Abstract
Rationale
The mesostriatal dopamine system plays a key role in mediating the reinforcing effects of psychostimulant drugs like cocaine. The muscarinic M4 acetylcholine receptor subtype is centrally involved in regulation of dopamine release in striatal areas. Consequently, striatal M4 receptors could be a novel target for modulating psychostimulant effects of cocaine.
Objectives
For the first time, we here addressed this issue by investigating the effects of a novel selective positive allosteric modulator of M4 receptors, VU0152100, on cocaine-induced behavioral and neurochemical effects in mice.
Methods
To investigate the effect of VU0152100 on the acute reinforcing effects of cocaine, we use an acute-cocaine self-administration model. We used in vivo microdialysis to investigate whether the effects of VU0152100 in the behavioral studies were mediated via effects on dopaminergic neurotransmission. In addition the effect of VU0152100 on cocaine-induced hyperactivity and rotarod performance was evaluated.
Results
We found that VU0152100 caused a prominent reduction in cocaine self-administration, cocaine-induced hyperlocomotion, and cocaine-induced striatal dopamine increase, without affecting motor performance. Consistent with these effects of VU0152100 being mediated via M4 receptors, its inhibitory effects on cocaine-induced increases in striatal dopamine were abolished in M4 receptor knockout mice. Furthermore, selective deletion of the M4 receptor gene in dopamine D1 receptor-expressing neurons resulted in a partial reduction of the VU0152100 effect, indicating that VU0152100 partly regulates dopaminergic neurotransmission via M4 receptors co-localized with D1 receptors.
Conclusions
These results show that positive allosteric modulators of the M4 receptor deserve attention as agents in the future treatment of cocaine abuse.
Keywords: Muscarinic acetylcholine (ACh) M4 receptor, positive allosteric modulator (PAM), cocaine, psychostimulants, dopamine, striatum
Introduction
Midbrain dopaminergic neurones projecting to the striatum are centrally involved in mediating reinforcing effects of psychostimulant drugs like cocaine (Wise, 1996; Koob et al. 1998). Acetylcholine (ACh) plays important roles in regulation of many fundamental functions of the central nervous system, including abuse-related effects of cocaine (Williams and Adinoff et al. 2008; Sofuoglu and Mooney, 2009). ACh exerts its actions through binding to two distinct classes of receptors, i.e. nicotinic and muscarinic receptors in the brain. The muscarinic receptor family consists of five subtypes (M1-M5), which control many important central and peripheral functions (Wess, 2004; Wess et al. 2007). Muscarinic M4 receptors are highly expressed in striatal areas (Levey, 1993; Yasuda et al. 1993) where the cholinergic and dopaminergic neurotransmitter systems converge (Di Chiara et al. 1994). In the striatum, M4 receptors are the predominant muscarinic autoreceptors on cholinergic interneurones (Zhang et al. 2002) but are also expressed on GABAergic projection neurones where they are primarily co-localized with dopamine D1 receptors, and to a lesser extent with D2 receptors (Weiner et al. 1990; Bernard et al. 1992; Ince et al. 1997). Striatal D1 receptors and M4 receptors exert opposing effects on intracellular cAMP formation (Onali and Olianas, 2002). Mice lacking the M4 receptor (M4-KO mice) display a ‘dopamine hypersensitivity’ behavioral phenotype (Gomeza et al. 1999; Felder et al. 2001; Tzavara et al. 2004), and we recently showed that M4 receptor deletion increases chronic cocaine self-administration, cocaine-induced dopamine increase in the nucleus accumbens, and cocaine-induced hyperlocomotor activity in mice (Schmidt et al. 2011). Taken together, these data suggest that compounds with agonistic effects on striatal M4 receptor could inhibit cocaine-induced behavioral effects and striatal dopamine increases.
Centrally active positive allosteric modulators (PAM) of the M4 receptor have recently been developed, including LY2033298, VU0152099, and VU0152100 (Brady et al. 2008; Chan et al. 2008). Consistent with findings in M4-KO mice, LY2033298 attenuated apomorphine-induced deficits in pre-pulse inhibition of the startle response and conditioned avoidance response in rats (Chan et al. 2008; Leach et al. 2010) and VU0152099/VU0152100 potently inhibited amphetamine-induced hyperlocomotion in rats (Brady et al. 2008). Until now, the effects of M4 receptor PAMs on the reinforcing properties of drugs of abuse have not been investigated. Here, we addressed this issue by studying the effects of the M4 receptor PAM, VU0152100, on behavioral and neurochemical effects of cocaine in mice.
Materials and methods
Animals
Male NMRI mice (Taconic, Denmark) and M4-KO (Schmidt et al. 2011) and D1-M4-KO (Jeon et al. 2010) mice on a C57BL/6 background weighing 28–31 g were used in all experiments, except for the acute self-administration experiment where mice weighing 20–22 g were used. All mice were housed in Makrolon cages (20 × 35 × 15 cm) enriched with cardboard housing and nesting material. The animals were kept at room temperature (22°C ± 2) in a 12-hour light/dark cycle (lights on at 6:00 A.M.) with free access to food and water. All experiments were performed in the middle of the light cycle between 9:00 A.M. and 4:00 P.M. The mice were allowed to acclimatize to the animal facility for at least 7 days prior to initiation of the experiments. Experiments in transgenic mice were performed on experimentally naïve 10–16 week-old mice. All procedures were conducted in accordance with guidelines from the Animal Experimentation Inspectorate, Denmark and the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Drugs used for behavioral studies
VU0152100 (3-amino-N-84-methoxybenzyl)-4,6-dimethylthieno[2,3-b]pyridine carboxamide) was obtained from Axon Medchem, (Groningen, The Netherlands). Cocaine hydrochloride was obtained from the Copenhagen University Hospital Pharmacy, Denmark. VU0152100 (0.1–10 mg/kg) was dissolved in 10% tween 80 and 0.9% saline solution with pH adjusted to 6–7 using sodium hydroxide (1 M). Cocaine was dissolved in saline. An initial dose-finding study revealed that VU0152100 10 mg/kg intraperitoneally (i.p.) was sufficient to reduce cocaine (30 mg/kg)-induced hyperlocomotion in mice (data not shown). In all experiments VU0152100 was administered (i.p.) 40 min prior to cocaine, which was administered subcutaneously (s.c.) or intravenously (i.v.). We have not evaluated the pharmacokinetic properties of VU0152100 in mice but have adapted to the data from the rat study (Brady et al. 2008). Haloperidol (Serenase; 5 mg/ml, Janssen-Cilag) was diluted in saline. All drugs were injected in a volume of 10 ml/kg.
Chemicals and reagents
Dopamine hydrochloride was obtained from Sigma (St. Louis, MO, USA). Metacam was obtained from Boehringer Ingelheim (Copenhagen, Denmark) and Sevoflurane from Baxter (Alleroed, Denmark). All other chemicals were purchased from Merck (Darmstadt, Germany) and Fluka Chemie (Buchs, Switzerland). Deionized water was obtained from a MilliQ water purification system (Billerica, Massachusetts, USA).
Acute cocaine self-administration
The procedure has been described in detail previously (Sørensen et al. 2008). The self-administration apparatus consisted of transparent plastic boxes (8×8×8 cm) with a centred frontal nose-poke hole (12 mm diameter) 1 cm above the floor and a centred posterior vertical opening (width 5 mm) through which the tail extended. Dual photocells projected an infrared beam 1 mm in front of the nose-poke hole. Eight NMRI mice were tested at the same time, with interposed shields, preventing the mice from seeing each other during the experiment. Immediately before being placed in the test boxes, the mice were left for approximately 3 min below a 150 W infrared light bulb to induce vasodilatation, thus facilitating the insertion of the needle into their tail veins. The tail was fixed onto a stripe of double-sided adhesive tape. The tape also fixed the tail at its base as well as the needle at the insertion point. A nose-poke interrupted the infrared beam, thus activating the photocell connected to an interface (SG 502, Med Associates Inc.) and thereafter a syringe pump (PHM-100A, Med Associates Inc.) holding a 2 ml syringe connected by 75 cm PVC tubing (0.9 mm inner diameter) to a 27 G infusion needle. A back-check valve between needle and tubing prevented reflux of blood. A fixed ratio 1 (FR-1) schedule was used, with no time-out between nose-poke and infusion, so that each nose-poke induced the i.v. infusion of 1.4 µl cocaine or saline solution. A computer and self-administration software (Ellegaard Systems, Denmark) were used to control infusions and to record nose-poking behaviour. After placing the mouse in the self-administration box for a 10 min habituation period during which nose-poking did not induce infusions, one priming infusion was given by the experimenter immediately before starting a 30-min session. Immediately after the session, the correct placement of the infusion needle was verified by manual infusion of the tested drug by an experimenter blind to the pretreatment as well as to the number of nose-pokes produced, and the animals were quickly sacrificed. Animals were excluded from further analysis if they had not produced at least five nose-pokes during the self-administration session or correct placement of the infusion needle could not be verified.
The mice received an i.p. injection of VU0152100 at doses of 0.1 (n=13) or 1.0 (n=11) mg/kg or corresponding vehicle (n=14) and, at 30 min later, they were subjected to the self-administration procedure described above with access to i.v. administration of cocaine at 0.03 mg/kg/infusion, previously shown to be the peak-dose for inducing self-administration in NMRI mice (22). A control group (n=11) received vehicle i.p. followed by saline i.v.
Locomotor activity measurements
The locomotor activity cages (Ellegaard Systems, Denmark) were equipped with 5×8 infrared light sources plus photocells. The light beams crossed the cage 1.8 cm above the bottom of the cage. During the test session, locomotor activity was recorded as crossings of infrared light beams. The recording of a motility count required interruption of two adjacent light beams, thus avoiding counts induced by stationary movements of the mice. All experiments were conducted in a clean cage with a scant lining of bedding material. Initially, NMRI mice were placed in the test cages for a 30-min habituation period followed by i.p. administration of VU0152100 (0.1–10 mg/kg) or vehicle. Forty min later, all mice were injected with 30 mg/kg of cocaine (s.c), and locomotor activity was measured for an additional 3 hours.
Rotorod test
The effects of VU0152100 on motor performance were evaluated using an accelerating rotorod model (Ugo Basile 7650, Italy). NMRI mice were trained once per day for 3 consecutive days on an accelerating protocol (4–40 rpm over 5 min). Animals that after 3 days could not stay on the rotorod for 5 min were excluded. Twenty min after the last training, mice received an i.p. injection of VU0152100 (10 mg/kg), haloperidol (0.5 mg/kg), or vehicle (10% tween 80). Forty min later (i.e. 1 hour after the last training session), the mice were tested again on the rotorod and the latency to fall was measured up to 5 min. Immediately after the last test, the animals were quickly decapitated, their brains were rapidly removed, frozen and stored at −80°C until cfos in situ hybridisation.
In Vivo microdialysis
The procedure was previously described (Weikop et al. 2004; Schmidt et al. 2011). Twenty to thirty min prior to surgery, NMRI or M4 transgenic mice were treated with analgesic (Metacam, 5 mg/kg, s.c., Boehringer Ingelheim), then deeply anaesthetized using sevoflurane (Baxter) and subsequently placed in a stereotaxic frame. Intracerebral guide cannulae (CMA Microdialysis AB, Sweden) were stereotaxically implanted into the brain to allow positioning of the dialysis probe in the striatum (AP: +1.4 mm, ML: +1.2 mm relative to bregma and DV: −3.0 mm relative to skull surface; Franklin and Paxinos, 2007) and fixed in place with one anchor screw and dental cement. After surgery, the animals were housed in individual cages and left to recover for at least 24 hours. Correct placement of the probes was verified histologically at the end of the experiment.
The 2 mm microdialysis probe (CMA/7) was perfused at a rate of 1.8 µl/min with an artificial cerebrospinal fluid solution (147 mM NaCl, 4 mM KC1 and 2.3 mM CaC2, adjusted to pH 6.5). Using a swivel and a bowl (Instech Laboratories, Pennsylvania, USA), the animals were able to move freely during dialysis. The first five 20-min fractions were discarded to obtain stable basal values. After this, three 20-min fractions were collected to establish dopamine baseline levels. Subsequently, mice were injected with VU0152100 (0.1, 1.0, and 10 mg/kg) or vehicle and two 20-min fractions were collected. Mice were then injected with s.c. cocaine (30 mg/kg) or saline vehicle. All fractions were assayed immediately after collection using high-performance liquid chromatography (HPLC) with electrochemical detection (Weikop et al. 2004).
Total dopamine concentration in striatal tissue
D1-M4-KO and WT mice were decapitated, the brains rapidly removed, the striatum dissected, frozen and stored at −80°C. On the day for the biochemical analysis the tissue from each mice was homogenised in 0.5 ml of 0.1 N perchloric acid containing 5% EDTA. After centrifugation (14,000 rpm for 30 min) 200 µl of the supernatant was filtered through a glass filter (0.22 µm) and 20 µl was used for the dopamine analysis as described below.
Dopamine measurement
Concentrations of dopamine in the microdialysis samples were determined by reversed-phase HPLC with electrochemical detection as previously described (Jeon et al. 2010). Briefly, the HPLC system consisted of a HPLC pump (LC-20AD, Shimadzu, Kyoto, Japan), a degasser (LC-27A, Waters, Denmark), a refrigerated microsampler (SIL-20AC, Shimadzu), an amperometric detector (2465 EC, Waters) and a computerized data acquisition system (Empower, Waters). The electrochemical detector cell was equipped with a glassy carbon electrode operating at +0.7 V vs. Ag/AgCl reference electrode. Typically, 15 µl samples were injected onto a Prodigy C18 column (100 × 2 mm I.D., 3-µm particle size, YMC Europe, Schermbeck, Germany). The mobile phase consisted of 93% of 94.2 mM NaH2PO4, 0.98 mM octanesulfonic acid, 0.06 mM Na2EDTA, adjusted to pH 3.7 with 1 M phosphoric acid and 7% acetonitrile (v/v). The flow-rate was 0.25 ml/min. The limit of detection (at signal-to-noise-ratio 3) for dopamine was 7 fmol/15 µl.
In Situ hybridisation
The procedure for in situ hybridisation was previously described (Hjaeresen et al. 2008; Sørensen et al. in press). Briefly, coronal brain sections (15 µm) were cut through the frontal cortex (bregma 1.94 → 1.70 mm), striatum (bregma 1.10 → 0.86 mm), and hippocampus (−1.70 → −2.18 mm, only in experiment with M4 transgenic mice) using a cryostat. Synthetic oligonucleotide DNA probes (DNA Technology) were used for visualization of cfos and M4 receptor mRNA. The probe sequences were: cfos, 5’-CGG GCA GTG GCA CGT CTG GAT GCC GGC TGC CTT GCC TTC TCT GAC TGC-3’; M4, 5’-GTG GTG GAC AGC TCT GTG GGT GGT CGT TCC TTG GTG TTC TGG GTG GCA-3’.
Data analysis
Data are presented as means ± standard errors of the means (SEM). Basal extracellular levels of dopamine in all groups were calculated as means of three fractions collected prior to drug or vehicle administration and were set to 100%. All other values were expressed on a relative scale (mean ± SEM) as percentage of the basal (control) levels. The data were examined with two-way repeated measures ANOVA followed by Bonferroni-corrected pairwise comparisons. Additionally, area-under-the curve (AUC) values for the 60–120 min (AUC60–120 min) treatment interval were calculated for each animal and converted to percent of the AUC value of vehicle-treated mice (set as 100%). Group differences in AUC data as well as cocaine self-administration, locomotor activity, and rotorod data were analysed using one-way ANOVA followed by post-hoc Dunnett’s multiple comparison tests. In the microdialysis studies with transgenic mice, two-way repeated measures ANOVA followed by post-hoc Bonferroni-corrected pairwise comparisons was used to compare treatment effects within the genotypes, as well as two-way ANOVA followed by post-hoc Bonferroni-corrected pairwise comparisons to compare treatment effects between the genotypes. In the self-administration data, a higher variance was seen with cocaine treatment as compared to saline, probably due to the fact that some animals failed to learn the self-administration paradigm during the single session. To counter this variance in homogeneity, the number of nose-pokes was square root transformed before statistical analysis (Sørensen et al. 2008). M4 receptor and cfos mRNA expression was determined for each animal with a total of six measurements for each brain area. The mean of these measurements was used for further statistical analysis. Two-tailed, unpaired t-tests were used to compare M4 expression in D1-M4-KO and WT mice. One-way ANOVA followed by Bonferroni-corrected pairwise comparisons was used to compare cfos expression and total striatal tissue dopamine content. A p-value <0.05 was considered statistically significant.
Results
VU152100 Attenuates Acute Cocaine Self-Administration and Cocaine-Induced Hyperlocomotion
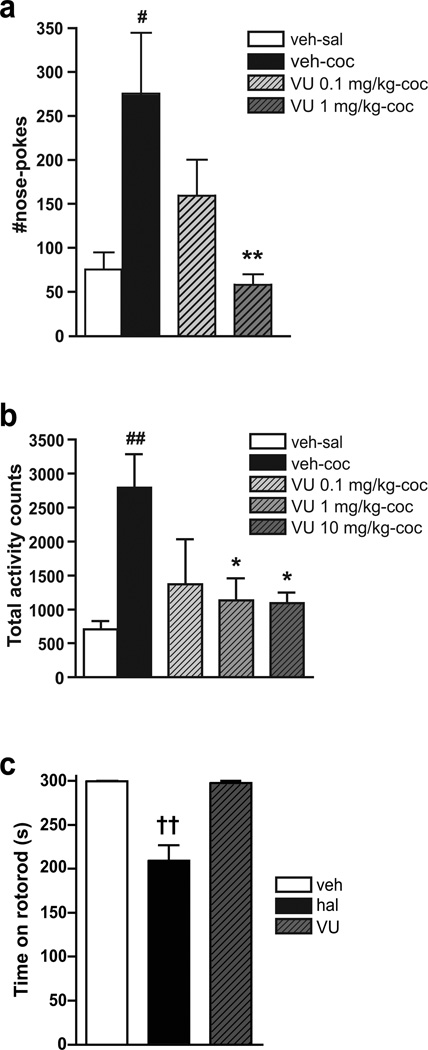
To investigate the effect of VU0152100 on the acute reinforcing effects of cocaine, we pretreated mice with VU0152100 (0.1–1.0 mg/kg) and then exposed them to the possibility to self-administer cocaine. One-way ANOVA showed a significant difference between test groups (F(3,44)=4.05, p=0.013). Post-hoc Dunnett’s multiple comparison test revealed that infusion of the cocaine peak dose (0.03 mg/kg/nose-poke) resulted in a significant increase in nose-poking during the 30 min session as compared to mice nose-poking for saline (p<0.05; Fig. 1a). Pretreatment with VU0152100 (0.1 and 1.0 mg/kg) dose-dependently reduced the self-administration of cocaine, reaching significance at the 1.0 mg/kg dose (p<0.01), where VU0152100 reduced self-administration to the level of saline (Fig. 1a). We also studied the effect of VU0152100 on cocaine-induced hyperlocomotion in mice. One-way ANOVA showed a significant difference between the test groups during the 3 h test period after treatment with cocaine 30 mg/kg (F(4,27)=4.38, p=0.009). As expected, post-hoc analysis showed that cocaine, as compared to saline, significantly increased locomotor activity measured as activity counts (p<0.01; Fig. lb). More importantly, pretreatment with VU0152100 (0.1–10 mg/kg) dose-dependently reduced the cocaine-induced hyperlocomotion by ~50–60%, reaching significance at the two highest doses (p<0.05; Fig. lb).
Fig. 1.
Behavioral effects of the M4 receptor PAM, VU0152100 in NMRI mice. a In the acute self-administration model, cocaine (0.03 mg/kg/inf., black column; n=14) induced a significant increase in the number of nose-pokes as compared to saline (white column; n=11). Pretreatment with VU0152100 (0.1 and 1 mg/kg, i.p., grey columns; n=13 and 11, respectively) dose-dependently inhibited nose-poking for cocaine, reaching significance at 1 mg/kg. b In the activity boxes, cocaine (30 mg/kg, s.c. black column; n=6) induced hyperlocomotion as revealed by a significant increase in the number of total activity counts as compared to saline (white column; n=6). Pretreatment with VU0152100 (0.1, 1, 10 mg/kg, i.p., grey columns; n=5–6) significantly reduced cocaine-induced hyperlocomotion. c Time spent on rotorod after i.p. pretreatment with vehicle (veh, white column), haloperidol (hal, 0.5 mg/kg; black column), or VU0152100 (VU, 10 mg/kg; grey column) (n=9 in each group). Haloperidol significantly reduced time spent on the rotorod when compared to vehicle. In contrast, VU0152100 had no effect on rotorod performance. All data represent group mean ± SEM. #p<0.05, ##p<0.01 vs. vehicle i.p. followed by saline (veh-sal); *p<0.05, **p<0.01 vs. vehicle i.p. followed by cocaine (veh-coc); ‹‹p<0.01 vs. veh (one-way ANOVA followed by Dunnett’s multiple comparison test)
VU0152100 Does Not Affect Motor Coordination and Balance
To examine the possibility that the effects of VU0152100 on cocaine self-administration and cocaine-induced hyperlocomotion might be due to unwanted side effects on motor coordination or balance, we subjected VU0152100-treated mice to the rotorod test. One-way ANOVA showed a significant difference between the three test groups (F(2,26)=25.03, p<0.001). Post-hoc analysis showed that pretreatment with the prototypical antipsychotic and dopamine D2 receptor antagonist haloperidol (0.5 mg/kg) significantly reduced the time spent on the rotorod (p<0.01), while a high dose of VU0152100 (10 mg/kg) was without effect (Fig. 1c).
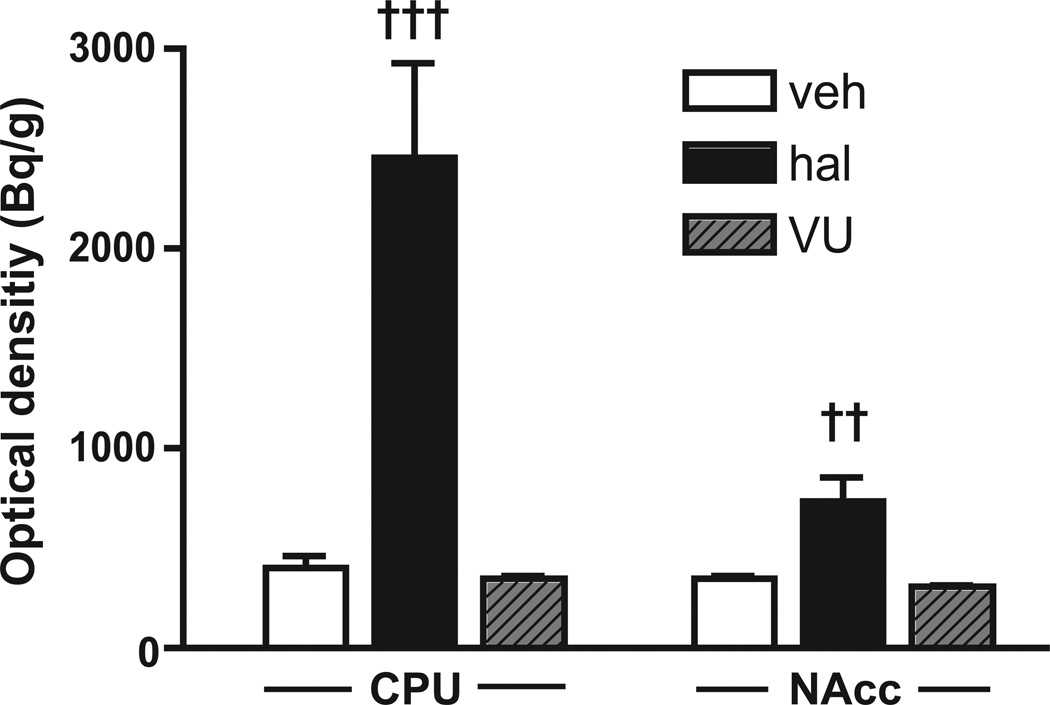
Previous studies have suggested that haloperidol-induced cfos activation, particularly in the caudate-putamen, reflects the manifestation of extrapyramidal motor side effects (Guo et al. 1992; Robertson et al. 1994). Consequently, we compared the effect of pretreatment with VU0152100 to that of haloperidol on rotorod-induced cfos mRNA expression in the caudate-putamen (one-way ANOVA: F(2,20)=19.59, p<0.001) and nucleus accumbens (one-way ANOVA: F(2,20)=10.97, p<0.001). Treatment with haloperidol (0.5 mg/kg) increased cfos mRNA expression in both areas (p<0.001 and p<0.01, respectively) compared to vehicle while VU0152100 (10 mg/kg) had no such effect (Fig. 2).
Fig. 2.
In situ hybridisation analysis of cfos mRNA expression in NMRI mice after motor activation in the rotorod test. Treatment with haloperidol (hal, 0.5 mg/kg, i.p.; black columns) induced a significant increase in cfos expression in both the striatal regions, caudate-putamen (CPU) and nucleus accumbens (NAcc) compared to vehicle control (veh, white columns). Treatment with VU0152100 (VU, 10 mg/kg, i.p.; grey columns) alone gave no significant increase in cfos expression compared to vehicle. All data represent group mean ± SEM (n=6 per group). ‹‹p<0.01, ‹‹‹p<0.001 vs. veh (one-way ANOVA followed by Bonferroni-corrected pairwise comparisons)
VU0152100 Potently Reduces Cocaine-Induced Increases in Striatal Dopamine by Activation of the M4 Receptor
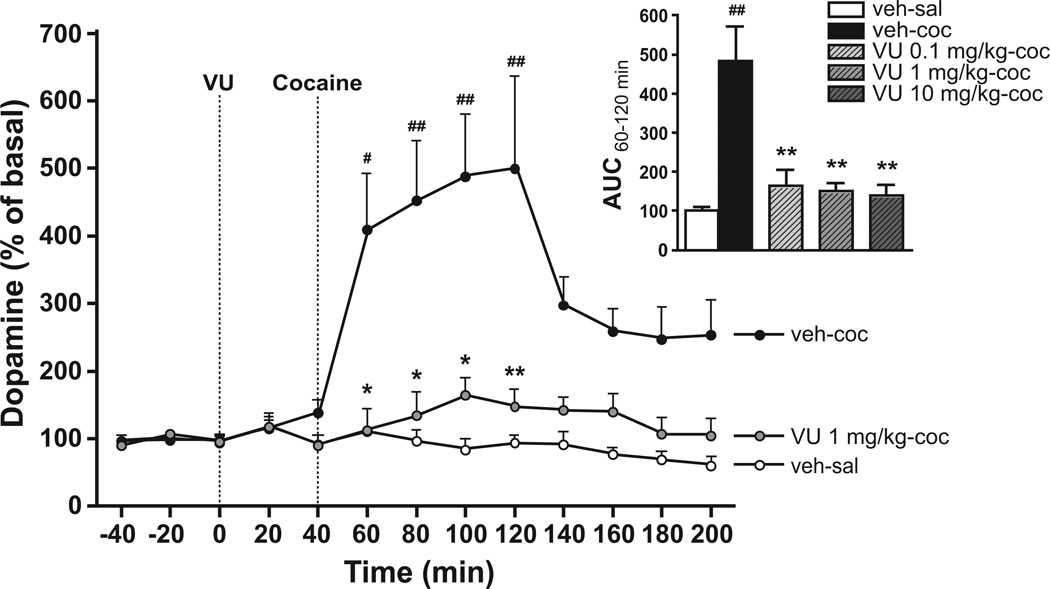
We used in vivo microdialysis to investigate whether the effects of VU0152100 in the behavioral studies might be mediated via effects on dopaminergic neurotransmission. The average baseline concentration of extracellular dopamine in the microdialysis samples from NMRI mice was 15 ± 1.2 fmol/15 µl. Two-way repeated measures ANOVA showed a significant effect of time (F(11,319)=26.62, p<0.001), treatment (F(5,319)=16.33, p<0.001), as well as a significant interaction (F(55,319)=5.82, p<0.001). As expected, Bonferroni-corrected post-hoc t-tests revealed that cocaine injection (30 mg/kg) induced a significant increase (498±74%) in extracellular dopamine levels (p<0.01–0.05; Fig. 3). Pretreatment with all three doses of VU0152100 caused a pronounced reduction in the cocaine-induced dopamine increase in the striatum close to vehicle levels during the 60–120 min time interval (see Fig. 3 for the effect of the 1.0 mg/kg dose of VU0152100, 193±25%). Analysing the AUC values using one-way ANOVA confirmed these findings (F(4,24)=10.97, p<0.001; see insert of Fig. 3 for the effect of all VU0152100 doses, p<0.01 all doses, Bonferroni-corrected post-hoc t-tests).
Fig. 3.
Extracellular striatal dopamine levels in response to cocaine and VU0152100 (VU) in NMRI mice. Cocaine (30 mg/kg, veh-coc, black circles; n=5) significantly increased extracellular dopamine compared to saline-treated controls (veh-sal, white circles; n=5). This response was significantly reduced by pretreatment with VU0152100 (1 mg/kg, i.p.; grey circles; n=5). #p<0.05, ##p<0.01 vs. veh-sal; *p<0.05, **p<0.01 vs. veh-coc (two-way repeated measures ANOVA followed by Bonferroni-corrected pairwise comparisons). The insert shows cocaine-induced dopamine levels as AUC from 60–120 min. All three doses of VU0152100 caused a significant reduction in dopamine levels (n=4–6). ##p<0.01 vs. veh-sal; **p<0.01 vs. veh-coc (one-way ANOVA followed by Dunnett’s multiple comparison tests). All data represent group mean ± SEM
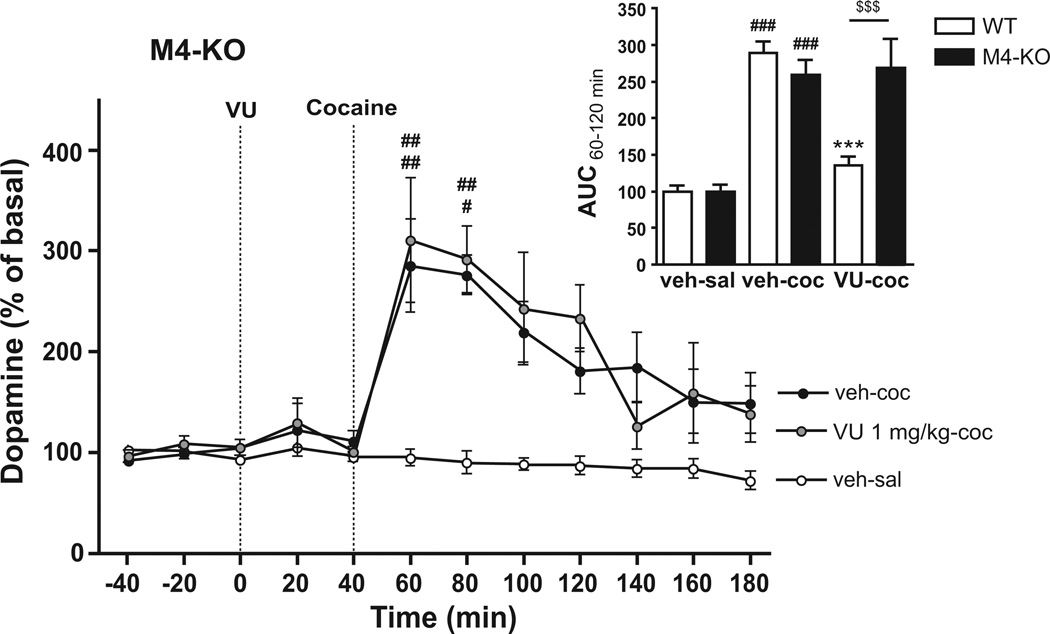
To confirm that the effects of VU0152100 were, indeed, mediated by M4 receptors, we next tested the effect of VU0152100 (1 mg/kg) on cocaine-induced striatal dopamine increases in M4-KO mice. Two-way ANOVA comparing AUCs from 60–120 min, covering the cocaine peak, showed a significant effect of genotype (F(1,31)=5.73, p<0.05), treatment (F(2,31)=48.62, p<0.001), and interaction (F(2,31)=12.13, p<0.001). As in NMRI mice, Bonferroni-corrected post-hoc t-tests revealed that injection of cocaine (30 mg/kg) compared to saline induced a significant dopamine increase in both wildtype (WT, 289±16%) littermate control and M4-KO mice (259±20%, both p<0.001; insert of Fig. 4). As above, this increase could be prominently attenuated by pretreatment with VU0152100 (1 mg/kg) in WT mice (135±13%, p<0.001; insert of Fig. 4). In contrast, in M4-KO mice, the cocaine-induced dopamine increase could not be attenuated by pretreatment with VU0152100 (1 mg/kg; 269±40% Fig. 4 and insert of Fig. 4), indicating that M4 receptors are necessary for the demonstrated effect of VU0152100.
Fig. 4.
Extracellular striatal dopamine levels in response to cocaine and VU0152100 in M4-KO mice. Cocaine (30 mg/kg, s.c., black circles; n=5) significantly increased dopamine compared to saline in M4-KO mice (white circles; n=6). The cocaine-induced dopamine increase could not be attenuated by pretreatment with VU0152100 (1 mg/kg, i.p., grey circles; n=4) in M4-KO mice. #p<0.05, ##p<0.01 vs. veh-sal in M4-KO mice (two-way repeated measures ANOVA followed by Bonferroni-corrected pairwise comparisons). The insert shows dopamine levels as AUC from 60–120 min in WT and M4-KO mice after saline (veh-sal), cocaine (veh-coc) or cocaine+VU0152100 (VU-coc) treatment. ###p<0.001 vs. veh-sal within genotype; ***p<0.001 vs. veh-coc within genotype, $$$p<0.001 vs. same treatment group between genotypes (two-way ANOVA followed by Bonferroni-corrected pairwise comparisons; n=4–8). All data represent group mean ± SEM
VU0152100 Reduces Cocaine-Induced Increases in Striatal Dopamine Partly by Activation of M4 Receptors Co-localized with Dopamine D1 Receptors
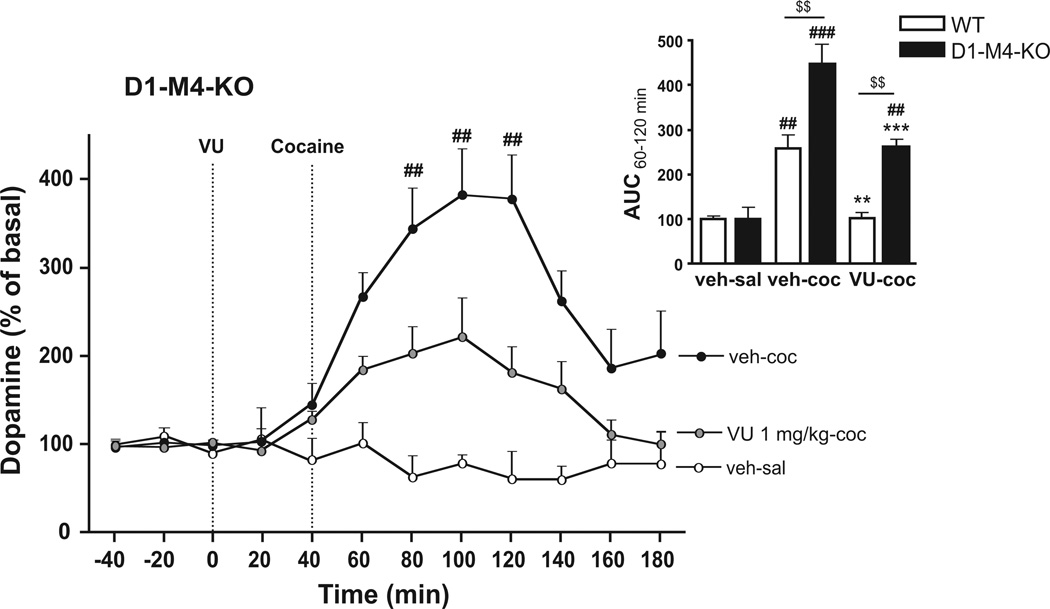
Studies in a transgenic mouse strain in which the M4 receptor has been selectively abrogated in D1 receptor-expressing cells (D1-M4-KO) indicated that this subpopulation of M4 receptors plays an import role in modulation of dopaminergic neurotransmission (Jeon et al. 2010; Dencker et al. 2011). To further investigate the role of this subpopulation of M4 receptors in mediating effects of VU0152100, we tested the ligand on cocaine-induced striatal dopamine levels in D1-M4-KO mice. Two-way ANOVA comparing AUCs from 60–120 min, covering the cocaine peak, showed a significant effect of genotype (F(1,22)=24.19, p<0.001), treatment (F(2,22)=40.86, p<0.001), and interaction (F(2,22)=6,20, p<0.01). Bonferroni-corrected post-hoc t-tests showed that cocaine injection (30 mg/kg) in WT mice induced a significant dopamine increase (298±15%, p<0.01, insert of Fig. 5), and this effect could be reversed completely to the levels of saline by pretreatment with VU0152100 (1 mg/kg; 118±3%, p<0.01, insert of Fig. 5). In D1-M4-KO mice, cocaine injection induced significantly higher dopamine levels than in WT mice (344±27%, p<0.01, insert of Fig. 5). Interestingly, in these mice, pretreatment with VU0152100 (1 mg/kg) only partially blocked the cocaine-induced dopamine increase (199±10%, p<0.001, Fig. 5 and insert of Fig. 5), indicating that M4 receptors on cells that do not express dopamine D1 receptors also contribute to mediating inhibitory effects of VU0152100 on cocaine-induced striatal extracellular dopamine. The effects of VU0152100 in the mutant mouse strains were independent of potential baseline differences in dopamine or DOPAC tissue levels since these were similar in striatal tissues from M4-KO, D1-M4-KO, and corresponding WT control mice (Table 1).
Fig. 5.
Extracellular striatal dopamine levels in response to cocaine and VU0152100 in D1-M4-KO mice. Cocaine (30 mg/kg, s.c., black circles; n=5) significantly increased extracellular dopamine compared to saline (white circles; n=4). Pretreatment with VU0152100 (1 mg/kg, i.p., grey circles; n=5) appeared to only partially reduce the effect of cocaine in D1-M4-KO mice. ##p<0.01 vs. veh-sal in D1-M4-KO mice (two-way repeated measures ANOVA followed by Bonferroni-corrected pairwise comparisons). The insert shows dopamine levels as AUC from 60–120 min in WT and D1-M4-KO mice after saline (veh-sal), cocaine (veh-coc) or cocaine+VU0152100 (VU-coc) treatment. ##p<0.01, ###p<0.001 vs. veh-sal within genotype, **p<0.01, ***p<0.001 vs. veh-coc within genotype, $$p<0.001 vs. same treatment group between genotypes (two-way ANOVA followed by Bonferroni-corrected pairwise comparisons; n=4–5). All data represent group mean ± SEM.
Table 1. Striatal tissue concentration of dopamine.
Total striatal tissue concentration of DA and DOPAC in M4-KO and D1-M4-KO mice and their respective WT controls. No significant differences were found between M4-KO and WT mice or D1-M4-KO and floxed WT mice (n=6–9)
| Genotype | DA (ng/g) Mean±SEM |
DOPAC (ng/g) Mean±SEM |
|---|---|---|
| M4-KO | 13.2 ± 1.2 | 3.7 ± 0.4 |
| WT | 15.8 ± 0.6 | 4.5 ± 0.1 |
| D1-M4-KO | 12.8 ± 1.8 | 5.7 ± 0.8 |
| Floxed WT | 12.7 ± 0.8 | 5.3 ± 0.4 |
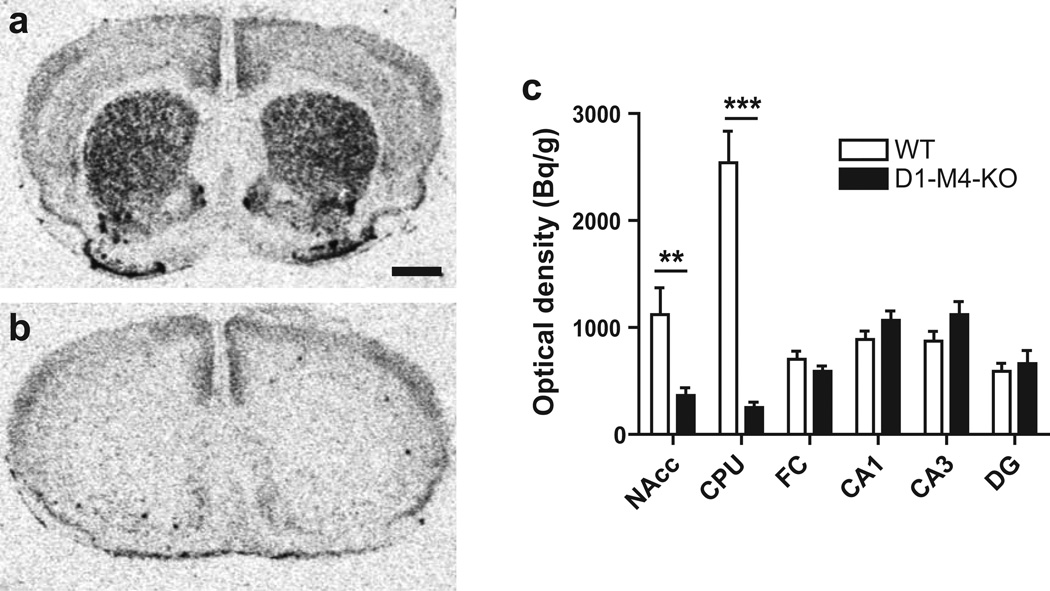
To determine to what extent M4 receptors are co-expressed with dopamine D1 receptors in our D1-M4-KO mice, we determined M4 receptor mRNA levels in the caudate-putamen, nucleus accumbens, frontal cortex, and hippocampal subregions using in situ hybridisation. M4 expression was found to be strongly reduced in the caudate-putamen (−90%; p<0.01, unpaired t-test) and nucleus accumbens (−79%; p<0.001) of D1-M4-KO compared to WT mice (Fig. 6a–c) while M4 receptor mRNA levels did not differ in the other examined areas. This suggests that, in the measured striatal areas, M4 receptors are predominantly expressed by D1-containing cells while in other measured brain regions M4 receptors are predominantly found on non-D1-containing cells.
Fig. 6.
In situ hybridisation autoradiograms showing M4 mRNA levels in treatment-naive floxed WT (a) and D1-M4-KO (b) mice at the level of the dorsal striatum. Magnification bar = 1 mm. (c) Quantification revealed a prominent reduction in the nucleus accumbens (NAcc) and the caudate-putamen (CPU) in D1-M4-KO mice compared to WT mice while there were no significant differences in the frontal cortex (FC), hippocampal dentate gyrus (DG) and subfields CA3/CA1. Data represent group mean ± SEM. **p<0.01, ***p<0.001 vs. WT (Student’s t-test; n=6)
Discussion
The present study demonstrates, for the first time, that activations of the M4 receptor, by PAM VU0152100, exerts prominent inhibitory effect on cocaine-induced behavioral and biochemical effects in mice. Thus VU0152100 (1.0 mg/kg) reversed cocaine effects in the acute cocaine self-administration model and in cocaine-induced hyperlocomotion (1 and 10 mg/kg). These findings are in agreement with our previous findings in a chronic self-administration model in M4-KO mice, showing that deficiency in M4 receptors results in increased cocaine self-administration (Schmidt et al. 2011). The present data however only investigates the effects of M4 receptor activation on the acute reinforcing properties of cocaine and investigation of the effects of VU0152100 in traditional chronic self-administration model is needed to evaluate the chronic reinforcing effects of cocaine as well as relapse.
An increase in extracellular dopamine levels in the striatum is central to the reinforcing effects of cocaine and other drugs of abuse (Wise, 1996; Koob et al. 1998). Using microdialysis, we found that the cocaine-induced striatal dopamine increase was almost abolished by pretreatment with VU0152100 at all three doses tested (0.1–10 mg/kg). This indicates that the inhibitory effects of VU0152100 on cocaine-mediated behaviours could be due to the reversal of cocaine effects on striatal dopamine levels. VU0152100 potentiates ACh-induced activation of M4 receptors without potentiating activity at M1 receptors or a number of other relevant G protein-coupled receptors (GPCRs), including dopamine D1 and D2 receptors and also the dopamine transporter (Brady et al. 2008, suppl. table 1). Besides weak antagonist activity at the 5HT2B receptor, no other significant activity was detected in a Millipore GPCR Profiler™ screen (Brady et al. 2008). Consequently, VU0152100 represents a valuable novel tool to study the physiological effects of M4 receptor activation. As further evidence that effects of VU0152100 on dopamine are, indeed, due to enhanced M4 receptor activity, we found that the attenuating effect of VU0152100 on cocaine-induced increase in striatal dopamine was abolished in M4-KO mice.
In the striatum, M4 receptors are expressed on cholinergic interneurones as well as GABAergic medium spiny projection neurones, where they are highly co-expressed with D1 receptors (Weiner et al. 1990; Bernard et al. 1992; Ince et al. 1997). Regulation of acetylcholine release by M4 receptors on striatal cholinergic interneurons (or cholinergic projections) can regulate dopamine release via β2-subunit-containing nicotinic receptors on dopaminergic axons. (Threlfell and Cragg 2011). On the other hand, activation of presynaptic M4 receptors on GABAergic MSNs will lead to a decrease in GABA released from these neurons. Through a direct monosynaptic connection this would be predicted to result in an increased release of dopamine. However, decreased GABAergic tone will also suppress GABA interneurons in the substantia nigra and the VTA, areas that do not express M4 receptors. Reduced inhibition of these GABA interneurons will increase the inhibitory input to dopaminergic neurons in the midbrain and thereby decreasing in dopamine release (Tzavara et al 2004). It has been proposed that M4 receptors localized on medium spiny neurones antagonize D1 receptor signalling (Jeon et al. 2010; Dencker et al. 2011). As in M4-KO mice, we found that the cocaine-induced striatal dopamine increase was significantly reduced by pretreatment with VU0152100 in D1-M4-KO mice, indicating that D1 receptor expressing neurones are importantly involved in mediating observed effects of VU0152100. However, since the cocaine-induced striatal dopamine increase was only partially abolished by VU0152100 in D1-M4-KO mice, M4 receptor populations other than those on D1 receptor expressing neurones must also play a role.
M4-KO mice have previously been reported to exhibit increased dopamine release in the nucleus accumbens after administration of dopamine agonists (Tzavara et al. 2004; Brady et al. 2008). In contrast, in the present study, we found no difference in the striatal dopamine response to cocaine between M4-KO and WT mice. The location of our microdialysis probe indicates that we must have sampled from both the dorsal (caudate-putamen) and ventral striatum (nucleus accumbens), with the majority of the dialysate deriving from the dorsal striatum. Threlfell and co-workers (2010) recently showed that ventral and dorsal striatal dopamine levels are differentially controlled by activation of muscarinic receptors on cholinergic interneurones. In the dorsal striatum, both M2 and M4 receptors are necessary for muscarinic regulation of dopamine release, while only M4 receptors are necessary in the ventral striatum (Threlfell et al. 2010). Together with our present data, this suggests that M2 receptors localized on cholinergic interneurones could be sufficient to compensate for the loss of M4 receptors in the regulation of dorsal striatal dopamine levels.
However, in D1-M4-KO mice, where M4 receptors are preserved on striatal cholinergic interneurones, but are absent on GABAergic medium spiny projection neurones, cocaine induced a significantly greater dorsal striatal dopamine response, compared to WT mice. This suggests opposing roles for M4 receptors on cholinergic interneurones and GABAergic projection neurones in the control of dopamine release. Since the reduction in M4 receptor gene expression was relatively smaller in D1-M4-KO mice in the nucleus accumbens (79%) compared to caudate-putamen (90%; see Fig. 6), this indicates that a relatively higher proportion of cells express M4 on non-D1 cells in the nucleus accumbens than in the caudate-putamen. This further suggests that M4 receptors on cholinergic interneurones may play a greater role in the regulation of dopamine levels in the ventral than in the dorsal striatum, consistent with the findings by Threlfell et al (2010). Clearly, more detailed studies are needed to address the differential roles of muscarinic receptor subpopulations in the regulation of dopamine release.
Our recent finding of an attenuated cataleptic response to antipsychotic drugs in M4-KO mice could raise the concern that activation of the M4 receptor might give rise to motor side effects (Fink-Jensen et al. 2011). However, VU0152100 had no effect on performance in the rotorod test at the highest dose used in the present study (10 mg/kg). This is in accordance with earlier studies in rats where doses of up to 100 mg/kg were used (Brady et al. 2008). Further consistent with this interpretation, we found no effect of VU0152100 on cfos induction after rotorod-induced motor activation. In contrast, haloperidol caused catalepsy that was accompanied by a pronounced increase in striatal cfos activation, which has been suggested to reflect the induction of motor side effects (Guo et al. 1992; Robertson et al. 1994). Thus the effects of VU0152100 in behavioral models reported here do not seem to be influenced by effects on baseline levels of motor performance. Neither, does VU0152100 seem to affect baseline dopamine levels during pretreatment time (0–40 min). However this has not been investigated further.
It remains to be established why VU0152100 potently reduced cocaine-induced behaviours and striatal dopamine increases without affecting motor performance. Our finding suggests that the cocaine-mediated acetylcholine release (Imperato et al. 1992) is required for VU0152100 to increase the activity of the M4 receptor and hereby decrease dopamine levels. In line with this result VU0152100 potently blocked cocaine-induced behavior, whereas we did not observe any effect of VU0152100 in the rotorod test, where the acetylcholine tone induced by motor-activation or stress (Imperato et al. 1989) most likely is moderate. The results obtained in the present study supports the findings in transgenic mice suggesting an important role for the M4 receptor in regulation of striatal dopaminergic neurotransmission. The development of selective PAM for the M4 receptor will be very useful in further experiment elucidating the role of M4 receptors in regulation of dopaminergic neurotransmission and for the investigations of the therapeutic potential of this receptor. To further evaluate the potential of the M4 receptor as a potential target for drug abuse chronic experiments are warranted.
This study indicates that PAMs of the M4 receptor deserve attention as potential agents for future treatment of drug addiction as well as several other severe CNS disorders, where enhanced dopaminergic neurotransmission is a hallmark e.g. schizophrenia.
Acknowledgments
The Ivan Nielsen Foundation, Aase and Einar Danielsens Foundation, Butcher Max Wørzner and wife Inger Wørzner Foundation, A.P. Møller foundation for the Advancement of Medical Science, and Lundbeck Foundation supported the present work. We thank Birgit H. Hansen for expert technical assistance.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- Bernard V, Normand E, Bloch B. Phenotypical characterization of the rat striatal neurons expressing muscarinic receptor genes. J Neurosci. 1992;12:3591–3600. doi: 10.1523/JNEUROSCI.12-09-03591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady AE, Jones CK, Bridges TM, Kennedy JP, Thompson AD, Heiman JU, Breininger ML, Gentry PR, Yin H, Jadhav SB, Shirey JK, Conn PJ. Centrally active allosteric potentiators of the M4 muscarinic acetylcholine receptor reverse amphetamine induced hyperlocomotor activity in rats. J Pharmacol Exp Ther. 2008;327:941–953. doi: 10.1124/jpet.108.140350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, McKinzie DL, Bose S, Mitchell SN, Witkin JM, Thompson RC, Christopoulos A, Lazareno S, Birdsall NJ, Bymaster FP, Felder CC. Allosteric modulation of the muscarinic M4 receptor as an approach to treating schizophrenia. Proc Natl Acad Sci U S A. 2008;105:10978–10983. doi: 10.1073/pnas.0800567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencker D, Wàrtwein G, Weikop P, Jeon J, Thomsen M, Sager TN, Mørk A, Woldbye DPD, Wess J, Fink-Jensen A. Involvement of a subpopulation of neuronal M4 muscarinic acetylcholine receptors in the antipsychotic-like effects of the M1/M4 preferring muscarinic receptor agonist xanomeline. J Neurosci. 2011;31:5905–5908. doi: 10.1523/JNEUROSCI.0370-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Felder CC, Porter AC, Skillman TL, Zhang L, Bymaster FP, Nathanson NM, Hamilton SE, Gomeza J, Wess J, McKinzie DL. Elucidating the role of muscarinic receptors in psychosis. Life Sci. 2001;68:2605–2613. doi: 10.1016/s0024-3205(01)01059-1. [DOI] [PubMed] [Google Scholar]
- Fink-Jensen A, Schmidt LS, Dencker D, Schülein C, Wess J, Wörtwein G, Woldbye DPD. Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic receptor. Eur J Pharmacol. 2011;656:39–44. doi: 10.1016/j.ejphar.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KJB, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Academic; 2007. [Google Scholar]
- Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng CX, Wess J. Enhancement of Dl dopamine receptor-mediated locomotor stimulation in M4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:10483–10488. doi: 10.1073/pnas.96.18.10483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N, Robertson GS, Fibiger HC. Scopolamine attenuates haloperidol-induced c-fos expression in the striatum. Brain Res. 1992;588:164–167. doi: 10.1016/0006-8993(92)91358-l. [DOI] [PubMed] [Google Scholar]
- Hjaeresen ML, Hageman I, Wortwein G, Plenge P, Jørgensen MB. Chronic electroconvulsive stimulation but not chronic restraint stress modulates mRNA expression of voltage dependent potassium channels Kv7.2 and Kvl 1.1 in the rat piriform cortex. Brain Res. 2008;1217:179–184. doi: 10.1016/j.brainres.2007.09.071. [DOI] [PubMed] [Google Scholar]
- Imperato A, Puglisi-Allegra S, Casolini P, Zocchi A, Angelucci L. Stress-induced 0 enhancement of dopamine and acetylcholine release in limbic structures: role of corticosterone. Eur J Pharmacol. 1989;165:337–338. doi: 10.1016/0014-2999(89)90735-8. [DOI] [PubMed] [Google Scholar]
- Imperato A, Demontis COMV, Gassa GL. Cocaine releases limbic acetylcholine through endogenous dopamine action on Dl receptors. Eur J Pharmacol. 1992;229:265–267. doi: 10.1016/0014-2999(92)90565-l. [DOI] [PubMed] [Google Scholar]
- Ince E, Ciliax BJ, Levey AI. Differential expression of Dl and D2 dopamine and M4 muscarinic acetylcholine receptor proteins in identified striatonigral neurons. Synapse. 1997;27:357–366. doi: 10.1002/(SICI)1098-2396(199712)27:4<357::AID-SYN9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Jeon J, Dencker D, Wörtwein G, Woldbye DPD, Cui Y, Davis AA, Levey AI, Schütz G, Sager TN, Mørk A, Li C, Deng C, Fink-Jensen A, Wess J. A subpopulation of neuronal M4 muscarinic acetylcholine receptors plays a critical role in modulating dopamine-dependent behaviors. J Neurosci. 2010;30:2396–2405. doi: 10.1523/JNEUROSCI.3843-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- Leach K, Loiacono RE, Felder CC, McKinzie DL, Mogg A, Shaw DB, Sexton PM, Christopoulos A. Molecular mechanisms of action and in vivo validation of an M4 muscarinic acetylcholine receptor allosteric modulator with potential antipsychotic properties. Neuropsychopharmacology. 2010;35:855–869. doi: 10.1038/npp.2009.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AI. Immunological localization of M1-M5 muscarinic acetylcholine receptors in peripheral tissues and brain. Life Sci. 1993;52:441–448. doi: 10.1016/0024-3205(93)90300-r. [DOI] [PubMed] [Google Scholar]
- Onali P, Olianas MC. Muscarinic M4 receptor inhibition of dopamine Dl-like receptor signaling in rat nucleus accumbens. Eur J Pharmacol. 2002;448:105–111. doi: 10.1016/s0014-2999(02)01910-6. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther. 1994;271:1058–1066. [PubMed] [Google Scholar]
- Schmidt LS, Thomsen M, Weikop P, Dencker D, Wess J, Woldbye DPD, Wörtwein G, Fink-Jensen A. Increased self-administration in M4 muscarinic acetylcholine receptor knockout mice. Psychopharmacology. 2011;216:367–378. doi: 10.1007/s00213-011-2225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M. Cholinergic function in stimulant addiction: Implications for medications development. CNS Drugs. 2009;23:939–952. doi: 10.2165/11310920-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Sager TN, Petersen JH, Brennum LT, Thøgersen P, Hee Bengtsen C, Thomsen M, Wörtwein G, Fink-Jensen A, Woldbye DP. Aripiprazole blocks acute self-administration of cocaine and is not self-administered in mice. Psychopharmacology. 2008;199:37–46. doi: 10.1007/s00213-008-1069-z. [DOI] [PubMed] [Google Scholar]
- Sørensen G, Jensen M, Weikop P, Dencker D, Christiansen SH, Løland CJ, Bengtsen CH, Petersen JH, Fink-Jensen A, Wörtwein G, Woldbye DPD. Neuropeptide Y Y5 receptor antagonism attenuates addiction-related effects of cocaine in mice. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2651-y. Epub DOI: 10.1007/s00213-012-2651-y. [DOI] [PubMed] [Google Scholar]
- Threlfell S, Clements MA, Khodai T, Pienaar IS, Exley R, Wess J, Cragg SJ. Striatal muscarinic receptors promote activity dependence of dopamine transmission via distinct receptor subtypes on cholinergic interneurons in ventral versus dorsal striatum. J Neurosci. 2010;30:3398–3408. doi: 10.1523/JNEUROSCI.5620-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ. Dopamine signalling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Sys Neurosci. 2011;5:1–10. doi: 10.3389/fnsys.2011.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG. M4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J. 2004;18:1410–1412. doi: 10.1096/fj.04-1575fje. [DOI] [PubMed] [Google Scholar]
- Weikop P, Egestad B, Kehr J. Application of triple-probe microdialysis for fast pharmacokinetic/pharmacodynamic evaluation of dopamimetic activity of drug candidates in the rat brain. J Neurosci Methods. 2004;140:59–65. doi: 10.1016/j.jneumeth.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor messenger-RNAs in rat basal ganglia. Proc Natl Acad Sci USA. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J. Muscarinic acetylcholine receptor knockout mice: Novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol. 2004;44:721–733. doi: 10.1146/annurev.pharmtox.44.101802.121622. [DOI] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov. 2007;6:721–733. doi: 10.1038/nrd2379. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Yasuda RP, Ciesla W, Flores LR, Wall SJ, Li M, Satkus SA, Weisstein JS, Spagnola BV, Wolfe BB. Development of antisera selective for M4 and M5 muscarinic cholinergic receptors -distribution of M4 and M5 receptors in rat-brain. Mol Pharmacol. 1993;43:149–157. [PubMed] [Google Scholar]
- Zhang W, Basile AS, Gomeza J, Volpicelli LA, Levey AI, Wess J. Characterization of central inhibitory muscarinic autoreceptors by the use of muscarinic acetylcholine receptor knock-out mice. J Neurosci. 2002;22:1709–1717. doi: 10.1523/JNEUROSCI.22-05-01709.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]