs induced equivalent early P14 cell proliferation, we asked whether Ag dose affects effector function by exposing P14 T cells to C-peptide, M-peptide or control peptide pulsed DCs in popLNs. P14 cells were harvested 20h or 48h later and restimulated ex vivo to assess effector activity. There was no significant difference in the frequency or magnitude of IFN-γ production in response to 1C or 100C DCs (Fig. 4A-B). Likewise, when P14 T cells were activated by either 1C or 100C DC for 48h, Ag-specific in vivo cytotoxicity was similar (Fig. 4C-D). Thus, minimal antigenic constellations that do not promote stable contacts with Ag-pulsed DCs can be sufficient to initiate TN cell activation and TEff differentiation.
Figure 4. Effector function is equivalent on whether or not T cells engage in stable contacts with DCs.
(A-B) Standard protocol (Figure 1a), with CD45.1 recipients who received 1C or 100C DCs in the right footpad and control DCs injected in left footpad to serve as internal controls. At 20h and 48h, popLN were harvested and IFN-γ production was measured by cell-surface capture. (A) Representative flow cytometry plots at 48h. (B) %IFN-γ positive transferred cells from 1C or 100C at 20h and 48h after P14 T cell transfer. IFN-γ positivity is calculated based on IFN-γ secretion from P14 T cells exposed to test peptide-pulsed DCs corrected for the amounts of secretion from P14 T cells exposed to control peptide-pulsed DCs. (%IFN-γ positivity by experiment γ SEM and MFI is presented per mouse, mean γ SD.; N = 4-5 mice 1C at 20 and 48h, N = 3-4 mice 100C at 20 and 48h; N=2 expt at 20h, N=3 expt at 48h). (C-D) Control peptide DC (left), 100C DC(middle) or 1C DC (right), were injected into the footpads of recipient congenic (CD45.1) mice. P14 T cells were injected iv 18h later. After an additional 48h, two target polyclonal B cell populations (one pulsed with 10μM M-peptide, labelled with 2μM CFSE; the other non-peptide pulsed, labelled with 0.1μM CFSE), were mixed at a 1:1 ratio and injected iv. 6h later, the ratio of CFSEhigh:CFSElow B cells was assessed in the popLN. (C) Representative plots of target cells after in vivo lysis (representative of 3-9 mice per group, N=2 (100C) or 4 (all other concentrations) expt). (D) Pooled specific lysis of Ag pulsed target cells (per mouse, mean ± SD. N=3-9 mice per group, N=2 (100C) or 4 (all other concentrations) expt). Percent specific lysis is calculated as (1-(ratio of unprimed/ratio primed))*100), where the ratio is %(CFSElo non-peptide pulsed)/(%CFSEhi peptide-pulsed) among transferred CFSE+ target B cells. Figure 4, see also Figure S2.
Memory differentiation correlates with Ag dose and stability of T cell-DC contacts
How does differential Ag presentation impact T cell memory differentiation? We first tried to address this question by transferring CD8+ T cells from CD45.2+ P14xTcra−/− donors (from Taconic) into congenic CD45.1+ recipients (from Jackson Labs or Taconic) to monitor long-term persistence and recall of CD45.2+ cells. However, the transferred cells disappeared within ~3-4 weeks (not shown), suggesting that differences between the P14xTcra−/− and both CD45.1+ strains (per their vendors on a C57BL/6 background) elicited a host response against cryptic alloantigens (Bhattacharya et al., 2006). Thus, we sought alternatives for tracking of non-congenic transferred T cells while avoiding concomitant activation of endogenous T cells recognizing C-peptide. Since the half-life of C-peptide in H-2Db is ~2.4h (Henrickson et al., 2008), encounters occurring before 18h after DC injection (when P14 TN cells are transferred) would expose endogenous T cells to higher Ag doses that presumably support stable contacts. P14 cell transfers were deliberately timed so that the cells accessed LNs only when the Ag dose on 1C DCs had fallen below the threshold at which stable contacts occur.
Using two distinct recipient strains that could neither mount an endogenous response against gp33-41 nor reject transferred P14xTcra−/− TN we could assess Ag specific recall responses mediated exclusively by transferred cells. In both settings, we applied our standard protocol for DC and P14 T cell transfer (Fig. 1a, but without anti-L-selectin), challenged with LCMV (103 pfu iv) after 30+ days and 5d later restimulated CD8 T cells ex vivo with gp33-41 to detect IFN-γ secreting P14 memory cells.
In the first model, we infused naive C57BL/6 mice with M-peptide, which depletes endogenous CD8+ T cells recognizing the gp33-41 epitope, creating a ‘hole’ in the endogenous T cell repertoire (Garza et al., 2000) and rendering mice incapable of responding to gp33-41 (Supp. Fig. 1). After LCMV rechallenge, recipients of 1C DCs or control DCs contained equivalently low numbers of gp33-41 reactive T cells, whereas 100C DC sensitized mice had generated abundant P14 memory cells (Fig. 5A). As a second strategy, we tested the same protocol using transgenic recipients (OT-IxRag1−/−) that expressed a single irrelevant TCR and could not respond to C-peptide or alloantigens. Again, recipients of control DCs or 1C DCs contained few gp33-41 reactive CD8+ T cells, whereas 100C DC recipients had generated ~5-fold more memory cells (Fig. 5B). In both experiments, virus-specific T cell responses were assessed as late as 5 days after LCMV challenge, providing ample time for even small memory populations to ‘catch up’. The fact that there was no statistical difference between 1C DC recipients and control mice that harbored only TN cells suggests that 1C DCs completely failed to induce memory.
Figure 5. Memory differentiation is impaired without stable DC-T cell contacts.
(a, b) Purified CD11c+ DCs were pulsed with 10 μM control-peptide (or no peptide), 1C or 100C and injected into the right footpad of recipient mice (in A, peptide depleted C57BL/6 recipients, in B, OT-I recipients) with LPS. 5 ×106 P14 T cells were injected iv 18h later. At d30+ post-T cell transfer each mouse was infected iv with 103 p.f.u. LCMV Armstrong, the spleens harvested at d5 p.i. and IFN-γ was stained by ICCS after a 5h ex vivo stimulation with (+) or without (−) 1μM C-peptide at 37°. (A) C57BL/6 recipients were treated at d-10, -7 and -4 with high dose M-peptide to deplete them of Ag specific cells. Upper graph shows a summary of CD8+ IFNγ+ and lower graph shows representative flow cytometry. (N=3 expt, 3-5 mice, per condition; mean ± SEM). (B) Recipients are OT-I Rag1−/−. Upper graph shows a CD8+ IFN-γ+ and lower graph shows representative flow cytometry. Of note, in the OT-I recipients there were occasionally animals with extreme splenomegaly and expansion of lymph nodes (in all conditions) which were excluded from analysis in all settings. (N=3-5 expt, 4-7 mice per condition, mean±SD per mouse).
Transcription profiles of T cells exposed to high and low dose antigenic constellations
To explore at a molecular level how 1C and 100C DC sensitization induce such distinct outcomes in cell-cell interactions and memory differentiation, we compared transcription profiles of P14 T cells using DNA microarrays (Haining et al., 2008). P14 cells were activated following our standard protocol (Fig. 1A) and sorted from single-cell suspensions of popLNs at 12 or 24h after transfer. This timing of T cell harvesting was chosen because previous MP-IVM results had shown that an intermediate Ag dose (10C DC) induces phase 2 interactions that are maximal by ~12h and resolve after ~20h (Mempel et al., 2004), suggesting that the conditions encountered by CD8+ TN cells within the first day set the stage for a differential fate decision resulting either in long-term memory or an abortive TEff response.
Activation by either 1C or 100C DCs altered the expression of several 1,000 genes that were significantly up- or down-regulated compared to TN cells. Many genes were similarly regulated in both conditions, but a roughly equal number changed in only one activating setting (Fig. 6A). These differentially expressed genes increased slightly in number from 12 to 24h (Fig. 6A). Among the transcripts that changed uniquely at 24h in the 100C setting, 57% were upregulated, while most uniquely regulated genes in the 1C setting were downregulated (67%). No genes were reciprocally altered relative to TN cells, i.e. no genes increased in one activation setting and decreased in the other. Principal components analysis (PCA) showed that P14 cell activation by 1C or 100C DCs resulted in transcriptional profiles that were distinct and divergent between 12 and 24h, but more similar to each other than to TN cells (Fig. 6B).
Figure 6. Comparison of cDNA profiles by DNA microarray at 12 and 24hr.
(A-E) Standard protocol (Figure 1a), with two types of recipients who received 1C or 100C or no DCs in the right footpad. The right popliteal LN was harvested at 12 or 24h post T cell transfer, single cell suspension created, and stained with CD4, B220 and CD19 (dump channel). Cells were then sorted on a FACSAria as non-doublets, CMFDA+ and dump channel negative. RNA was extracted using standard phenol-chloroform techniques, and then concentrated and cleaned with the Agencourt RNAdvance tissue kit. Of the total RNA extracted, an aliquot was then amplified using commercially available kits (NuGEN Pico). Following RNA amplification, aliquots of cDNA from each sample were assayed using the Agilent 2100 bioanalyzer to ensure high-quality amplification prior to fragmentation, labelling and hybridization to microarray. cDNA was then hybridized on Affymetrix 430_2 arrays for analysis of gene expression patterns at a core facility. (A) Venn diagrams of differentially expressed genes. Left column, 12h timepoint, right column, 24h timepoint. Upper row represents the number of genes that were upregulated for vs naïve, and the lower row represents the number of genes that were downregulated vs naïve. For each Venn diagram, on the left are genes that are differentially expressed between T cells exposed to 1C vs. adoptively transferred naïve T cells not exposed to DCs and on the right are genes differentially expressed between T cells exposed to 100C vs. adoptively transferred naïve T cells not exposed to DCs. Genes included in these diagrams have fold change statistics > 0.5 Wilcoxon p-value <= 0.001. (B) 2D principal components analysis (PCA) of all 6 conditions (control, 1C and 100C, each at 12 and 24h with each color indicating a separate concentration at a specific timept), with arrows drawn from 12h to 24h data for each antigenic dose. Principle component (PC) 1 accounts for 28.3% and PC 2 accounts for 11.32%. (C) Enrichment of the d8 effector signature (Wherry et al., 2007) for the samples from T cells exposed to 1C and 100C samples at 24h. (D) Heatmap of the 30 most differentially expressed genes for each of three sample types (T cells exposed to no DCs, T cells exposed to 1C and T cells exposed to 100C all at 24h) with some genes of interest noted by arrows on the right margin. (E) Gene expression volcano plot, with –log 10 of the SAM p-value on the y axis and log 2 fold change on the x axis, such that genes with higher expression in 1C are on the left and genes with higher expression in 100C are on the right. Genes plotted were expert selected for their known relevance in T cell effector and memory differentiation. (arrays: N = 3 for control T cells at 24h, N= 10 for 1C at 24h, N=7 for 100C at 24h; N= 3 for control T cells at 12h; N= 6 for 1C at 12h; N= 6 for 100C at 12h). Figure 6, see also Figure S1 and Table S1-S3.
A gene-set enrichment analysis (GSEA) (Haining and Wherry, 2010) was performed to compare our results to published gene expression profiles from P14 TEff cells on day 8 after LCMV infection (Wherry et al., 2007). Compared to TN cells, the d8 effector gene set was significantly enriched in T cells exposed to 1C DCs (P<0.001, FDR < 0.0001) and 100C DCs (P<0.001, FDR <0.002) at both at 12h and 24h after stimulation (Fig. 6C), indicating that effector differentiation commences as early as 12h after activation.
There were also differences between transcriptional states in the 1C and 100C settings, which were evident when results were presented as a heatmap (Fig. 6D) or volcano plot (Fig. 6E). The list of genes differentially expressed between 1C and 100C T cells included several transcriptional regulators, suggesting that day 1 may constitute a cell-fate branch-point for CD8+ T cells (Supp. Table 1A-B). We also used GSEA with curated gene sets to assess how gene families involved in specific cellular processes or signaling pathways are modified (Supp. Table 2A-B), compared specific molecules of interest, including costimulatory and inhibitory receptors, cytokines and chemokines and their receptors, components of the apoptosis pathway, and genes involved in CD8 T cell exhaustion and effector states (Supp. Table 3A), and investigated genes of interest within gene sets identified by GSEA (Supp. Tables 3B-E).
Transcripts for actin and other cytoskeletal genes were upregulated in T cells exposed to 1C DCs (Supp. Table 2A-B; 2D), consistent with the sustained high T cell motility in this condition and formation of serial kinapses with APCs (Moreau et al., 2012). In T cells exposed to 100C DCs, effector cytokines and lineage-specific transcription factors were significantly upregulated compared to the 1C DC setting, including IL-2 (at 24h) and IFN-γ (at 12h and 24h; Supp. Table 1A, 3E). Accordingly, there was a marked enrichment in transcription factors associated with effector and memory differentiation (Cui and Kaech, Imm Rev, 2010), including TBX21 (Tbet) and Jun, in 100C DC exposed T cells (Supp. Table 3A,C). Given the transcriptional differences in IFN-γ and other cytokines, despite equivalent IFN-γ protein secretion upon in vitro re-stimulation at 20h and 48h (Fig. 4A-B), we examined IFN-γ production at 96h (Supp. Fig 2). Consistent with the preceding upregulation of IFN-γ mRNA, T cells exposed to 100C DCs secreted significantly higher amounts of IFN-γ at 96h than T cells that had encountered 1C DCs. 100C DC exposed T cells also upregulated the Stat3 pathway (Supp. Table 3A; C), which is upregulated in memory precursors (Cui et al., 2011; Siegel et al., 2011). This differential pattern of transcription factors and regulatory molecules is consistent with the finding that stimulation with 1C versus 100C DCs profoundly influences the fate of P14 cells. Finally, 100C DC exposed T cells selectively upregulated many co-inhibitory molecules (Odorizzi and Wherry, 2012; Pardoll, 2012; Youngblood et al., 2011), including PD-1, CD200, LAG-3 and CTLA-4 (Suppl. Table 1A, 2A), which might reflect a mechanism to protect activated T cells from activation induced cell death in an ongoing response.
DISCUSSION
Optimal CD8+ T cell responses ensue when mature DCs present Ag at a dose and duration sufficient to result in TN cell activation and differentiation into short-lived TEff and long-lived self-renewing memory cells. We know little about the signals that determine whether, how and what kind of memory cells arise, but these decisions are thought to be regulated, in part, by the dynamics of TN cell interactions with DCs (Hugues, 2010). During the first 48h after TN cell entry into a LN containing DCs pulsed with a high dose of Ag these interactions usually follow a 3-phase program (Mempel et al., 2004). The duration of phase 1, wherein TN cells undergo short, serial encounters with DCs, is inversely correlated with Ag dose (Henrickson et al., 2008). Phase 2 features prolonged, stable conjugates during which T cells commence cytokine production. Finally, during phase 3 T cells return to short contacts and proliferate rapidly (Henrickson et al., 2008; Mempel et al., 2004).
It has been unclear how the interaction dynamics during each phase influence immunological outcome. An early clue came from in vitro imaging of T cells interacting with DCs in a collagen gel, which showed that T cells proliferate even when they undergo only transient interactions (Gunzer et al., 2000). While engaging in transient contacts in vivo, T cells are thought to integrate stimuli during each APC encounter until the cumulative signal exceeds a threshold for phase 2 transition (Henrickson et al., 2008). The mechanism for in vivo integration of serial activation signals is unknown, but in vitro work has implicated the Ras family of GTPases (Das et al., 2009) and the Akt pathway (Kim et al., 2012). Phase 2 begins when the median duration between TN cells and DCs exceeds 30min. This definition was chosen because TN cells require ~30 min to form a mature immunological synapse, a dynamic structure at the interface with APCs that is stabilized by LFA-1 on T cells binding the adhesion molecule ICAM-1 on APCs (Dustin and Groves, 2012; Lee et al., 2002). LFA-1–ICAM-1 interactions are also needed for stable Ag-driven T cell–DC contacts in LNs, and ICAM-1 deficient DCs presenting a high Ag dose fail to retain TEff cells at d12 after activation (Scholer et al., 2008). Experimental manipulations that induce T cell tolerance rather than TEff responses, e.g. Ag targeting to immature DCs or promotion of co-inhibitory signaling in T cells, result in enhanced T cell motility and lack of stable T cell–DC contacts (Fife et al., 2009; Hugues et al., 2004; Schneider et al., 2006). However, for T cell priming by fully mature wildtype DCs, it had been widely assumed that tight interactions with sustained TCR signaling are needed (Fooksman et al., 2009; Iezzi et al., 1998). Indeed, many MP-IVM studies use reduced T cell motility and clustering with DCs as a surrogate parameter for T cell activation.
Our earlier studies with M-peptide, which has a higher affinity and longer half-life in H2-Db than C-peptide, seem consistent with this idea (Henrickson et al., 2008). Although pMHC complexes with either C- or M-peptide have similar affinity for the P14 TCR, DCs pulsed with the threshold peptide dose needed to induce P14 proliferation support distinct interaction dynamics; P14 cells that encounter threshold dose M-peptide pulsed DCs transition to phase 2 (Henrickson et al., 2008), but they remained in phase 1 when C-peptide was presented (this study). The most likely explanation for this differential response is the distinct half-life of complexes formed by M- versus C-peptide with H2-Db (~6h versus ~2.4h, respectively (Henrickson et al., 2008)). Thus, after P14 cells encounter DCs presenting an amount of Ag that is sufficient to trigger TCR signaling, but insufficient to promote rapid transition to stable contacts, the T cells continue to migrate, accumulating serial activation signals. During this information gathering period, pMHC complexes disintegrate at a constant rate, which is higher for C-than M-peptide. After several hours, the P14 cells activate LFA-1 enabling sustained contacts, but only with DCs presenting a sufficient amount of Ag to support synapse formation because TCR signaling is needed for LFA-1 to assume a high affinity state and/or to redistribute toward and within the emerging synapse. Long-lived pMHC complexes with M-peptide remain available at this timepoint, but C-peptide dissipates much faster, so P14 cells cannot find suitable binding partners to engage in phase 2 interactions.
How do the dynamics of T cell–APC interactions affect T cell responses? Clearly, stable contacts are not needed to initiate effector responses by CD8+ T cells in vivo; both 1C and 100C DC induced efficient population-wide proliferation and TEff differentiation of LN-resident Ag-specific TN cells However, they did so with distinct kinetics and consequences: exposure to high dose Ag triggered a transient early apoptotic contraction of the responder population (likely due to AICD), followed by vigorous and sustained expansion of LN-resident T cells for ~96h and long-term memory formation. By contrast, at the 1C threshold dose, early T cell apoptosis was modest, but after 48h the effector pool underwent accelerated attrition ultimately leading to a complete loss of Ag-experienced cells. While cytokine production and cytotoxicity were equivalent for T cells exposed to 1C and 100C DCs through 48h, proliferation and IFNγ production by 1C T cells were markedly lower at 96h, thus reflecting at a protein level the transcriptional differences that appeared as early as 24h after Ag encounter.
We used two approaches to study memory differentiation, which each allowed transfer of P14 TN cells into recipients that lacked gp33-41 responsive T cells, so the transferred cells could be identified later based on their Ag responsiveness. Both models restricted a short-lived non-proliferating Ag to a small number of DCs in a single popLN. To ensure that the number of P14 cells that encountered Ag was sufficient for detection and analysis, we transferred 5 million P14 TN cells of which <0.1% were activated before the Ag disappeared. Consequently, unlike in a systemic viral infection, a substantial population of unactivated transferred cells remained in recipients. To measure memory differentiation, this residual TN population had to be distinguished from true memory cells that arose from Ag encounters in the popLN. This was possible because memory cells respond more rapidly than TN cells to Ag challenge, so memory cells could be revealed by measuring early Ag induced IFN-γ secretion. No difference was found between recipients of P14 TN cells that had received 1C DCs or no Ag, but there was a substantial increase in IFN-γ secreting cells in recipients of 100C DCs, indicating that the latter had developed memory.
These results establish that CD8+ T cells make differential memory fate decisions in vivo as a consequence of the Ag dose presented by DCs. In this context, we must consider three parameters that distinguish how TN cells experience encounters with 1C versus 100C DCs: 1. the difference in density of cognate pMHC complexes on a DC affects the number of TCRs that are triggered simultaneously (instantaneous signal intensity); 2. the time interval between T cell entry into the LN until cognate pMHC complexes have disappeared from DCs (signal persistence); 3. 100C DCs, unlike 1C DCs, support phase 2-like contacts, so TCR stimulation changes from an intermittent to a continuous mode (signal duration) (Tkach and Altan-Bonnet, 2013). It remains to be determined whether and to what extent each of these parameters contributes to memory differentiation. Moreover, only a small fraction of P14 cells that encountered 100C DCs ultimately entered the memory pool, and it will be important to understand how this subset is selected.
Regardless of the initial C-peptide pulsing dose and mode of interaction, the very short half life of pMHC complexes is expected to result in a 99.9995% loss of C-peptide from pulsed DCs at 24h after T cell injection (42h after DC pulsing), so P14 cells in the present study did not experience cognate Ag beyond the first day. Thus, signals received by CD8+ TN cells from DCs within a single day can precipitate a fate choice between a transient effector burst and long-term memory commitment. Accordingly, a recent study using anti-pMHC antibodies to terminate CD8+ TN cell access to Ag showed that Ag accessibility must exceed a threshold duration for optimal memory cell differentiation (Blair et al., 2011). Similarly, during microbial infections LN dwell time, proliferation and memory differentiation of CD8+ T cell are inversely correlated with TCR affinity for microbial Ags (Zehn et al., 2009).
It should be noted that the rules of memory fate commitment are almost certainly more complex when CD8+ T cells respond to infections. Aside from the concomitant stimulation of CD4+ T cells and other leukocytes that influence CD8+ T cells, even pathogen-derived Ags with low affinity for MHC may afford long signal persistence because proliferating pathogens continue to produce antigenic material, and DCs phagocytose and store such material for cross-presentation (Trombetta and Mellman, 2005), while the short peptides used here are not cross-presented (Cebrian et al., 2011). Of note, DCs in our system did not present antigenic moieties to stimulate CD4+ T cells, so P14 cells did not receive ‘help’ (Williams and Bevan, 2007), however, we co-injected LPS with DCs to promote in vivo DC maturation. The experimental system described here reflects a reductionist approach allowing quantitative study of early T cell fate decisions. Our approach delivers a single Ag pulse with a known (short) half-life on mature DCs providing a level of control that is not feasible with an infectious Ag source. In terms of clinical correlate, this model resembles immunization with a subunit vaccine or autologous DC therapy.
Previous work on early CD8+ memory differentiation has traditionally examined Ag-experienced T cells expressing specific markers that predict effector or memory fate (Kaech et al., 2003) and are associated with widespread changes in gene expression (Haining et al., 2008; Kaech et al., 2002; Sarkar et al., 2008; Wherry et al., 2007). There are also transcriptional differences between TEff and memory T cells and between memory T cells and exhausted T cells (Doering et al., 2012), as well as memory precursor T cells in mice (Sarkar et al., 2008) and humans (Chowdhury et al., 2011). However, as known memory fate markers arise only several days after the initial stimulus, the earliest transcriptional signatures of memory precursors were obtained from this late interval, and the timepoint when activated T cells first reach a memory fate checkpoint has been unclear (Best et al., 2013). The present results suggest that this checkpoint is reached in vivo within less than a day of priming.
To begin to address the determinants and consequences of this early checkpoint, we conducted a transcriptome analysis of P14 cells that were sorted from popLNs 12h or 24h after exposure to 1C or 100C DCs. Our results reveal a dramatic and progressive divergence in transcriptional profiles even though all environmental parameters other then Ag dose were presumably identical. Interestingly, at 24h of stimulation, the 100C condition was associated with preferential upregulation of at least 3 co-inhibitory molecules, CTLA-4, LAG-3 and PD-1. Since tight T cell–DC conjugates dissociate around the 24h timepoint (Mempel et al., 2004), it is possible that these molecules are involved in terminating phase 2. Several studies report that co-inhibitory signals antagonize the ‘stop’ signal that T cells receive upon TCR engagement (Fife et al., 2009; Schneider et al., 2006). This signal attenuation may also protect T cells from continued Ag stimulation, which might drive T cells toward an apoptosis-prone TEff phenotype (Mitchison, 1964). Thus, phase 2 may be needed to induce a transcriptional program that in aggregate dampens further TCR signaling during the subsequent effector phase and promotes survival and differentiation toward a memory phenotype.
In summary, we describe an experimental strategy in which Ag-specific CD8+ TN cells encounter in LNs mature DCs that were pulsed with either a low or a high dose of a naturally occurring cognate viral Ag.
Only DCs presenting a high Ag dose supported phase 2-like tight interactions, while DCs pulsed with either Ag dose induced vigorous early T cell proliferation and TEff differentiation. The differential interactive behavior induced by high- versus low-dose pulsed DCs was paralleled by distinct transcriptional programs in activated T cells, and only T cells that interacted with DCs presenting the high Ag dose gave rise to sustained immunological memory. This suggests that information exchange in phase 2 allows T cells to pass through a critical early checkpoint that fosters Ag specific long-term protection and avoids immunological amnesia.
Experimental Procedures
Mice
Male C57BL/6 mice (Charles River Laboratories), congenic C57BL/6 (CD45.1+) mice (Taconic Farms or Jackson Laboratories), OT-I Rag1−/− and P14 Tcra−/− mice (Taconic Farms) were used at 6–10 weeks of age. Experiments were performed in accordance with NIH guidelines and approved by the IACUC of Harvard Medical School.
Reagents
M-peptide (KAVYNFATM), C-peptide (KAVYNFATC) and SIINFEKL were purchased from New England Peptides and resuspended in deionized H20. Anti-L-selectin mAb (Mel-14) was purchased from BD Pharmingen or BioExpress. All other mAbs were from BD Pharmingen.
Cell Isolation for Adoptive Cell Transfer
DCs were purified by immunomagnetic cell sorting (~98% CD11c+, Miltenyi Biotec) from spleens of C57BL/6 mice that had been implanted with a Flt-3L secreting melanoma, as described (Mora et al., 2003). CD8+ T cells from LNs and spleens of P14 Tcra−/− and OT-I Rag1 −/− mice were purified by negative immunomagnetic sorting (Miltenyi Biotec).
Flow cytometry
Phenotyping of DCs and T cells was performed on a FACSCalibur or FACSCanto analyzer (Becton Dickinson).
LCMV infections
Mice were infected iv with 103 p.f.u. LCMV Armstrong at various time points after DC transfer with or without P14 T cell transfer. At d5 post-infection, mice were sacrificed and spleens and popLNs removed for flow cytometry of intracellular IFN-γ expression in CD8+ T cells.
Multiphoton intravital microscopy
DCs were pulsed with peptide and labeled for 20 min at 37°C with 10μM 5-(and 6-)-(((4-chloromethyl)benzoyl) amino)tetramethylrhodamine (CMTMR) or 7-amino-4-chloromethylcoumarin (CMAC; Invitrogen). 5×105 DCs in 20μl IMDM (with 10% FCS) containing 10ng E. coli LPS (Sigma) were injected into the right hind footpad of recipient mice. T cell populations were labeled for 15min at 37°C with 4μM 5-chloromethylfluorescein diacetate (CMFDA; Invitrogen) or for 25min at 37°C with 10μM CMAC (dyes were swapped between experiments). 5 × 106 cells of each subset (1:1 ratio) were given to recipients iv 18h after DC injection. After 2h, animals received 100 μg Mel-14 iv. The right popliteal LN was prepared for MP-IVM on a BioRad 2100MP system as described (Mempel et al., 2004).
Statistics
When appropriate, significance of differences was calculated using Mann-Whitney or Student's T test. The bootstrap correction mean was calculated for interaction durations and meandering indices for each dataset (n = 100 interactions) (Manly, 1997).
Microarray data acquisition
Total RNA was isolated from TriZol using the Agencourt RNAdvance Tissue Kit (Beckman Coulter, Brea CA), and was amplified using the WT-Ovation Pico RNA Amplification and Labeling System (NuGEN, San Carlos, CA). The cDNA was fragmented, labeled and hybridized to Affymetrix 430_2 microarrays (Affymetrix, Santa Clara, CA). The gene expression dataset has been submitted to the NCBI/ Genbank GEO database (series entry GSE49274).
Supplementary Material
Highlights.
Stable DC-CD8+ T cell contacts are not required for effector T cell differentiation
Memory differentiation of CD8+ T cells is correlated with stable DC-T cell contacts
Low and high dose antigen initiate distinct transcriptional programs in T cells
CD8 T cells make a memory fate decision within 24h after antigen encounter
Acknowledgements
We thank T. Buschman for assistance with programming; G. Cheng and M. Perdue for technical and secretarial assistance, respectively. This work was supported by NIH grants AI069259 and AI072252 to U.H.v.A.; MSTP funding at Harvard Medical School and a Osaka University Immunology Frontier Research Center (iFREC) Postdoctoral Fellowship to S.E.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Achour A, Michaelsson J, Harris RA, Odeberg J, Grufman P, Sandberg JK, Levitsky V, Karre K, Sandalova T, Schneider G. A structural basis for LCMV immune evasion: subversion of H-2D(b) and H-2K(b) presentation of gp33 revealed by comparative crystal structure analyses. Immunity. 2002;17:757–768. doi: 10.1016/s1074-7613(02)00478-8. [DOI] [PubMed] [Google Scholar]
- Best JA, Blair DA, Knell J, Yang E, Mayya V, Doedens A, Dustin ML, Goldrath AW, Immunological Genome Project, C. Transcriptional insights into the CD8(+) T cell response to infection and memory T cell formation. Nat Immunol. 2013;14:404–412. doi: 10.1038/ni.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya D, Rossi DJ, Bryder D, Weissman IL. Purified hematopoietic stem cell engraftment of rare niches corrects severe lymphoid deficiencies without host conditioning. J Exp Med. 2006;203:73–85. doi: 10.1084/jem.20051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattman JN, Antia R, Sourdive DJ, Wang X, Kaech SM, Murali-Krishna K, Altman JD, Ahmed R. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J. Exp. Med. 2002;195:657–664. doi: 10.1084/jem.20001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter JM, Schmitz N, Sewell AK, Godkin AJ, Bachmann MF, Gallimore AM. Potent T cell agonism mediated by a very rapid TCR/pMHC interaction. Eur J Immunol. 2007;37:798–806. doi: 10.1002/eji.200636743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casrouge A, Beaudoing E, Dalle S, Pannetier C, Kanellopoulos J, Kourilsky P. Size estimate of the alpha beta TCR repertoire of naive mouse splenocytes. J Immunol. 2000;164:5782–5787. doi: 10.4049/jimmunol.164.11.5782. [DOI] [PubMed] [Google Scholar]
- Cebrian I, Visentin G, Blanchard N, Jouve M, Bobard A, Moita C, Enninga J, Moita LF, Amigorena S, Savina A. Sec22b regulates phagosomal maturation and antigen crosspresentation by dendritic cells. Cell. 2011;147:1355–1368. doi: 10.1016/j.cell.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Chowdhury FZ, Ramos HJ, Davis LS, Forman J, Farrar JD. IL-12 selectively programs effector pathways that are stably expressed in human CD8+ effector memory T cells in vivo. Blood. 2011;118:3890–3900. doi: 10.1182/blood-2011-05-357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui W, Liu Y, Weinstein J, Craft J, Kaech S. An Interleukin-21- Interleukin-10-STAT3 Pathway Is Critical for Functional Maturation of Memory CD8+ T Cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, Chakraborty AK, Roose JP. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–351. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Groves JT. Receptor signaling clusters in the immune synapse. Annu Rev Biophys. 2012;41:543–556. doi: 10.1146/annurev-biophys-042910-155238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fife BT, Pauken KE, Eagar TN, Obu T, Wu J, Tang Q, Azuma M, Krummel MF, Bluestone JA. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol. 2009;10:1185–1192. doi: 10.1038/ni.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair D, Waite J, Sacristan C, Victora G, Zanin-Zhorov A, Dustin ML. Functional Anatomy of T Cell Activation and Synapse Formation. Annu Rev Immunol. 2009 doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza KM, Chan SM, Suri R, Nguyen LT, Odermatt B, Schoenberger SP, Ohashi PS. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J Exp Med. 2000;191:2021–2027. doi: 10.1084/jem.191.11.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain RN, Stefanova I. The dynamics of T cell receptor signaling: complex orchestration and the key roles of tempo and cooperation. Annu Rev Immunol. 1999;17:467–522. doi: 10.1146/annurev.immunol.17.1.467. [DOI] [PubMed] [Google Scholar]
- Gunzer M, Schafer A, Borgmann S, Grabbe S, Zanker KS, Brocker EB, Kampgen E, Friedl P. Antigen presentation in extracellular matrix: interactions of T cells with dendritic cells are dynamic, short lived, and sequential. Immunity. 2000;13:323–332. doi: 10.1016/s1074-7613(00)00032-7. [DOI] [PubMed] [Google Scholar]
- Haining WN, Ebert BL, Subrmanian A, Wherry EJ, Eichbaum Q, Evans JW, Mak R, Rivoli S, Pretz J, Angelosanto J, et al. Identification of an evolutionarily conserved transcriptional signature of CD8 memory differentiation that is shared by T and B cells. J Immunol. 2008;181:1859–1868. doi: 10.4049/jimmunol.181.3.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Wherry EJ. Integrating Genomic Signatures for Immunologic Discovery. Immunity. 2010;32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Henrickson SE, Mempel TR, Mazo IB, Liu B, Zheng H, Flynn M, Artymov MN, Junt T, Wong HC, Chakraborty AK, Von Andrian UH. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson SE, von Andrian UH. Single-cell dynamics of T-cell priming. Curr Opin Immunol. 2007;19:249–258. doi: 10.1016/j.coi.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Hugues S. Dynamics of dendritic cell-T cell interactions: a role in T cell outcome. Semin Immunopathol. 2010;32:227–238. doi: 10.1007/s00281-010-0211-2. [DOI] [PubMed] [Google Scholar]
- Hugues S, Fetler L, Bonifaz L, Helft J, Amblard F, Amigorena S. Distinct T celldynamics in lymph nodes during the induction of tolerance and immunity. Nat Immunol. 2004;5:1235–1242. doi: 10.1038/ni1134. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Karjalainen K, Lanzavecchia A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzavecchia A, Sallusto F. From synapses to immunological memory: the role of sustained T cell stimulation. Curr Opin Immunol. 2000;12:92–98. doi: 10.1016/s0952-7915(99)00056-4. [DOI] [PubMed] [Google Scholar]
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296:346–349. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, Bootstrap and Monte Carlo Methods in Biology. 2nd Ed. Boca Raton; FL: 1997. [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proc. Natl. Acad. Sci. U. S. A. 2004a;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Safrina O, Parker I, Cahalan MD. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J Exp Med. 2004b;200:847–856. doi: 10.1084/jem.20041236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison NA. Induction of Immunological Paralysis in Two Zones of Dosage. Proc R Soc Lond B Biol Sci. 1964;161:275–292. doi: 10.1098/rspb.1964.0093. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Moreau HD, Lemaitre F, Terriac E, Azar G, Piel M, Lennon-Dumenil AM, Bousso P. Dynamic in situ cytometry uncovers T cell receptor signaling during immunological synapses and kinapses in vivo. Immunity. 2012;37:351–363. doi: 10.1016/j.immuni.2012.05.014. [DOI] [PubMed] [Google Scholar]
- Odorizzi PM, Wherry EJ. Inhibitory receptors on lymphocytes: insights from infections. J Immunol. 2012;188:2957–2965. doi: 10.4049/jimmunol.1100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–561. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Kalia V, Haining WN, Konieczny BT, Subramaniam S, Ahmed R. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J Exp Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Downey J, Smith A, Zinselmeyer BH, Rush C, Brewer JM, Wei B, Hogg N, Garside P, Rudd CE. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- Scholer A, Hugues S, Boissonnas A, Fetler L, Amigorena S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity. 2008;28:258–270. doi: 10.1016/j.immuni.2007.12.016. [DOI] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, Douek DC, Fahle GH, Cohen JI, Holland SM, Milner JD. A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity. 2011;35:806–818. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, Schwickert T, Nussenzweig MC, Dustin ML. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007 doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- Stoll S, Delon J, Brotz TM, Germain RN. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Science. 2002;296:1873–1876. doi: 10.1126/science.1071065. [DOI] [PubMed] [Google Scholar]
- Tkach K, Altan-Bonnet G. T cell responses to antigen: hasty proposals resolved through long engagements. Curr Opin Immunol. 2013;25:120–125. doi: 10.1016/j.coi.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu.Rev.Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Trombetta ES, Mellman I. Cell biology of antigen processing in vitro and in vivo. Annu Rev Immunol. 2005;23:975–1028. doi: 10.1146/annurev.immunol.22.012703.104538. [DOI] [PubMed] [Google Scholar]
- von Andrian UH, Mempel TR. Homing and cellular traffic in lymph nodes. Nature Reviews Immunology. 2003;3:867–878. doi: 10.1038/nri1222. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Youngblood B, Oestreich KJ, Ha SJ, Duraiswamy J, Akondy RS, West EE, Wei Z, Lu P, Austin JW, Riley JL, et al. Chronic virus infection enforces demethylation of the locus that encodes PD-1 in antigen-specific CD8(+) T cells. Immunity. 2011;35:400–412. doi: 10.1016/j.immuni.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel RM, Doherty PC. Immunological surveillance against altered self components by sensitised T lymphocytes in lymphocytic choriomeningitis. Nature. 1974;251:547–548. doi: 10.1038/251547a0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.