Abstract
ALT-803, a complex of an interleukin-15 (IL-15) superagonist mutant and a dimeric IL-15 receptor α/Fc fusion protein, was found to exhibit significantly stronger in vivo biological activity on NK and T cells than IL-15. In this study, we show that a single dose of ALT-803, but not IL-15 alone, eliminated well-established 5T33P and MOPC-315P myeloma cells in the bone marrow of tumor-bearing mice. ALT-803 treatment also significantly prolonged survival of myeloma-bearing mice and provided resistance to rechallenge with the same tumor cells through a CD8+ T cell-dependent mechanism. ALT-803 treatment stimulated CD8+ T cells to secrete large amounts of interferon-γ (IFN-γ) and promoted rapid expansion of CD8+CD44high memory T cells in vivo. These memory CD8+ T cells exhibited ALT-803-mediated up-regulation of NKG2D (KLRK1) but not PD-1 (PDCD1) or CD25 (IL2RA) on their cell surfaces. ALT-803-activated CD8+ memory T cells also exhibited non-specific cytotoxicity against myeloma and other tumor cells in vitro, whereas IFN-γ had no direct effect on myeloma cell growth. ALT-803 lost its anti-myeloma activity in tumor-bearing IFN-γ knockout mice but retained the ability to promote CD8+CD44high memory T cell proliferation, indicating that ALT-803-mediated stimulation of CD8+CD44high memory T cells is IFN-γ-independent. Thus, besides well-known IL-15 biological functions in host immunity, this study demonstrates that IL-15-based ALT-803 could activate CD8+CD44high memory T cells to acquire a unique innate-like phenotype and secrete IFN-γ for non-specific tumor cell killing. This unique immune modulatory property of ALT-803 strongly supports its clinical development as a novel immunotherapeutic agent against cancer and viral infections.
Keywords: Interleukin-15, Interleukin-15Rα, Myeloma, CD8+ T cells, NK cells
Introduction
Multiple myeloma (MM) is a plasma cell malignancy, accounting for over 1% of neoplastic diseases and 14% of all hematological cancers (1). MM tumor cells are susceptible to immune cell recognition and elimination, as demonstrated by the potentially curative graft-versus-myeloma activity observed in some patients following allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusion therapies (2). However, these approaches are limited by transplantation-related mortality ranging from 30% to 50% and disease relapse in a majority of patients. Immunomodulatory chemotherapies, such as lenalidomide, are also thought to provide therapeutic benefit via mechanisms due in part to stimulation of T-cell and/or natural killer (NK) cell activity against myeloma cells (3). Although survival of MM patients has improved significantly by the use of these novel agents, MM remains incurable due to the persistence of minimal residual disease (4, 5). Thus, novel modalities are needed to complement or improve the current treatment options for MM.
Interleukin-15 (IL-15) is a critical cytokine for the development, proliferation and activation of effector NK cells and CD8+ memory T cells (6, 7). IL-15 binds to the IL-15 receptor α (IL-15Rα) and is presented in trans to the IL-2/IL-15 receptor β - common γ chain (IL-15Rβγc) complex on effector cells. IL-15 and IL-2 share binding to the IL-15Rβγc and signal through STAT3 and STAT5 pathways. However, unlike IL-2, IL-15 does not support maintenance of CD4+CD25+FoxP3+ regulatory T (Treg) cells or induce cell death of activated CD8+ T cells (6), effects that may have limited the therapeutic activity of IL-2 against MM (8). Additionally, IL-15 is the only cytokine known to provide anti-apoptotic signaling to effector CD8+ T cells (9). IL-15, either administered alone or as a complex with the IL-15Rα, exhibits potent anti-tumor activities against well-established solid tumors in experimental animal models and, thus, has been identified as one of the most promising immunotherapeutic drugs that could potentially cure cancer (10–17). However, there have been no reports showing efficacy of IL-15 against hematologic tumors.
To facilitate clinical development of an IL-15-based cancer therapeutic, we previously identified a novel IL-15 mutant with increased biological activity compared to IL-15 (18). The pharmacokinetics and biological activity of this IL-15 super-agonist (IL-15N72D) was further improved by the creation of IL-15N72D:IL-15Rα/Fc fusion complex (ALT-803), such that the super agonist complex has at least 25-times the activity of the native cytokine in vivo (19). Thus, we hypothesized that ALT-803 could potentially provide durable, immune cell-mediated anti-tumor efficacy. We evaluated this hypothesis by employing two multiple myeloma models in syngeneic immunocompetent mice. The study also revealed that ALT-803 employs a novel mechanism of action against myeloma.
Materials and Methods
Mice and tumor cell lines
C57BL/6NHsd and BALB/c mice (5–6 week old females, Harlan Laboratories) and interferon-γ (IFN-γ) knockout (KO) [B6.129S7-Ifngtm1Ts/J] and perforin KO [C57BL/6-Prf1tm1Sdz/J] mice (5–6 week old females, The Jackson Laboratory) were housed in the animal facilities at Altor BioScience. All animal studies were performed according to NIH animal care guidelines under IACUC approved protocols.
The murine 5T33 multiple myeloma cell line (20) was kindly provided by Dr. Ulrich von Andrian, (Harvard Medical School, Boston, MA). The murine MOPC-315 myeloma cell line was purchased from American Type Culture Collection (ATCC). Tumor cell sublines, 5T33P and MOPC-315P, were developed by passage of the parental myeloma cells in C57BL/6NHsd and BALB/c mice, respectively. All cells were routinely cultured in I-10 media at 37°C with 5% CO2 and harvested for animal injection at 80–90% confluency.
Tumor models
Following intravenous (i.v.) injection with 1 × 107 5T33P cells/mouse, 100% of C57BL/6NHsd mice developed tumor-induced hind leg paralysis between 20–30 days. Similar tumor take rates were observed in BALB/c mice following i.v. injection of 1 × 107 MOPC-315P cells/mouse. Tumor-bearing mice were monitored daily for hind leg paralysis, signs of overt disease progression and mortality.
ALT-803 (IL-15N72D:IL-15RαSu/Fc) was generated as described previously (19). Recombinant human IL-15 (21) was kindly provided by Dr. Jason Yovandich (NCI, Fredrick, MD). ALT-803 at 0.2 mg/kg/dose (or as indicated), IL-15 at 0.056 mg/kg/dose (IL-15 molar equivalent dose of 0.2 mg/kg ALT-803) or PBS as control was administered i.v. via the lateral tail vein to tumor-bearing mice. Levels of BM myeloma cells and hind leg paralysis or survival were assessed as study endpoints.
Flow cytometry and ELISA analysis
To quantitate levels of murine lymphocyte subsets, BM, spleen, lymph node and blood were collected separately from each mouse, cells were prepared and stained with fluor-labeled antibodies (Abs) specific to CD4, CD8, CD11c, CD19, CD25, CD40, CD44, CD80, CD107a, I-A(b), IFN-γ, IgG2b, IgA, NK1.1, NKG2D, NKp46, and/or PD-1, and appropriate isotype controls (eBiosciences, BD Biosciences, and Biolegend) as indicated in figure legends. Cell staining was analyzed on a FACSverse (BD Biosciences). The sorting of NKG2DnegCD25negCD8+CD44high T cells was conducted with FACS Aria and analyzed with Diva software (BD Biosciences).
Levels of 5T33P and MOPC-316P cells in BM preparations, and IFN-γ in splenocytes were assessed by intracellular staining with Abs specific to IgG2b, IgA and IFN-γ, respectively.
IFN-γ levels in mouse serum were quantitated by ELISA using anti-IFN-γ Ab (AN-18) capture and biotinylated anti-IFN-γ Ab (R4-6A2) detection following the manufacturer’s instruction (Biolegend).
In vivo depletion of mouse NK1.1+ cells and CD8+ T cells
For in vivo depletion of NK1.1+ cells and CD8+ T cells, mice were injected intraperitoneally (i.p.) with 200 μg/dose anti-NK1.1 (PK136, ATCC) and/or 500 μg/dose anti-CD8 (53-6.72, ATCC) Abs. Control mice received PBS (0.2 mL). In pilot studies, the efficiency of NK1.1+ cell and CD8+ T-cell depletion was monitored by flow cytometry following staining of PBMCs and BM cells with appropriate Abs.
T cell labeling and adoptive transfer
CD3+ enriched cells (prepared with Mouse CD3+ T Cell Enrichment Column, R&D System), CD8+ enriched T cells [positive, CD8a (Ly-2) MicroBeads, mouse, Miltenyi Biotech] or sorted NKG2DnegCD25negCD8+CD44high memoryT cells from spleens and lymph nodes of donor C57BL/6NHsd or IFN-γ KO B6 mice were labeled with Celltrace™ Violet (Invitrogen) at 1.5 μM/1× 106 cells/ml, and then 1 to 1.5 × 106 violet labeled cells were adoptively transferred into syngeneic C57BL/6NHsd or IFN-γ KO B6 recipients on day 0 (SD0). On SD2, mice were treated (i.v.) with the following test articles 0.02 mg/kg ALT803, 0.2 mg/kg ALT-803 or PBS. On SD6, spleens were harvested and splenocytes were analyzed for proliferation of donor cells (violet label) or staining with antibodies specific to CD25, PD-1, CD44, CD8a, and NKG2D.
In vitro cytotoxicity assay
Tumor target cells (i.e., 5T33P, A20) were labeled with PKH67 (Sigma-Aldrich) according to the manufacturer’s instructions. CD8+ T cell enriched spleen cells from normal, IFN-γ KO, and perforin KO B6 mice were isolated (untouched, CD8a+ T Cell Isolation Kit II, mouse, Miltenyi Biotech). Effector populations were produced by culturing prepared cells (2×107) in RPMI-1640 complete media containing ALT-803 (200 ng/mL) for 72hr. Resulting effector cells were harvested, washed twice, and re-plated into 24 well plates with PKH-labeled tumor target cells (E:T ratio; 10:1) in media containing varying doses of ALT-803. After incubation for 20–24 hrs at 37°C with 5% CO2, target cell killing was assessed by analysis of PI staining of PKH67-labeled tumor cells on a BD FACScan.
Data analysis
Data are expressed as the mean ± SE. Survival data was analyzed using the log-rank test and Kaplan-Meier method. Comparisons of continuous variables were done using Student’s t tests or ANOVA (two-tailed) (GraphPad Prism Version 4.03). P values of less than or equal to 0.05 are considered significant.
Results
Efficacy of ALT-803 in murine myeloma models
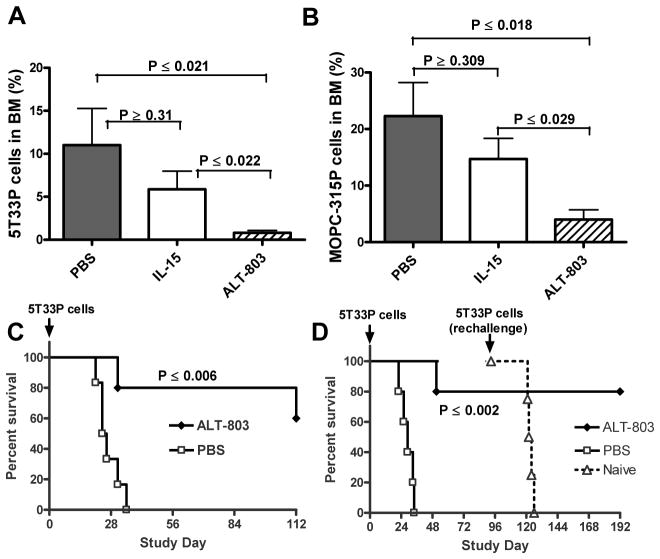
To conduct efficacy studies in hematologic tumor models, we derived highly tumorigenic myeloma lines 5T33P and MOPC-315P from the well-characterized 5T33 and MOPC-315 parental lines, respectively, and found that these cells could populate the BM and cause paralysis following i.v. inoculation of syngeneic mice. Tumor development in 5T33P-bearing C57BL/6NHsd mice and MOPC-315P-bearing BALB/c mice was assessed by staining myeloma cells in isolated BM cell preparations for intracellular 5T33P-specific IgG2b and MOPC-315P-specific IgA paraproteins. In C57BL/6NHsd mice, IgG2b paraprotein-positive myeloma cell levels increased to over 20% of the total BM cells by 21 days after 5T33P tumor cell inoculation (Supplementary Fig. S1). A single i.v. treatment of ALT-803 (0.2 mg/kg) had a marked effect on 5T33P cells in the BM of mice with well-established tumors (14 days after tumor implantation), providing >90% reduction in BM IgG2b+ myeloma cells four days after treatment compared to controls (0.8% versus 11.0%, P ≤ 0.02) (Fig. 1A). However, a molar equivalent dose of IL-15 was much less effective and only reduced BM 5T33P cells by 53% compared to PBS-treated mice (P ≥0.31). Dose response studies indicated that a single dose of ALT-803 at as low as 0.05 mg/kg was capable of reducing 90% of the BM 5T33P myeloma cells (Supplementary Fig. S2A). Similar studies in BALB/c mice bearing well-established MOPC-315P tumors confirmed that treatment with ALT-803, but not IL-15, resulted in a significant decrease in BM myeloma cells compared to controls (P ≤ 0.02, ALT-803 vs. PBS; P ≥0.31, IL-15 vs. PBS) (Fig. 1B). No toxicity was observed following treatment, indicating that ALT-803 administration and its anti-tumor effects, which resulted in the rapid killing of a large number of myeloma cells over a short duration, were well tolerated by mice.
Figure 1.
Anti-tumor effects of ALT-803 in murine myeloma models. A & B, effect of ALT-803 or IL-15 on myeloma cells in BM of 5T33P or MOPC-315P bearing mice. Female mice (5 mice/group) were injected i.v. with 5T33P (A) or MOPC-315P (B) myeloma cells (1 × 107/mouse) on day 0. ALT-803 (0.2 mg/kg), IL-15 (0.056 mg/kg, an IL-15 molar equivalent dose to 0.2 mg/kg ALT-803), or PBS (dose volume equivalent) was then administered as a single i.v. injection on day 15 (5T33P) or 14 (MOPC-315P). BM cells were collected 4 days after study drug treatments. The cells were then stained with PE-conjugated rat anti-mouse IgG2b or IgA Ab to evaluate the percentage of 5T33P or MOPC-315P cells in BM, respectively. The plotted values represent the mean ± SE; P values are presented. C, ALT-803 treatment prolonged survival of the examined mice bearing murine myeloma cells. Female C57BL/6NHsd mice (n = 5/group) were injected i.v. with murine 5T33P myeloma cells (1 × 107 cells/mouse) on day 0. A single-dose of ALT-803 was administered i.v. at 0.2 mg/kg on day 4. Control mice were treated with PBS on day 4. Survival (or morbidity due to hind leg paralysis) was monitored as a study endpoint. D, ALT-803 treatment prolongs survival of C57BL/6NHsd mice following subsequent rechallenge with 5T33P myeloma cells. 5T33P tumor-bearing mice (n = 5) were treated with ALT-803 days 1 and 7 or with PBS as in 2A. ALT-803 treated mice that survived (n = 4) were rechallenged with 5T33P cells (1 × 107) on day 93. Five treatment-naïve mice were also administered 5T33P cells (1 × 107) on day 93 as a control for tumor development.
ALT-803 effects on mouse survival were also evaluated in these myeloma models. 5T33P-bearing C57BL/6NHsd mice treated with a single 0.2 mg/kg dose of ALT-803 showed significantly increased survival when compared to PBS-treated mice, which all exhibited hind leg paralysis (survival endpoint) between 21 to 35 days post tumor cell injection with a median survival time (MST) of 25 days (P ≤0.006) (Fig. 1C). Two or three weekly doses of ALT-803 also provided a significant survival benefit in this model (P ≤0.002, ALT-803 vs. PBS) (Fig. 1D) and in BALB/c mice bearing MOPC-315P tumors (Supplementary Fig. S2B).
Since ALT-803 treatment was capable of essentially curing mice bearing 5T33P myeloma, we evaluated whether these mice retain immunological memory against the tumor cells. As shown in Fig. 1D, C57BL/6NHsd mice that survived initial 5T33P inoculation due to ALT-803 treatment were not affected by 5T33P cell upon rechallenge 3 months later, even in the absence of additional ALT-803 administration. These mice continued to survive over 190 days from the initial tumor cell inoculation. In contrast, all of the treatment-naïve mice administered 5T33P cells on the same study day subsequently exhibited paralysis with a MST of 29 days post tumor cell injection. Together, these results demonstrate that a short course of ALT-803 treatment has significantly greater anti-tumor activity against established BM myeloma cells than IL-15 treatment, resulting in prolonged survival of myeloma-tumor bearing mice. ALT-803 was also capable of inducing long-lasting protective immunologic memory against subsequent tumor cell rechallenge.
CD8+ T cells mediate efficacy of ALT-803 against myeloma cells
Since ALT-803 treatment effectively eliminated myeloma cells in vivo, we tested whether ALT-803 had a direct effect on the viability and proliferation of 5T33P and MOPC-315P cells in vitro. Neither a decrease in cell numbers nor an increase in apoptotic cells was observed following incubation of tumor cells with ALT-803 even at high concentrations (Supplementary Fig. S3). Thus, ALT-803 anti-myeloma activity in vivo is likely due to activation of immune responses rather than direct killing of tumor cells.
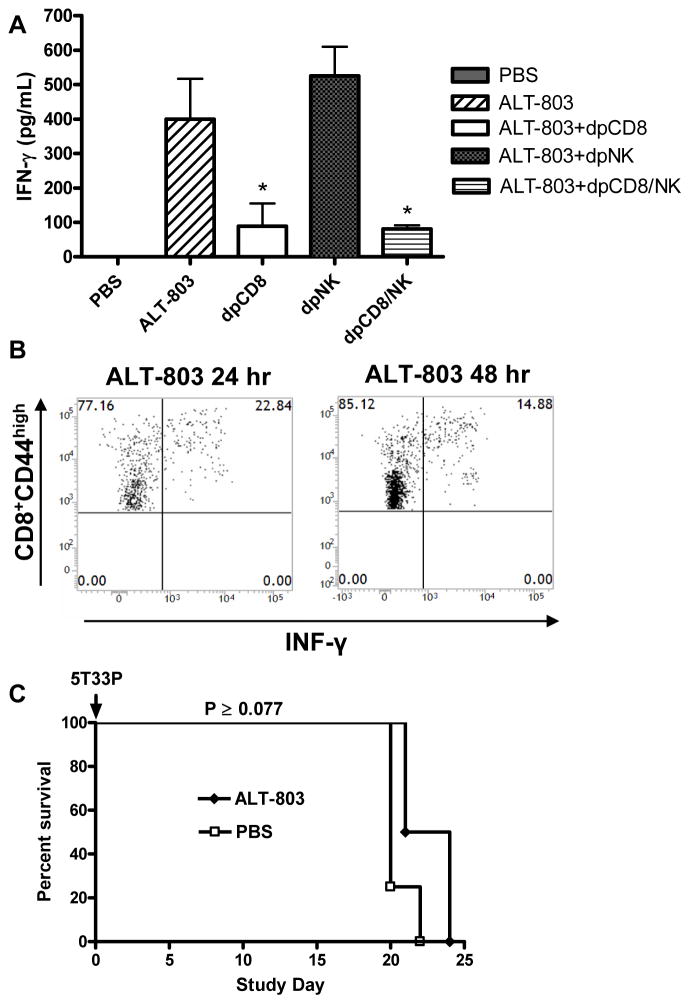
ALT-803 treatment is capable of significantly increasing the number of NK and T cells in vivo (19). To determine if these immune cells were responsible ALT-803-mediated anti-myeloma efficacy, Ab-immunodepletion of CD8+ T cells and NK1.1+ cells was performed in tumor-bearing mice prior to ALT-803 treatment. Effective depletion of these immune cell subsets could be achieved by i.p administration of anti-CD8 and/or anti-NK1.1 antibodies starting with injections 48 h and 24 h prior to tumor inoculation and weekly post-tumor inoculation (data not shown). When ALT-803 efficacy was examined in 5T33P-bearing mice, it was found that CD8+ T-cell depletion alone or in combination with NK1.1+ cell depletion, but not NK1.1+ cell depletion alone, eliminated the anti-tumor effects of ALT-803 on BM 5T33P myeloma cells (Fig. 2A). Consistent with these results, anti-tumor activity correlated with ALT-803-mediated increases in BM CD8+ T-cell and not NK cell levels (Fig. 2A). We also conducted immune cell depletion studies in 5T33P-bearing C57BL/6NHsd mice treated with ALT-803 using survival as the efficacy endpoint. As described above, ALT-803 treatment effectively cured myeloma-bearing mice that otherwise developed paralysis within 28 days (Fig. 2B). Depletion of NK1.1+ cells had no effect on the anti-tumor activity of ALT-803, whereas depletion of CD8+ T cells or both CD8+ T cells and NK1.1+ cells significantly reduced the ALT-803–mediated survival benefit to 5T33P-bearing mice (P <0.013). These results support our conclusion that CD8+ T cells, not NK1.1+ cells, play a major role in ALT-803-mediated activity against 5T33P cells in C57BL/6NHsd mice.
Figure 2.
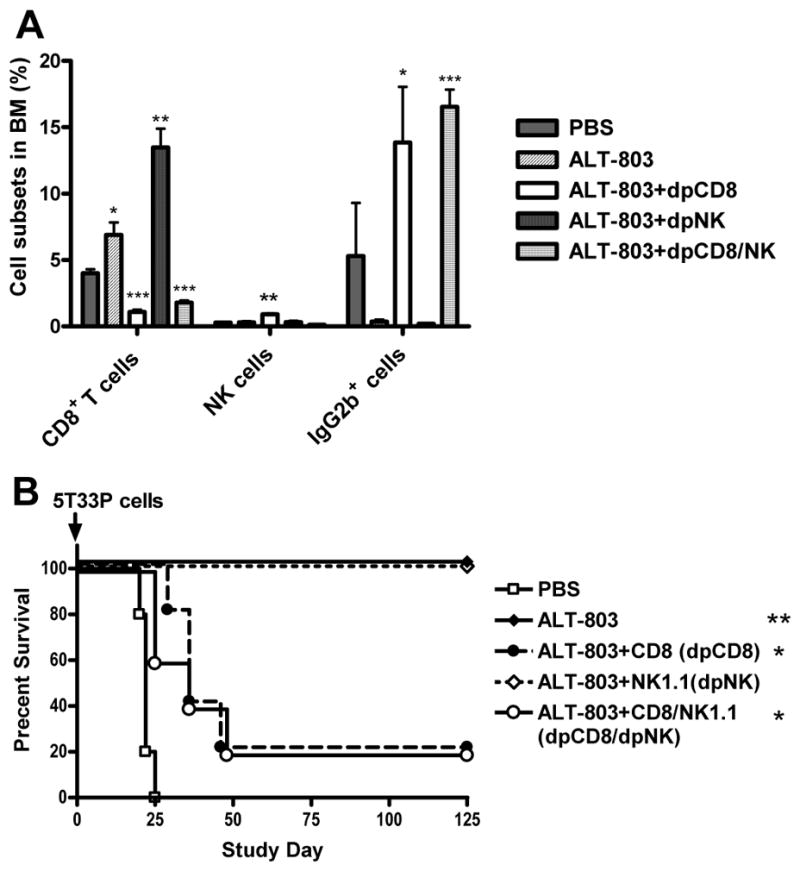
Immune cell effects on the anti-myeloma activity of ALT-803. A, female C57BL/6NHsd mice (n = 5/group) were depleted of CD8+ T cells (dpCD8), NK1.1+ cells (dpNK), or both (dpCD8/NK) by i.p. treatment with anti-CD8 and/or anti-NK1.1 Abs on days -2 and -1. Mice were then injected with 5T33P myeloma cells (1 × 107) on day 0 and treated with anti-CD8 and/or anti- NK1.1 Abs on days 7 and 14. The mice were treated with ALT-803 on day 14. Undepleted 5T33P-bearing mice receiving PBS served as controls. Four days after ALT-803 treatment, BM cells were isolated and stained with FITC-anti-CD8b, PE-anti-NKp46 and FITC-anti-IgG2b Abs and analyzed by flow cytometry. The percentage of CD8+ T cells, NKp46+ NK cells and IgG2b+ 5T33P myeloma cells in BM are shown. Bars represent the mean ± SE. B, female C57BL/6NHsd mice (n = 5/group) were depleted of CD8+ T cells (dpCD8), NK1.1+ cells (dpNK), or both (dpCD8/NK) by treatment with anti-CD8 and/or anti-NK1.1 Abs on days -2 and -1 as described in Fig. 2A. Mice were then injected with 5T33P myeloma cells (1 × 107) on day 0 and treated with anti-CD8 and/or anti-NK1.1 Abs and then weekly for 8 weeks. ALT-803 was administered i.v. at 0.2 mg/kg on days 4 and 11. Mice receiving 5T33P myeloma cells (1 × 107) on day 0 and PBS on days 4 and 11 were used as control. For comparison of ALT-801 vs. PBS or ALT-801+Ab depletion vs. ALT-801, *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001.
ALT-803 induces CD8+CD44high memory T cells to expand, up-regulate innate receptors and exhibit non-specific cytotoxic activity
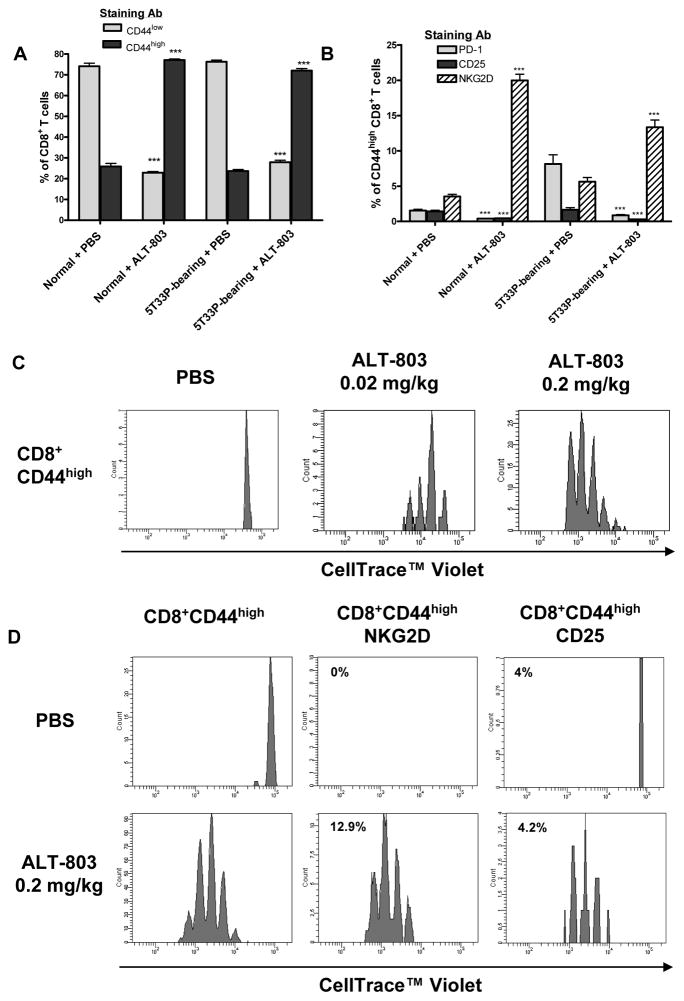
We have previously shown that a single dose of ALT- 803 at 0.2 mg/kg dose level, but not IL-15, could significantly increase the CD8+ T cells and NK cells in naïve mice (19). As shown in Fig. 3A, we found that a single dose of ALT-803 (0.2 mg/kg) administered to either normal or 5T33P-bearing C57BL/6NHsd mice resulted in a similar 3 fold increase in the percentage of CD8+CD44high memory T cells. This is consistent with observations by others that certain cytokines, such as IL-12, IL-18, IFN-γ or IL-15, can promote proliferation of CD8+CD44high T cells, but not the naïve CD8+ T cells, in vivo (22–24).
Figure 3.
ALT-803 induces CD8+CD44high memory T cell proliferation and up-regulation of NKG2D. A & B, female C57BL/6NHsd mice (5–6 weeks-old, 6 mice/group) were untreated (normal) or injected i.v. with 5T33P myeloma cells (1 × 107/mouse) (5T33P-bearing) on day 0. ALT-803 (0.2 mg/kg) or PBS (dose volume equivalent) was administered i.v. on day 14. Four days after treatment, mouse splenocytes were isolated and stained with Abs specific to CD44 (PE-Cy7), NKG2D (APC), PD-1 (FITC), CD25 (PE), and CD8 (PerCP-Cy5.5). Stained cells were analyzed by flow cytometry. The percentage of CD44low and CD44high in CD8+ T cells (A) and percentage of PD-1-, CD25- and NKG2D-positive cells in CD8+CD44high memory T cell population (B) are shown. For comparison of ALT-801 vs. PBS treatment effects in normal or 5T33P-bearing mice, ***, P ≤ 0.001. C, CD3+ enriched cells from spleens of donor C57BL/6NHsd mice were labeled with Celltrace™ Violet and then adoptively transferred (1.5 × 106 cells/mouse) into syngeneic recipients (3 mice/group) on day 0 (SD0). On SD2, mice were treated (i.v.) with 0.02 mg/kg of ALT803, 0.2 mg/kg of ALT-803 or PBS (dose volume equivalent). On SD6, spleens were harvested and analyzed individually by flow cytometry for donor cells (violet label) and positive staining with Abs specific to CD44 (PE-Cy7), NKG2D (APC), PD-1 (FITC), CD25 (PE), and CD8 (PerCP-Cy5.5). Histograms show proliferation of violet-labeled CD8+CD44high memory T cell population. D, NKG2DnegCD25negCD8+CD44high memory T cells from spleens and lymph nodes of donor C57BL/6NHsd mice were sorted with BD FACS Aria (Supplemental Fig. S4) and labeled with Celltrace™ Violet. Donor cells (1 × 106 cells/mouse) were then adoptively transferred into syngeneic recipients (3 mice/group) on SD0. On SD2, mice were treated (i.v.) 0.2 mg/kg ALT-803 or PBS (dose volume equivalent). On SD6, spleens were harvested and analyzed by flow cytometry as described in 3C. Histograms show proliferation of violet-labeled CD8+ CD44highmemory T cell population and CD8+CD44highNKG2D+ and CD8+CD44highCD25+ subpopulations. The value indicates the percentage of NKG2D+ or CD25+ cells in the donor CD8+CD44high memory T cell population.
A recent study also showed that certain immunotherapies promote antigen-nonspecific expansion of memory CD8+ T cells with innate-type cell receptors (25). Unlike the memory CD8+ T cells stimulated by antigen-dependent TCR signaling which up-regulate PD-1 and CD25 cell surface molecules, the immunotherapy-mediated expanded memory CD8+ T cells express NKG2D, granzyme B, and possess broadly antigen-nonspecific lytic capability. Interestingly, we found that the splenic memory CD8+ T cells expanded in vivo by ALT-803 treatment also expressed NKG2D and not CD25 or PD-1 (Fig. 3B). To examine ALT-803-mediated changes in this cell population, we isolated CD3+ enriched cells from spleens and lymph nodes of C57BL/6NHsd mice and labeled them with Celltrace™ Violet, and then adoptively transferred these cells into syngeneic recipients. Two days after transfer, the mice were treated with PBS or ALT-803 (0.02 mg/kg or 0.2 mg/kg) and the phenotype and proliferation of the adoptively transferred cells were examined 4 days later. As shown in Fig. 3C, ALT-803 treatment resulted in a significant, dose-dependent increase in proliferation of donor CD8+CD44high T cells isolated from spleens of recipient mice, whereas donor memory CD8+ T cells did not proliferate in PBS-treated mice. In the expanded memory CD8+ T-cell population from 0.2 mg/kg ALT-803 treated mice, over 90% expressed NKG2D with increased positive staining in cells that underwent multiple rounds of proliferation. To rule out the possibility that this is due to an enormous expansion of a small population of NKG2D+ cells following ALT-803 treatment, we conducted similar adoptive transfer studies with sorted NKG2DnegCD25negCD8+CD44high T cells labeled with Celltrace™ Violet. Treatment of recipient mice with 0.2 mg/kg ALT-803 caused an increase in NKG2D+ memory CD8+ T cells from 0% to 13% (Fig. 3D; see gating strategy in Supplemental Fig. S4). Thus, ALT-803 treatment not only induced the proliferation of the memory CD8+ T cells but also up-regulated the NKG2D receptor on their surface. Donor memory CD8+ T cell expressing CD25 also proliferated following ALT-803 treatment but the percentage of these cells (~4%) was the same in ALT-803- and PBS-treated mice, consistent with the findings in 5T33P tumor-bearing mice.
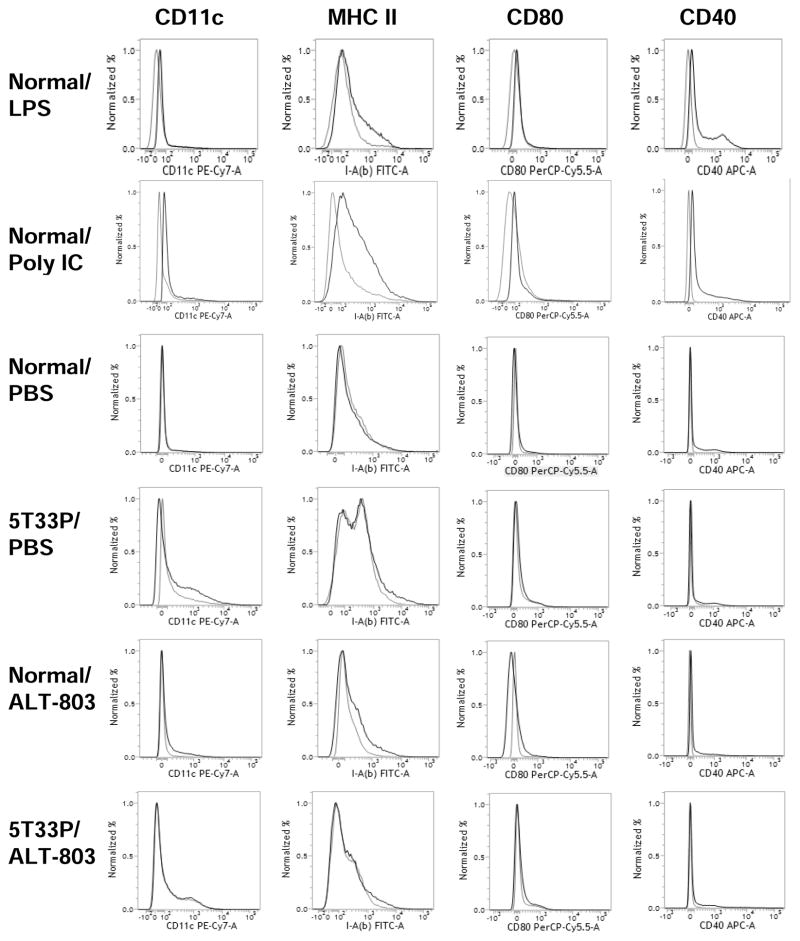
To assess whether the induced CD8+ T cell responses were associated with changes in antigen presentation potential in vivo, we administered ALT-803 (0.2 mg/kg), LPS (12.5 μg/mouse) or poly IC (10 μg/mouse) to normal and 5T33P-bearing C57BL/6NHsd mice and examined the up-regulation of activation/maturation markers on BM dendritic cells (DCs). We found that ALT-803, unlike poly IC or LPS, did not increase MHC II (I-Ab), CD80 or CD40 levels on BM DCs (Fig. 4). Similar results were found for splenic DCs. Thus, the rapid expansion of CD8+CD44high memory T-cell population stimulated by ALT-803 is unlikely a result of increased antigen-specific responses, consistent with the results of others demonstrating antigen-independent activation of innate-type memory T cells following immunotherapy or microbial or viral infection (26–28).
Figure 4.
ALT-803 did not increase expression maturation markers on BM dendritic cells. Female C57BL/6NHsd mice (5–6 weeks-old, 3 mice/group) were untreated (normal) or injected i.v. with 5T33P myeloma cells (1 × 107/mouse) (5T33P-bearing) on day 0. ALT-803 (0.2 mg/kg) or PBS (dose volume equivalent) was administered i.v. on day 14. Four days after treatment, BM cells were isolated, pooled and stained with Abs specific to CD11c (PE-Cy7), MHC II [I-A(b)] (FITC), CD80 (PerCP-Cy5.5), and CD40 (APC), then analyzed by flow cytometry. Mice treated i.p. either with 12.5 μg of LPS (E. coli 055:B5, Sigma-Aldrich) and sacrificed 12 hrs later or with 10 μg of Poly IC (InvivoGene) and sacrificed 24 hrs later served as positive controls. Histograms show staining with positive Abs (black line) or isotype controls (gray line).
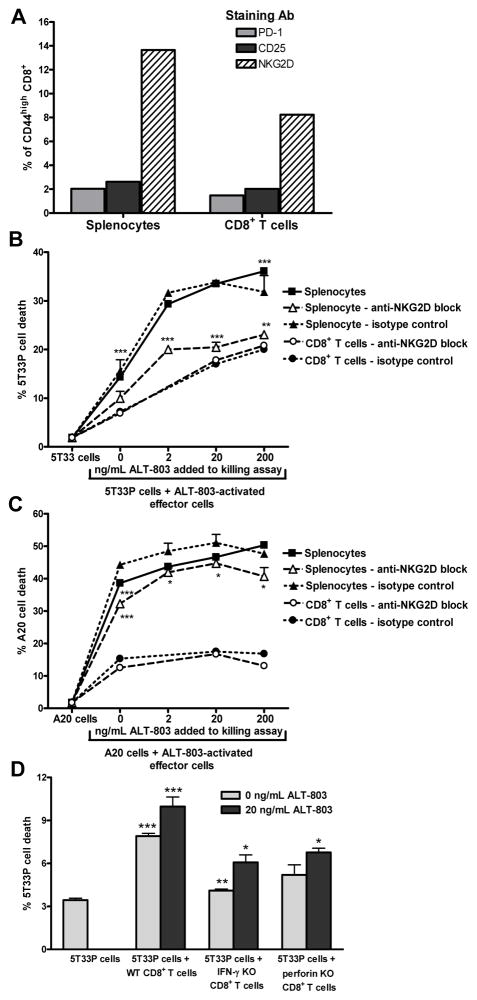
The cytotoxic activity of ALT-803-treated immune cells was also examined in vitro. CD8+CD44high T cells increased 5-fold in splenocytes and 3-fold in CD8+ enriched splenic T cells from normal C57BL/6NHsd mice following a 3-day incubation with 0.2 μg/mL ALT-803. Similar to the findings in vivo, up-regulation of NKG2D but not CD25 or PD-1 was observed on memory CD8+ T cells following ALT-803 incubation (Fig. 5A). The ALT-803-stimulated splenocytes and CD8+ enriched splenic T cells exhibited elevated cytolytic activity against 5T33P cells (Fig. 5B) as well as A20 lymphoma cell lines (Fig. 5C). Killing of 5T33P cells was further enhanced by inclusion of ALT- 803 during the cytotoxicity assay, suggesting a continued activation of immune cell anti-tumor activity by ALT-803. Interestingly, 5T33P myeloma-targeted cytotoxicity of ALT-803-stimulated CD8+ enriched splenocytes was not affected by inclusion of an NKG2D blocking antibody, whereas this antibody reduced 5T33P killing by whole splenocyte cultures (Fig. 5B). These results suggest that in vitro cytotoxicity of the NK cells in the whole splenocyte cultures are dependent on NKG2D whereas that of CD8+ T cells does not require NKG2D but may be mediated through other innate-like activating receptors induced by ALT-803. The cytotoxicity of CD8+ T cells was partially dependent of perforin expression since CD8+ T cells obtained from perforin knock-out mice showed reduced 5T33P cell killing in this assay (Fig. 5D).
Figure 5.
In vitro cytotoxic activity of ALT-803-treated immune cells. A, unfractionated or CD8+ T cell enriched splenocytes (untouched) from normal C57BL/6NHsd mice (pool of 3/group) were cultured with 200 ng/mL of ALT-803 for 72 hrs. Cells were then harvested, stained with Abs specific to CD44 (PE-Cy7), NKG2D (APC), PD-1 (FITC), CD25 (PE), and CD8 (PerCP-Cy5.5), and analyzed by flow cytometry for expansion of CD8+CD44high memory T cell populations. B & C, unfractionated or CD8+ T cell enriched splenocytes were activated as described in A, then washed thoroughly and re-plated in duplicate wells (1×106 cells/well) containing 0, 20, or 200 ng/mL ALT-803. NKG2D blocking antibody (10 μg/mL) or isotype control antibody (10 μg/mL) was added to appropriate wells as indicated. PHK-67 labeled 5T33P (1×105 cells/well) (B) or A20 tumor cells (1×105 cells/well) (C) were added (E:T ratio = 10:1) and incubated for 24 hrs. Target cell killing of the individual cultures was assessed by analysis of PI staining of PKH-67 labeled tumor cells on a BD FACScan. The level of PI staining in cultured PHK-67 labeled 5T33P or A20 cells alone served as a background control. The results are representative of two independent studies. For comparison of target cells+activated splenocytes vs. target cells alone or target cells+activated splenocytes+200 ng/mL ALT-801 vs. target cells+activated splenocytes, ***, P ≤ 0.001. For comparison of the effects of anti-NKG2D antibody vs. isotype control, *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001. D, in vitro 5T33P killing assay of CD8+ T cell enriched spleen cells from normal, IFN-γ KO B6, and perforin KO B6 mice (pool of 3/group). As described above, enriched CD8+ T cells (2×107) were incubated with ALT-803 (0.2 μg/mL) for 72 hrs and then re-plated into triplicated wells (3×106 cells/well) without or with ALT-803 (20ng/ml). PHK-67 labeled 5T33P tumor cells (3×105 cells/well) were added as target cells (E:T ratio = 10:1). After incubation for 20 hrs, target cell killing was assessed as described above. The percentage of PI-positive 5T33P cells is shown. For comparison of target cells+effector cells vs. target cells alone or target cells+KO effector cells vs. target cells+WT effector cells under the same culture conditions, *, P ≤ 0.05; **, P ≤ 0.01; and ***, P ≤ 0.001.
Overall, these studies indicate that ALT-803 potently induces CD8+CD44high T cells and up-regulates innate-cell receptor NKG2D without the requirement of antigen-specific stimulation. Also, this type of ALT-803-stimuated CD8+ memory T cells exhibit cytotoxic activity against myeloma and other tumor cells.
Serum IFN-γ is elevated by ALT-803 treatment in a CD8+ T cell-dependent manner and is required for ALT-803-mediated efficacy
In addition to stimulating immune cells, we found that a single dose of ALT-803 to C57BL/6NHsd mice was found to significantly increase serum IFN-γ levels (Fig. 6A). Immune-depletion studies were then carried out to identify the immune cell type responsible for IFN-γ production after ALT-803 treatment. As shown in Fig. 6A, depletion of CD8+ T cells, but not NK1.1+ cells, eliminated most of the high-level expression of serum IFN-γ, indicating that CD8+ T cells were the dominant source of ALT-803-induced IFN-γ. To further determine whether CD8+CD44high memory or CD8+CD44low naïve T cells were the primary producers of IFN-γ after ALT-803 treatment, we analyzed IFN-γ production of splenic CD8+ T cells from ALT-803-treated mice. Intracellular IFN-γ was detectable as early as 12 hrs after ALT-803 treatment in the CD8+CD44high memory T-cell population and the percentage of IFN-γ producing memory T cells continued to remain elevated for at least 48 hrs after ALT-803 treatment (Fig. 6B). Significant ALT-803-mediated induction of intracellular IFN-γ was not observed in CD8+CD44low naïve T cells. Thus, ALT-803 activates CD8+CD44high memory T cells to proliferate and secrete IFN-γ via an antigen-independent pathway.
Figure 6.
CD8+ T cell production of IFN-γ plays a role in ALT-803-mediated efficacy. A, ALT-803 induce high level of serum IFN-γ via CD8+ T cells. C57BL/6NHsd mice (n = 5) received three doses of anti-CD8 Ab (dpCD8), anti-NK1.1 Ab (dpNK) or both Abs (dpCD8/NK) i.p. on days -2, -1 and 7. On day 8, a single i.v. dose of ALT-803 (0.2 mg/kg) was administrated and two days later (day 10) serum IFN-γ levels were examined. Bars represent the mean ± SE. For comparison of ALT-803+Ab depletion vs. ALT-803, *, P ≤ 0.05. B, C57BL/6NHsd mice (n = 3) were administrated a single i.v. dose of ALT-803 (0.2 mg/kg) on day 1 or day 2 respectively. On day 3, isolated splenocytes were stained with Abs to CD44 (PE-Cy7), and CD8 (PerCP-Cy5.5), and then intracellularly stained with FITC-anti-IFN-γ Ab. Dot plots show the percentage of IFN-γ producing CD8+CD44high memory T cells. C, IFN-γ is required for ALT-803 anti-myeloma activity. Female IFN-γ KO B6 mice (n = 4/group) were injected i.v. with 5T33P myeloma cells (1 × 107 cells/mouse) on day 0. ALT-803 (0.2 mg/kg) or PBS was administered i.v. on days 4 and 11. Survival (or morbidity due to hind leg paralysis) was monitored as a study endpoint.
To determine whether induced IFN-γ plays a role in the anti-myeloma activity of ALT-803, treatment effects on survival were evaluated in IFN-γ KO B6 mice bearing 5T33P cells. Similar to the findings in myeloma-bearing C57BL/6NHsd mice following CD8+ T cells depletion, ALT-803 treatment provided little or no protection from mortality to IFN-γ KO mice after 5T33P cell inoculation, indicating IFN-γ is required for ALT-803 efficacy (Fig. 6C). However, IFN-γ had no direct effect on 5T33P cell growth or apoptosis in vitro (Supplementary Fig. S3), consistent with previous reports (29). These results support a mechanism where ALT-803 activates IFN-γ production and cytotoxic activity of CD8+ memory T cells and together these responses promote rapid elimination of myeloma cells and prolonged survival of tumor bearing mice.
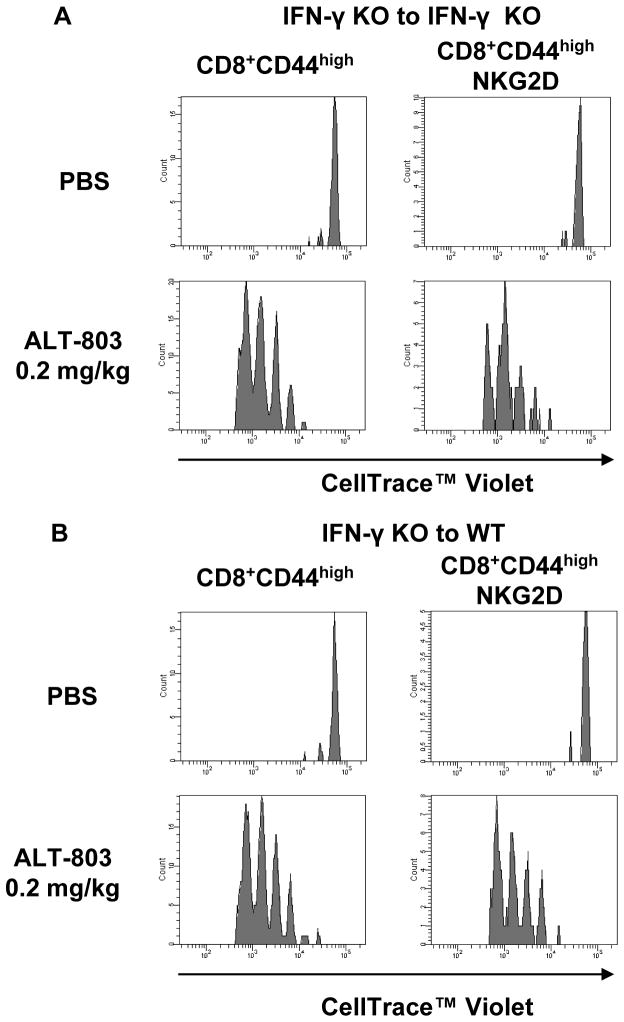
To assess whether IFN-γ is needed for ALT-803-mediated effects on CD8+ memory T-cell responses, adoptive cell transfer studies were conducted using donor Celltrace™ Violet-labeled CD8+ T cells from IFN-γ KO mice transferred into IFN-γ KO and wild-type recipient mice. As shown in Fig. 7, ALT-803 treatment of IFN-γ KO or wild-type recipients induced comparable CD8+CD44high memory T-cell proliferation and up-regulation of NKG2D of the adoptively-transferred cells. This indicates that ALT-803-induced CD8+CD44high memory T cell responses were IFN-γ independent. Interestingly, we also found that that CD8+ T cells isolated from IFN-γ KO mice exhibited less ALT-803-stimulated in vitro cytotoxic activity against 5T33P cells than was observed in CD8+ T cells from normal C57BL/6NHsd mice (Fig. 5D). Together, these results suggest that while IFN-γ is not required for ALT-803-mediated activation and expansion of CD8+ memory T cells, it still plays a role in augmenting the cytotoxicity of these cells against tumors via an as yet undetermined mechanism.
Figure 7.
ALT-803 induction of CD8+CD44high memory T cell responses was not dependent on IFN-γ. A & B, enriched CD8+ T cells (positive selection) from splenocytes and lymph nodes of IFN-γ KO B6 mice (6 weeks old) were labeled with Celltrace™ Violet and adoptively transferred (1.5 × 106 cell/mouse) into IFN-γ KO B6 recipients (KO, n=5) (A) or wild-type C57BL/6NHsd recipients (WT, n=5) (B) on day 0 (SD0). On SD2, 3 KO and 3 WT mice were treated with 0.2 mg/kg ALT-803 (i.v.) and the remaining 2 KO and 2 WT mice received PBS (i.v.) as controls. On SD6, spleens were harvested and analyzed individually by flow cytometry for donor cells (violet label) and positive staining with Abs specific to CD44 (PE-Cy7), NKG2D (APC), and CD8 (PerCP-Cy5.5). Histograms show proliferation of violet-labeled CD8+CD44high and CD8+CD44highNKG2D+ memory T cell population
Discussion
IL-15 and IL-15Rα are co-expressed and form a protein complex in antigen-presenting cells for trans-presentation to T and NK cells (6). Studies have shown that soluble IL-15:IL-15Rα complexes exhibit a 50 fold better immune stimulatory activity in vivo than IL-15 alone (10) and potent efficacy against solid and metastatic tumors in various mouse models (11–13); however, its activity against hematologic tumors has not been reported. In this study, we describe the anti-myeloma activity and mechanism-of-action of ALT-803, a protein complex consisting of an IL-15 super-agonist mutant associated with a dimeric IL-15Rα/Fc fusion protein (18, 19). We found that a single dose of ALT-803 was much more effective than IL-15 at reducing the levels of well-established murine 5T33P and MOPC-315P myeloma cells in the BM of tumor-bearing immunocompetent mice. ALT-803 was also found to prolong survival of 5T33P and MOPC-315P tumor-bearing mice and effectively cured a majority of the mice of tumors. Moreover, 5T33P-bearing mice cured by prior ALT-803 treatment were protected against subsequent 5T33P rechallenge, indicating that ALT-803-mediated the induction of long lasting anti-myeloma immune memory responses. These results are consistent with our previous report that ALT-803 exhibited significantly better activity compared to IL-15 in stimulating NK cell and CD8+ T-cell responses in vivo (19). This enhanced immunostimulatory activity is likely the result of a combination of the increased in vivo half-life of ALT-803 compared to IL-15 (25 h vs. <40 min) and the dimeric nature of the cytokine domain in the complex increasing its binding avidity to IL-15Rβγc (19). It is also possible that the Fc domain of the complex enables trans-presentation of the cytokine to IL-15Rβγc receptor-bearing NK and T cells via binding to the Fc-γ receptors (FcγR) on the surface of dendritic cells, macrophages, NK cells and other cell types (11). We have recently created an FcγR-binding deficient derivative of ALT-803 for studies to further evaluate the contribution of the Fc-γ domain to ALT-803–mediated immune stimulation.
Previous studies have shown that IL-15 and IL-15:IL-15Rα complexes can stimulate anti-tumor activity via either effector NK cells or T cells, demonstrating the remarkable capacity of IL-15 to induce different effector cell responses against diverse tumor types and tumor microenvironments (11–14, 16). In the 5T33P myeloma model reported here, we found that treatment with ALT-803 resulted in an increase in CD8+ T-cell levels in the BM of tumor-bearing mice that correlated with the complex’s ability to reduce BM 5T33P-cell burden. However, systemic depletion of CD8+ T cells, but not NK1.1+ cells, was shown to largely eliminate the anti-tumor activity of ALT-803 on BM myeloma cells, the treatment-related survival benefit in 5T33P-bearing mice. This indicates that CD8+ T cells, but not NK1.1+ cells, play a pivotal role in ALT-803 anti-myeloma activity. This finding is perplexing since we found that a single i.v. treatment of ALT-803 (0.2 mg/kg) had a marked effect on 5T33P cells in the BM of mice with well-established tumors, providing >90% reduction in BM IgG2b+ myeloma cells four days after treatment. Such a robust and rapid onset of immune responses is generally believed to only be associated with the innate immune system. Additionally, we further found that a single dose of ALT-803 was capable of inducing high serum levels of IFN-γ and promoting the proliferation of CD8+ cells in non-tumor bearing mice shortly after treatment. The source of serum IFN-γ was largely from CD8+CD44high T cells, not NK1.1+ cells, based on our immune-depletion analysis. Therefore, we questioned whether the activation of CD8+ T cells and subsequent anti-tumor activity mediated by ALT-803 was antigen-dependent. To address this, we examined whether ALT-803 induced dendritic cell activation/maturation. Our finding that ALT-803 treatment did not up-regulate CD86, CD80, MHC-II and CD40 in splenic DCs from either tumor- or non-tumor-bearing mice suggested that ALT-803 did not promote antigen presentation at the initial phase of the immune response. Thus, it appears unlikely that antigen-dependent clonal expansion of naïve CD8+ T cells immediately after ALT-803 treatment is responsible for the potent anti-myeloma activity observed in mice bearing established 5T33P and MOPC-315P tumors.
The proliferation of memory-phenotype (CD44high) CD8+ T cells, but not naïve CD8+ T cells, can be induced in vivo by the cytokines IL-12, IL-18 and IFN-γ, most likely via production of IL-15, or directly by IL-15 (22–24). A recent study also showed that cytokine-mediated stimulation could promote antigen-nonspecific expansion of memory CD8+ T cells with a unique phenotype (25). Unlike TCR signaling that up-regulates PD-1 and CD25 surface markers on memory CD8+ T cells, treatment with IL-2 in combination with anti-CD40 antibody resulted in expansion of memory CD8+ T cells that express NKG2D, granzyme B, and possess broad lytic capabilities. These cells have been suggested to be responsible for the dramatic anti-tumor effects of this therapy in animal models (26–28). Herein, using the adoptive-cell transfer approach, we demonstrated that ALT-803 alone could also induce CD8+CD44high memory T cells, but not naïve T cells, to acquire innate cell receptors, such as NKG2D, without inducing PD-1, in vitro and in vivo. ALT-803 appears to act by both inducing CD8+ memory T cell proliferation and up-regulating NKG2D expression rather than preferentially expanding pre-existing CD8+CD44high memory T cells carrying this receptor. In vitro, the ALT-803-activated CD8+CD44high memory T cells exhibited antigen-nonspecific and potent anti-tumor activity against 5T33P myeloma. Due to the presence of the large numbers of the CD8+CD44high memory T cells after ALT-803 treatment with an innate -like phenotype and their high anti-tumor activity, it is conceivable that these cells represented the main effector cells responsible for mounting robust and rapid immune responses against myeloma in the initial phase after ALT-803 infusion.
A single dose of ALT-803 was capable of inducing high serum levels of IFN-γ in mice. This activity appeared to be different from that in previous studies in which monotherapy with IL-15 or single-chain IL-15:IL-15Rα complexes was shown to induce mouse immune cell proliferation, but not to affect serum IFN-γ levels (30, 31). IL-15 has been reported to elevate IFN-γ levels in vivo when co-administered with IL-12, IL-18 or other immune-stimulatory molecules via a cytokine feedback cascade involving NK cells and macrophages (7). In contrast, we show that the effect of ALT-803 on serum IFN-γ levels was largely dependent on CD8+CD44high memory T cells and not NK1.1+ cells. It has been found that treatment of mice with IL-15:IL-15Rα/Fc complexes similar to ALT-803 can cause naïve CD8+ T cells to expand and acquire an activated phenotype that includes the ability to secrete IFN-γ and mediate antigen-specific cytolytic function (10). These responses were dependent on MHC class I molecules, TCR avidity and were enhanced in the presence of peptide antigen (32), suggesting that IL-15:IL-15Rα/Fc complexes increase the sensitivity and responsiveness of naïve CD8+ T cells to endogenous antigen presentation. In contrast, ALT-803 has the unique feature of inducing high levels of serum IFN-γ by activating CD8+ memory T cells in an antigen-independent fashion in vivo. Although IFN-γ has no direct effects on growth or induction of apoptosis of 5T33P tumor cells in vitro as shown in this study, the loss of treatment-mediated anti-myeloma activity in the IFN-γ KO mice bearing 5T33 tumors demonstrates the pivotal role of IFN-γ in the therapeutic potency of ALT-803. The effect of IFN-γ on ALT-803 anti-tumor activity is apparently via an indirect mechanism since ALT-803 did not lose its ability to induce IFN-γ-deficient CD8+CD44high memory T cells in IFN-γ KO mice.
IFN-γ is a remarkable cytokine that orchestrates a diverse array of cellular programs through transcriptional regulation of immunologically relevant genes (33). IFN-γ skews the immune response toward a Th1 phenotype by inducing T-bet, a critical transcription factor of Th1 cells, which directly induces many Th1 cell-related genes, but indirectly suppresses the Th2 cell-related genes (34). IFN-γ also orchestrates the trafficking of specific immune cells to sites of inflammation (e.g., tumor sites) through up-regulating expression of adhesion molecules (e.g., ICAM-1, VCAM-1) and chemokines (e.g., IP-10, MCP-1, MIG-1α/β, RANTES) (35–42). Thus, the loss of IFN-γ could lead to the loss of the Th1 cell-type anti-tumor environment and the inability to up-regulate the necessary chemokine receptors and/or adhesion molecules on the ALT-803-activated CD8+CD44high T cells for trafficking to the tumor site. In addition, IFN-γ is a potent activator of macrophage which kill pathogens and tumor cells by producing reactive oxygen species and reactive nitrogen intermediates via induction of NADPH oxidase system and INOS (43–45). IFN-γ is also known to repolarize the stage M2 tumor-promoting tumor-associated macrophages (TAMs) to M1 tumor-destroying macrophages at the tumor sites, which in turn could mount an effective immune response against tumors (46, 47). Thus, IFN-γ secreted by ALT-803-activated memory T cells could significantly contribute to the anti-tumor potency of ALT-803 by directly activating macrophages to enhance their tumor-killing activities or to repolarize the TAMs for tumor destruction.
In summary, we reveal the novel mechanism of action of ALT-803, an IL-15 super-agonist complex, against multiple myeloma that acts mainly through its stimulation of CD8+CD44high memory T cells to expand, acquire an innate-type phenotype and secrete IFN-γ independent of antigen requirement resulting in enhancement of host survival. These findings suggest a novel therapeutic strategy of exploiting the innate-cell function of adoptive immune cells. While our results suggest clinical application for treatment of multiple myeloma, this IL-15-based approach may also be efficacious for other cancers and infectious diseases.
Supplementary Material
Acknowledgments
Financial support: National Institutes of Health (CA156740) (H. C. Wong).
We thank Dr. Ulrich von Andrian, Harvard Medical School, for providing the 5T33 myeloma cell line and Dr. Jason Yovandich, Biological Resources Branch, National Cancer Institute-Frederick, for providing recombinant human IL-15. We thank Dr. Dean Taylor for his critical reading of the manuscript. This work was supported by the National Institutes of Health grant CA156740 (H.C.W.).
Footnotes
Competing interests statement: Certain authors are employees and/or shareholders of Altor BioScience Corp. and declare competing financial interests.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Bladé J, Rosiñol L, Cibeira MT, Rovira M, Carreras E. Hematopoietic stem cell transplantation for multiple myeloma beyond 2010. Blood. 2010;115:3655–3663. doi: 10.1182/blood-2009-08-238196. [DOI] [PubMed] [Google Scholar]
- 3.Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, Verma A. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009;2:36. doi: 10.1186/1756-8722-2-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palumbo A, Attal M, Roussel M. Shifts in the therapeutic paradigm for patients newly diagnosed with multiple myeloma: maintenance therapy and overall survival. Clin Cancer Res. 2011;17:1253–1263. doi: 10.1158/1078-0432.CCR-10-1925. [DOI] [PubMed] [Google Scholar]
- 5.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17:1264–1277. doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]
- 6.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 7.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 8.Peest D, Leo R, Bloche S, Hein R, Stannat-Kiessling S, Tschechne B, Fett W, Harms P, Hoffmann L, Bartl R, et al. Br J Haematol. 1995;89:328–337. doi: 10.1111/j.1365-2141.1995.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 9.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 12.Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, Hamerman JA, Goldrath AW, Turley SJ. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 2008;68:2972–2983. doi: 10.1158/0008-5472.CAN-08-0045. [DOI] [PubMed] [Google Scholar]
- 13.Bessard A, Sole V, Bouchaud G, Quemener A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther. 2009;8:2736–2745. doi: 10.1158/1535-7163.MCT-09-0275. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Yao Z, Dubois S, Ju W, Muller JR, Waldmann TA. Interleukin-15 combined with an anti-CD40 antibody provides enhanced therapeutic efficacy for murine models of colon cancer. Proc Natl Acad Sci U S A. 2009;106:7513–7518. doi: 10.1073/pnas.0902637106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu P, Steel JC, Zhang M, Morris JC, Waldmann TA. Simultaneous blockade of multiple immune system inhibitory checkpoints enhances antitumor activity mediated by interleukin-15 in a murine metastatic colon carcinoma model. Clin Cancer Res. 2010;16:6019–6028. doi: 10.1158/1078-0432.CCR-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu P, Steel JC, Zhang M, Morris JC, Waitz R, Fasso M, Allison JP, Waldmann TA. Simultaneous inhibition of two regulatory T-cell subsets enhanced Interleukin-15 efficacy in a prostate tumor model. Proc Natl Acad Sci U S A. 2012;109:6187–6192. doi: 10.1073/pnas.1203479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev. 2008;222:357–368. doi: 10.1111/j.1600-065X.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Marcus WD, Xu W, Lee HI, Han K, Egan JO, Yovandich JL, Rhode PR, Wong HC. Novel human interleukin-15 agonists. J Immunol. 2009;183:3598–3607. doi: 10.4049/jimmunol.0901244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han KP, Zhu X, Liu B, Jeng E, Kong L, Yovandich JL, Vyas VV, Marcus WD, Chavaillaz PA, Romero CA, Rhode PR, Wong HC. IL-15:IL-15 receptor alpha superagonist complex: High-level co-expression in recombinant mammalian cells, purification and characterization. Cytokine. 2011;56:804–810. doi: 10.1016/j.cyto.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radl J, Croese JW, Zurcher C, Van den Enden-Vieveen MH, de Leeuw AM. Animal model of human disease. Multiple myeloma. Am J Pathol. 1988;132:593–597. [PMC free article] [PubMed] [Google Scholar]
- 21.Waldmann TA, Lugli E, Roederer M, Perera LP, Smedley JV, Macallister RP, Goldman CK, Bryant BR, Decker JM, Fleisher TA, Lane HC, Sneller MC, Kurlander RJ, Kleiner DE, Pletcher JM, Figg WD, Yovandich JL, Creekmore SP. Safety (toxicity), pharmacokinetics, immunogenicity, and impact on elements of the normal immune system of recombinant human IL-15 in rhesus macaques. Blood. 2011;117:4787–4795. doi: 10.1182/blood-2010-10-311456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 23.Tough DF, Zhang X, Sprent J. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J Immunol. 2001;166:6007–6011. doi: 10.4049/jimmunol.166.10.6007. [DOI] [PubMed] [Google Scholar]
- 24.Sprent J, Zhang X, Sun S, Tough D. T-cell proliferation in vivo and the role of cytokines. Philos Trans R Soc Lond B Biol Sci. 2000;355:317–322. doi: 10.1098/rstb.2000.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, Ames E, Bruhn KW, Craft N, Wiltrout RH, Longo DL, Lanier LL, Blazar BR, Redelman D, Murphy WJ. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berner V, Liu H, Zhou Q, Alderson KL, Sun K, Weiss JM, Back TC, Longo DL, Blazar BR, Wiltrout RH, Welniak LA, Redelman D, Murphy WJ. IFN-gamma mediates CD4+ T-cell loss and impairs secondary antitumor responses after successful initial immunotherapy. Nat Med. 2007;13:354–360. doi: 10.1038/nm1554. [DOI] [PubMed] [Google Scholar]
- 27.Murphy WJ, Welniak L, Back T, Hixon J, Subleski J, Seki N, Wigginton JM, Wilson SE, Blazar BR, Malyguine AM, Sayers TJ, Wiltrout RH. Synergistic anti-tumor responses after administration of agonistic antibodies to CD40 and IL-2: coordination of dendritic and CD8+ cell responses. J Immunol. 2003;170:2727–2733. doi: 10.4049/jimmunol.170.5.2727. [DOI] [PubMed] [Google Scholar]
- 28.Soudja SM, Ruiz AL, Marie JC, Lauvau G. Inflammatory Monocytes Activate Memory CD8(+) T and Innate NK Lymphocytes Independent of Cognate Antigen during Microbial Pathogen Invasion. Immunity. 2012;37:549–562. doi: 10.1016/j.immuni.2012.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manning LS, Chamberlain NL, Leahy MF, Cordingley FT. Assessment of the therapeutic potential of cytokines, cytotoxic drugs and effector cell populations for the treatment of multiple myeloma using the 5T33 murine myeloma model. Immunol Cell Biol. 1995;73:326–332. doi: 10.1038/icb.1995.50. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- 31.Chang CM, Lo CH, Shih YM, Chen Y, Wu PY, Tsuneyama K, Roffler SR, Tao MH. Treatment of hepatocellular carcinoma with adeno-associated virus encoding interleukin-15 superagonist. Hum Gene Ther. 2010;21:611–621. doi: 10.1089/hum.2009.187. [DOI] [PubMed] [Google Scholar]
- 32.Stoklasek TA, Colpitts SL, Smilowitz HM, Lefrançois L. MHC Class I and TCR Avidity Control the CD8 T Cell Response to IL-15/IL-15Ralpha Complex. J Immunol. 2010;185:6857–6865. doi: 10.4049/jimmunol.1001601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, Feigenbaum L, Zhao K, Paul WE. The Transcription Factor T-bet Is Induced by Multiple Pathways and Prevents an Endogenous Th2 Cell Program during Th1 Cell Responses. Immunity. 2012;37:660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou J, Baichwal V, Cao Z. Regulatory elements and transcription factors controlling basal and cytokine-induced expression of the gene encoding intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1994;91:11641–11645. doi: 10.1073/pnas.91.24.11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jesse TL, LaChance R, Iademarco MF, Dean DC. Interferon regulatory factor-2 is a transcriptional activator in muscle where It regulates expression of vascular cell adhesion molecule-1. J Cell Biol. 1998;140:1265–1276. doi: 10.1083/jcb.140.5.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gil MP, Bohn E, O’Guin AK, Ramana CV, Levine B, Stark GR, Virgin HW, Schreiber RD. Biologic consequences of Stat1-independent IFN signaling. Proc Natl Acad Sci U S A. 2001;98:6680–6685. doi: 10.1073/pnas.111163898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taub DD, Lloyd AR, Conlon K, Wang JM, Ortaldo JR, Harada A, Matsushima K, Kelvin DJ, Oppenheim JJ. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and T lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1814. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rollins BJ, Yoshimura T, Leonard EJ, Pober JS. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am J Pathol. 1990;136:1229–1233. [PMC free article] [PubMed] [Google Scholar]
- 40.Liao F, Rabin RL, Yannelli JR, Koniaris LG, Vanguri P, Farber JM. Human Mig chemokine: biochemical and functional characterization. J Exp Med. 1995;182:1301–1314. doi: 10.1084/jem.182.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1 alpha and MIP-1 beta. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 42.Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. doi: 10.1016/s1471-4906(00)01812-3. [DOI] [PubMed] [Google Scholar]
- 43.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 44.Bolscher BG, Koenderman L, Tool AT, Stokman PM, Roos D. NADPH:O2 oxidoreductase of human eosinophils in the cell-free system. FEBS Lett. 1990;268:269–273. doi: 10.1016/0014-5793(90)81025-j. [DOI] [PubMed] [Google Scholar]
- 45.Roos D, Bolscher BG, Weening RS, Verhoeven AJ. Formation of reactive oxygen species by phagocytic cells: functions and dysfunctions. Acta Paediatr Hung. 1988;29:83–91. [PubMed] [Google Scholar]
- 46.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 47.Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.