Abstract
The Goal:
The goal of this study was to determine the effect of psychoactive substances (drugs) on the presence and frequency of oral Candida species and Candida dubliniensis.
Materials and methods:
For the purpose of achieving the set goals, we chose a sample. Sixty bed-ridden patients from the Institute for Alcoholism and Other Addictions in Sarajevo Canton, both males and females between 18 and 60 years of age, were included in the research and assigned to two different groups (alcohol addicts and opiate addicts). After extensive anamnesis and a clinical examination, samples of oral epithelia were taken for microbiological identification. Two confirmatory methods were used for the identification of Candida species: the blastesis test and cultivation in a chromatophilic medium (Chrom agar). A yeast assimilation test (API test) was used for the identification of non-albicans Candida. A separate test was used to identify Candida dubliniensis (PAL agar).
Results:
The results of the microbiological analysis confirmed the frequency of Candida albicans (43%) in psychoactive substance addicts, as well as an increase in non-albicans Candida regardless of the type of addiction (34%). The presence of Candida dubliniensis was proven in psychoactive substance addicts (23%) and it was confirmed that the frequency of bacterial adherence of Candida dubliniensis is directly proportional to the duration of the drug-addiction.
Conclusion:
The abuse of psychoactive substances has an effect on the frequency of albicans and non-albicans species of oral Candida. Based on the findings, we have concluded that psychoactive substances (opiates and alcohol) lead to an increase in oral Candida dubliniensis regardless of the type of addictions.
Key words: Psychoactive substances (drugs), oral Candida species, Candida dubliniensis.
1. INTRODUCTION
The World Health Organization has defined drugs or psychoactive substance as any natural or synthesized drug that, when introduced into the organism, can alter one or several functions of the organism. Drugs are divided into hard and soft drugs. Hard drugs cause strong psychological and physical addiction (heroin, cocaine). Soft drugs have a moderate potential for psychological addiction, but a low grade of physical addiction. These are: alcohol, amphetamines, nicotine, hallucinogenics, caffeine, marijuana (hashish), organic solvents etc. (1, 2, 3).
Psychoactive substances may be introduced into the organism in several ways: orally (alcohol, pills), by smoking, inhaling (marijuana, cocaine, opium), nasally (cocaine, heroin), subcutaneously and intravenously (heroin, cocaine).
Psychoactive substances (drugs) have a vast effect on oral health. Oral changes depend on the type of drug and the duration of use. Changes in the oral epithelia are not pathognomonic to psychoactive substance addicts. The most common oral changes that can be related to psychoactive substance addiction are: Candidiasis mucosae oris; Cheilitis angularis, Glossitis rhombica mediana, Candida leukoplakia, Hyperceratisis mucousae oris, Xerostomia, Gingivostomatitis ulceromembranacea, Lingua nigra villosa, Stomatitis aphthosis recurrens (SAR), Erosiae mucosae oris, Herpes simplex, Morsicatio mucosae oris, Bruxism.(4). Psychoactive substance (alcohol, opiates) addiction in patients leads to immunosuppression, which favors an increased incidence of opportunistic infections. Oral candidiasis is an opportunistic infective state caused by fungus of the Candida genus, of which Candida albicans is the most pathogenic, and selectively pathologically adheres to oral epithelia and causes oral diseases (5). Of other non-albicans species with a high incidence of clinically manifested candidiases, the following stand out: Candida dubliniensis, Candida glabrata, Candida tropicalis, Candida crusei, Candida parapsilosis, Candida stelatoidea, Candida kefyr, Candida guillermondi (6). A large number of epidemiological studies show an increased incidence of infections (epithelial, dermatological, hematogenous, and disseminated) caused by fungus which are not Candida albicans and belong to the non-albicans species.(5, 6) The most recent research is aimed towards the identification of Candida dubliniensis due to its pathogenicity, frequency, and resistance to antimicotics, especially in HIV patients, most of whom are intravenous drug users. Candida dubliniensis, as compared to Candida albicans, is a rare member of endogenous microflora in immunocompromised test subjects, and has a lesser ability for colonization and infection (7, 8).
Modern findings point to a relation between the consumption of psychoactive substances (drugs), the duration of the addiction and the type of drug used, and the frequency of oral Candida dubliniensis. The research of Rocchi et al. in 2007 confirmed the correlation between Candida dubliniensis and the duration of heroin use (9). In 2009, Rooban et al. tested oral health in addicts to various types of drugs (opiates, cocaine, marijuana) and determined a highly significant correlation between oral Candida dubliniensis and the duration of drug use, as well as the type of drug used (10).
Psychoactive substance addicts are high-risk patients for AIDS and Candida infections, the incidence of which is constantly increasing. These patients often show high resistance to therapeutic treatment, therefore, special significance is given to specific microbiological procedure which isolate and confirm the presence of non-albicans species, as well as the targeted identification of Candida dubliniensis.
2. MATERIALS AND METHODS
Sixty bed-ridden patients from the Institute for Alcoholism and Other Addictions in Sarajevo Canton were included in the study; these were separated into two groups: Group A – Alcohol addicts and Group B – Opiate addicts, ranging in age from 18 to 60, and including both genders. After extensive anamnesis, both extra oral and intraoral examinations of oral epithelia were performed, and an oral epithelia sample was taken for microbiological identification. The microbiological procedure was performed at the Institute of Microbiology, Immunology and Parasitology at the University Clinical Center. The cultivation of Candida species was first performed on Saboraud dextrose agar and was then placed inside a thermostat set to 37°C for 48 hours. A growth in the colonies was detected after the incubation period, and showed divergence in color, shape, and size. Yeast assimilation test (API test) was used for the identification of the non-albicans Candidae. As Candida dubliniensis is a variety of Candida albicans, specific tests were used for its identification (PAL agar). Two confirmatory methods were used to identify the Candida species: the blastesis test, and cultivation in a chromatophilic medium (Chrom agar). The blastesis test was performed by incubating the yeast suspension in a thermostat for 3 to 4 hours; following the incubation, a drop of the suspension was placed on a slide and observed under a microscope at a magnification of 40x. A positive test was the germination test, which pointed to an occurrence of Candida albicans. The positive culture was placed in a chromatophilic medium (Chrom agar) and, following the incubation period of 24 to 48 hours at a temperature of 37°C, was tested for 4 species of Candida, based on color differentiation (Candida albicans, Candida tropicalis, Candida glabrata, Candida crusei).
During further analyses, all cultures of Candida albicans (confirmed by the aforementioned methods) were placed in PAL agar medium. The culture incubated at 30°C for 24 to 48 hours (Candida dubliniensis requires higher temperatures for growth). If an increase in yeast colony size was noted, a solution was prepared and observed under a microscope at a magnification of 40x. A positive finding of chlamydospores confirmed that the observed yeast was Candida dubliniensis.
3. RESULTS OF MICROBIOLOGICAL ANALYSIS
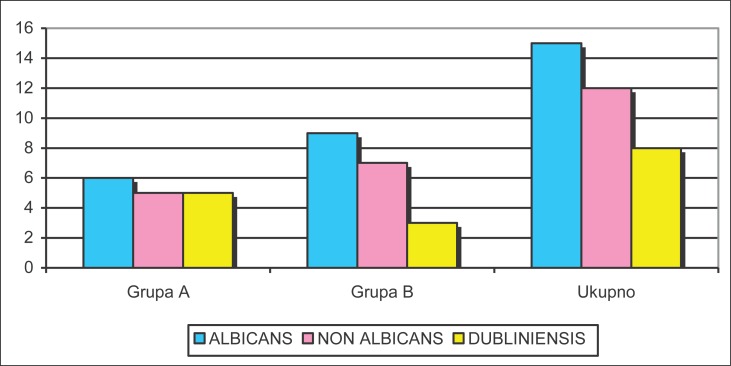
Within the examined sample, Candida albicans was present in 43% of patients. Non-albicans species were isolated in 34.3% of patients. Candida dubliniensis was identified in 23% of the patients who were addicted to psychoactive substances. The following Candida species were found in Group A (alcoholics): Candida albicans – 6 patients (37.5%), Candida tropicalis – 1 patient (6.25%), Candida glabrata – 3 patients (19%) and Candida crusei – 1 patient (6.2%). In Group B (opiate addicts), the following were found: Candida albicans – 9 patients (47.4%), Candida tropicalis – 1 patient (5.3%), Candida glabrata – 1 patient (5.3%) and Candida crusei – 5 patients (26.3%). Candida dubliniensis was identified in 8 patients–5 patients in Group A (31.25%) and 3 patients in Group B (15.8%). (Table 1, Figure 1).
Table 1.
Frequency of Candida species by groups (non-albicans species conjoined)
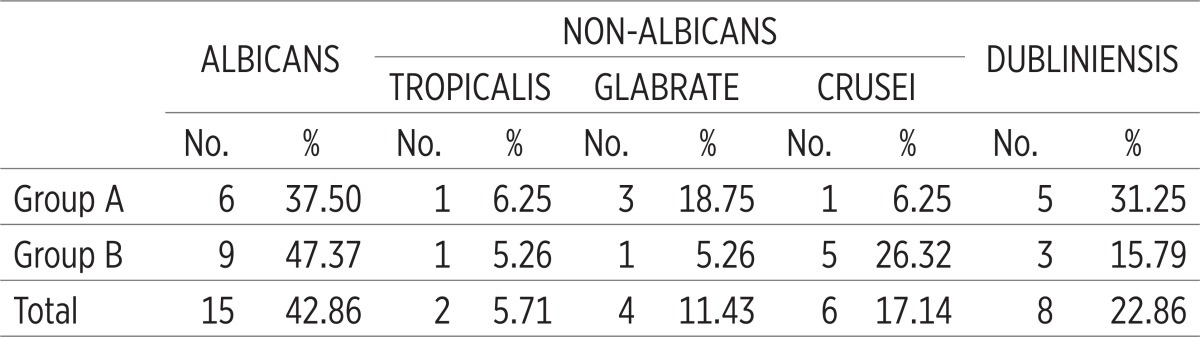 |
Figure 1.
The incidence of Candida by groups (non-albicans conjoined).
The chi-square test (p=0,55297) confirmed that there is no significant deviation in Candida incidence frequency between the two groups. Candida albicans was the most common, found in 42.86% of patients: 37.5% in Group A and 47.5% in Group B. Group B had 3 patients with confirmed Candida dubliniensis (15.8%), while there were 5 such patients (31.25%) in group A.
Candida dubliniensis was found in 8 patients, i.e. in 13.33% of all patients. 5 patients were identified in Group A (62.50%), and 9 (37.50%) in Group B. All patients in Group A found to have Candida dubliniensis consumed alcohol for more than 15 years, while in Group B the appearance of Candidae dubliniensis is shown in the first two observed time intervals. The presence of Candida dubliniensis in psychoactive substance addicts was shown (23%), and it was confirmed that the frequency of bacterial adherence of Candida dubliniensis is directly proportional to the time interval of the drug addiction. (Table 2)
Table 2.
Incidence of Candida dubliniensis in correlation with the duration of drug use
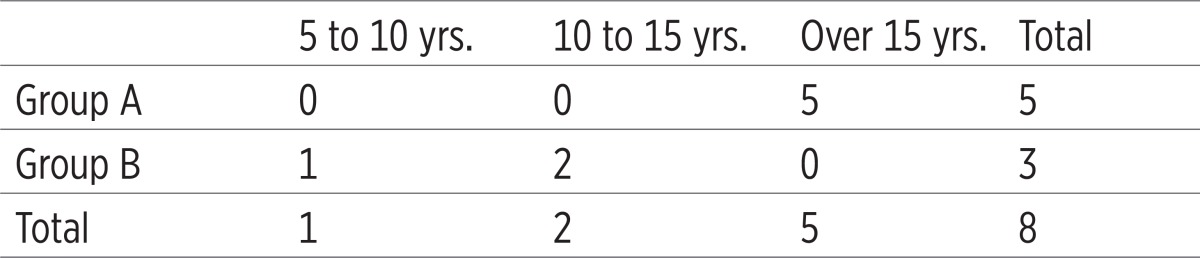 |
4. DISCUSSION
The implications of Candida species on the oral cavity are common. The Candida species is an opportunist and will lead to an infection if there are predisposing factors present. The virulence of Candida depends on the ability to adhere to the oral epithelia of the host, the production of filamentous growth, and the discharge of hydrolytic enzymes. The continued increase in fungal infection incidence, new fungal species, and resistance to antifungal therapy presented a challenge to research in this domain (11). In their work, Baradkar et al. tested the clinical and microbiological state of oral epithelia in intravenous addicts. More than 60 patients were included in the study. Candida species were found in 50 patients. The most common was Candida albicans at 70%, Candida parapsilosis at 15%, Candida Glabrata at 7.5%, Candida tropicalis at 5% and Candida dubliniensis in 1 patient. This study has shown the prevalence of oral candidiasis in intravenous addicts (12). The research of Kosalec et al. showed Candida albicans as the most commonly occurring, but also found a rise in non-albicans species (30%) (13). Immunosuppresive states and antiviral therapies are significantly linked to the increase in candidiases (14). Our research, performed on a sample of 60 patients addicted to alcohol and opiates, identified Candida albicans in 43% of patients. Group A showed 38%, while Group B showed 48%. Of the non-albicans species, the most commonly occurring was Candida crusei with 26% in Group B and 6% in Group A. Candida glabrata was present in 10% of the patients in Group A and 5.2% of Group B. Candida tropicalis was also identified and was equally distributed among both groups. The chi-squared test (p=0,55297) confirmed there is no significant deviation in the appearance of Candida between the groups. The most common is Candida albicans. Our results concur with the results of the authors cited herein. New studies show that the incidence of non-albicans species and Candida dubliniensis continues to increase. Candida dubliniensis is a fungal species whose growth was long considered to be pathognomonic to HIV infections, but its presence is now being proven in other diseases as well. Polacheck et al. have isolated Candida dubliniensis in hospitalized patients in Jerusalem. They believe the high incidence of Candida in psychoactive substance addicts is caused by the activity of alpha-adrenergic receptors in the blood vessels of salivary glands, leading to vasoconstriction and thus causing a decrease in the production of saliva (15,16). Suhail et al. confirmed a significant incidence of Candida dubliniensis in immunosuppresed patients; of the 7 isolated Candida dubliniensis samples, 5 were found in the saliva and one each in a vaginal sample and a urine sample (17). Jewtuchowicz et al. identified Candida dubliniensis in a sample of 240 patients who were alcohol and drug addicts. The incidence of Candida dubliniensis was 4.6% in the subgingival biofilm (18, 19). Our results concur with the results of the aforementioned authors on the incidence of Candida dubliniensis in HIV-negative patients addicted to psychoactive substances. Candida dubliniensis was found in 31.25% (5 patients) of patients in Group A (alcoholics) and 16% (3 patients) in Group B, which confirms that alcohol addiction with all its immunological imbalances and local condition of oral health provides an ideal medium for the development of Candida dubliniensis.
5. CONCLUSION
All of the above may lead to conclude that the abuse of psychoactive substances has an effect on the incidence of Candida species (Candida albicans and non-albicans species). The presence of Candida dubliniensis was proven in psychoactive substance addicts, regardless of the type of addiction, and a direct correlation between the frequency of bacterial adherence of Candida dubliniensis and the duration of substance addiction was confirmed as well.
Conflict of interest
None declared.
REFERENCES
- 1.Cerić I, Mehić BN, et al. Abuse of psychoactive substances and medication. Faculty of Medicine, University of Sarajevo; 2007. [Google Scholar]
- 2.Mehić BN, Cerić I, Hasečić H. Plan and Program of fighting drug abuse in FB&H; 13th International Conference of Reduction of Drug Related Harm; 3-7 March, 2002; Ljubljana. [Google Scholar]
- 3.Araujo MW, Dermen K, Connors G, et al. Oral and health among in patients in treatment for alcohol use disorders: pilot study. J Int Acad Periodontol. 2004;6(4):125–130. [PubMed] [Google Scholar]
- 4.Hamamoto DT, Rhodus NL. Journal Of Oral Disease. Vol. 15. USA: School of Dentistry, University of Minnesota; 2009. Methamphetamine abuse and dentistry; pp. 27–37. [DOI] [PubMed] [Google Scholar]
- 5.Nucci MK, Marr A. Emerging fungal disease. Clin Infect Dis. 2005;41:521–526. doi: 10.1086/432060. [DOI] [PubMed] [Google Scholar]
- 6.Mavor AL, Thewes S. Systemic fungal infections caused by Candida species: epidemiology, infection process and virulence attributes. Curr Drug Targets. 2005;6:674–863. doi: 10.2174/138945005774912735. [DOI] [PubMed] [Google Scholar]
- 7.Arjuna N, Ellepola B, Zia U. Prevalence of Candida dubliniensis among Oral Candida isolates in Patients Attending the Kuwait University Dental Clinic. Med Princ Pract. 2011;20:271–276. doi: 10.1159/000323440. [DOI] [PubMed] [Google Scholar]
- 8.Mosaid A, Sullivan DJ, Coleman DC. Differentiation of Candida dubliniensis from Candida albicans on Pal’s agar. J Clin Microbiol. 2003;41:4787–4789. doi: 10.1128/JCM.41.10.4787-4789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocchi L, D’ Alessandro D, Fabiani M. Association between oral candidiasis and immunity level in a population of drug addicts in a center for prevention and treatment of drug addictions in Roma, Italy; 17th. European Congress of Clinical Microbiology and Infectious Disease; 31 Mar–04 Apr; Germani. 2007. p. 73. [Google Scholar]
- 10.Rooban T, Rao A, Joshua E. Dental and health status in drug abusers in Chennai, India: A cross-sectional study, Department of Oral and Maxillofacial Pathology. Ragas Dental College and Hospital, Uthandi, Chennai, 600-119; 2008. p. 100. [Google Scholar]
- 11.Kempa H, Sadlk-Nowicka J, et al. Candida infections of the oral mucosa - not only a dental problem. Przgel Lek. 2006;63(59):257–260. [PubMed] [Google Scholar]
- 12.Baradkar VP, Kumar S. Species identification of Candida isolates obtained from oral lesions of HIV infected patients. Indian J Dermatol. 2009;54(4):385–386. doi: 10.4103/0019-5154.57622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kosalec I, Matica B, Jarža-Davila N, et al. Haemolytic activity of Candida albicans and Candida dubliniensis isolated from the oral cavity of immunocompromised patients. Clinical Microbiology and infection. 2006;12(supplement 4) [Google Scholar]
- 14.Cerqueira DF, Portela MB, Ponarico L, et al. Oral Candida colonisation and its relation with predisposing factors in HIV-infected children and their urinfected. siblings in Brasil: the era of highly active antretroviral therapy. J Oral Pathol Med. 2010;39(2):188–94. doi: 10.1111/j.1600-0714.2009.00857.x. 99. [DOI] [PubMed] [Google Scholar]
- 15.Gery P, David M, Coleman C, Derek J. Candida albicans versus Candida dubliniensis: Why is Candida albicans pathogenic? Int J Microbiol. 2011 doi: 10.1155/2012/205921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polacheck I, Strahilevitz J, Sullivan D, et al. Recovery of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Israel. J Clin Microbiol. 2000;38(1):170–174. doi: 10.1128/jcm.38.1.170-174.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suhail A, Zaiba K, Eiman M, Khan UZ. Isolation and molecular identification of Candida dubliniensis from non–human immunodeficiency virus-infected patients in Kuwait. Journal of Medical Microbiology. 2004;53:633–637. doi: 10.1099/jmm.0.05315-0. [DOI] [PubMed] [Google Scholar]
- 18.Jewtuchowicz VM, Mijuca MF, et al. Genetic relatedness of subgingival and buccal Candida dubliniensis isolates in immunocompetent subject assessed by RAPD-PCR. Journal of Oral Microbiology. 2009 doi: 10.3402/jom.v1i0.2003. doi:10-3402/jom.v.1io.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Sullivan EM. Prevalence of oral mucosal abnormalities in addiction treatment center residents in Southern Ireland. Oral Oncol. 2011 May;47(5):395–399. doi: 10.1016/j.oraloncology.2011.03.003. [DOI] [PubMed] [Google Scholar]