Abstract
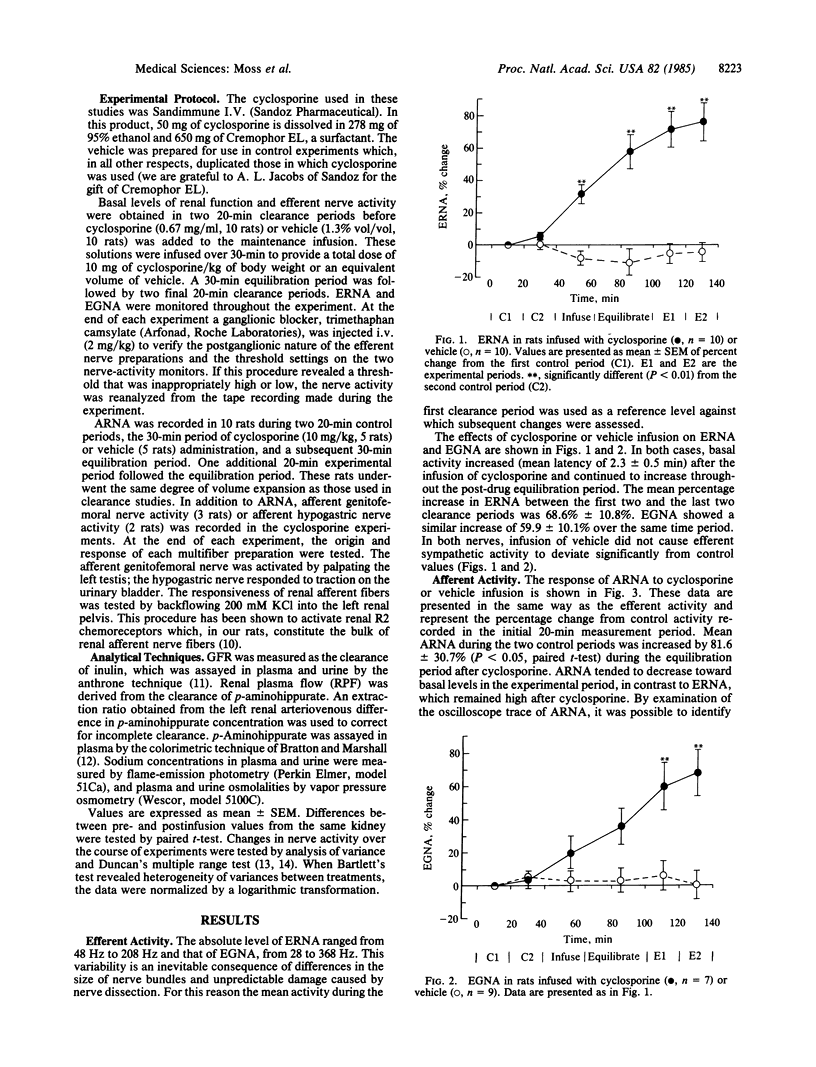
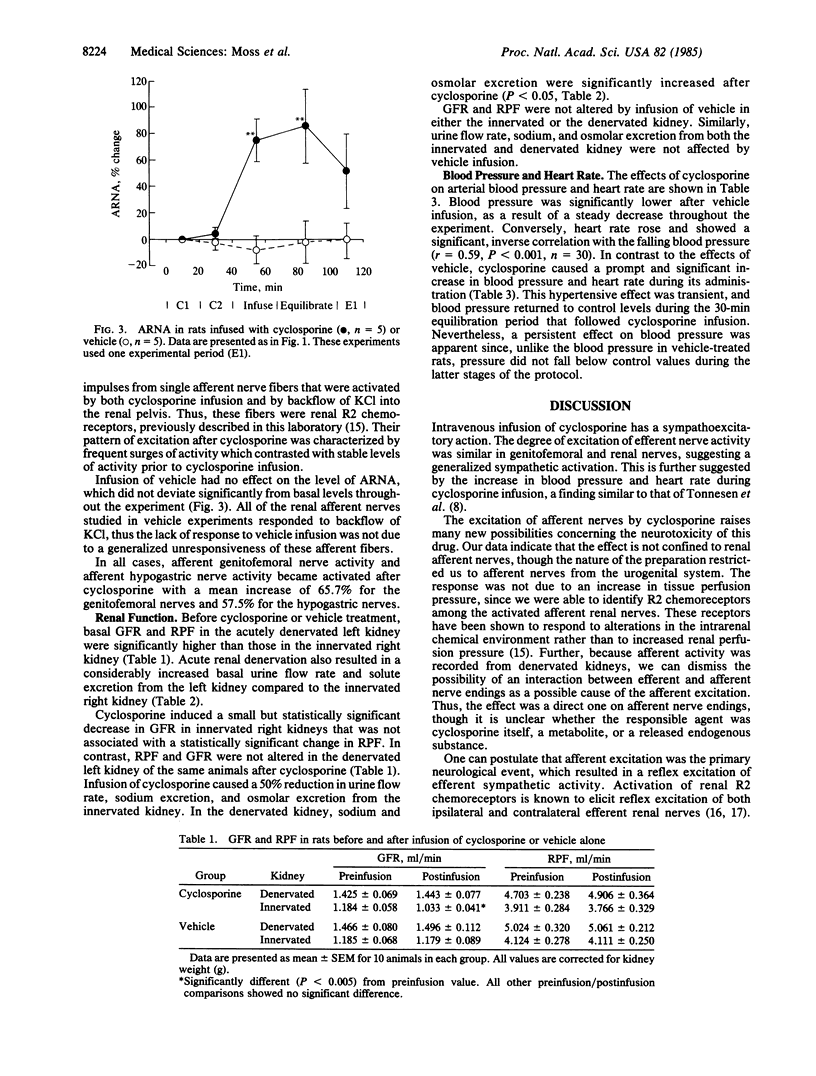
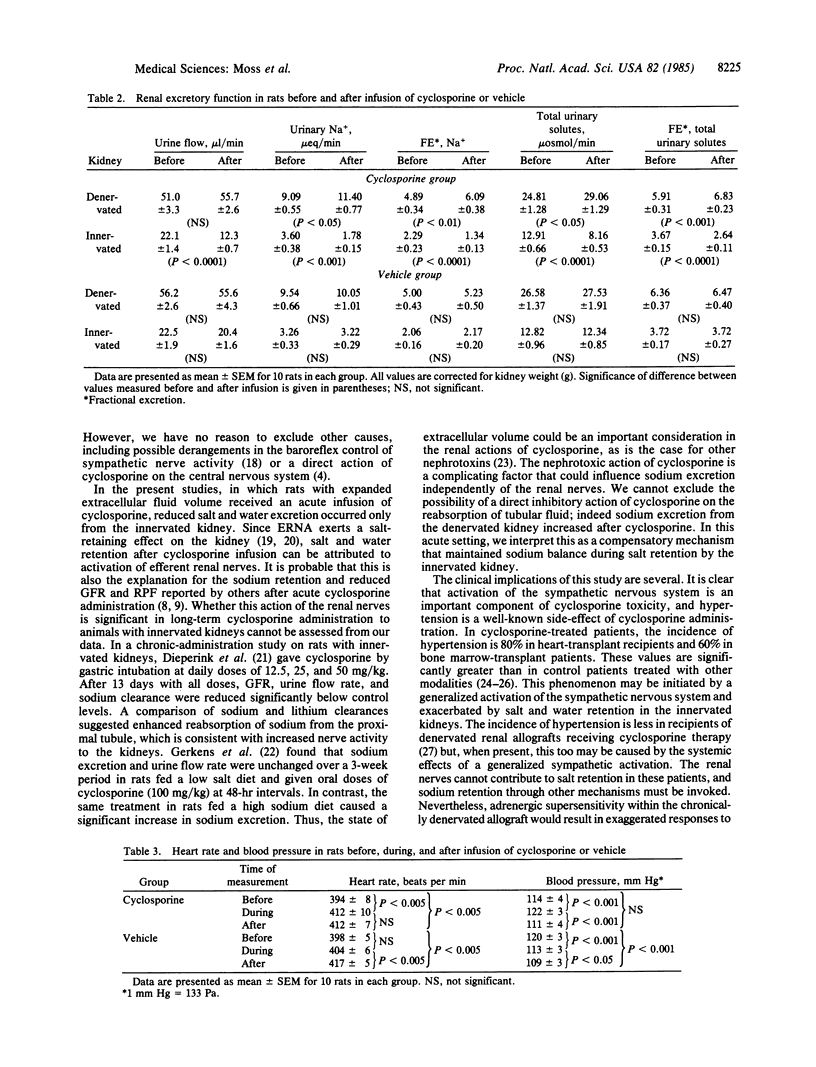
The effect of acute intravenous infusion of cyclosporine (10 mg/kg) on efferent renal and genitofemoral nerve activity and afferent renal nerve activity was studied in anesthetized rats. All animals were studied after unilateral renal denervation and extracellular fluid volume expansion. Activity of both efferent sympathetic nerves was increased significantly by cyclosporine infusion (renal, 69%; genitofemoral, 60%). Afferent renal nerve activity was increased 82% after cyclosporine (P less than 0.05). Urine flow rate and both absolute and fractional sodium excretion from the innervated kidney were reduced 50% after cyclosporine infusion (P less than 0.01). Absolute and fractional sodium excretion from the denervated kidney were significantly increased after cyclosporine. Infusion of vehicle had no significant effect on any measured variable in innervated or denervated kidneys. These studies demonstrate the capacity of cyclosporine to increase efferent sympathetic nerve activity and afferent nerve activity. It is also shown that sodium retention resulting from acute infusion of cyclosporine can be attributed to the increase in efferent renal nerve activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K., Biggs J., Darveniza P., Boland J., Concannon A., Dodds A. Cyclosporin-associated central nervous system toxicity after allogeneic bone marrow transplantation. Transplantation. 1984 Jul;38(1):34–37. doi: 10.1097/00007890-198407000-00009. [DOI] [PubMed] [Google Scholar]
- BERNE R. M., HOFFMAN W. K., Jr, KAGAN A., LEVY M. N. Response of the normal and denervated kidney to L'epinephrine and L'nor-epinephrine. Am J Physiol. 1952 Dec;171(3):564–571. doi: 10.1152/ajplegacy.1952.171.3.564. [DOI] [PubMed] [Google Scholar]
- Bell-Reuss E., Trevino D. L., Gottschalk C. W. Effect of renal sympathetic nerve stimulation on proximal water and sodium reabsorption. J Clin Invest. 1976 Apr;57(4):1104–1107. doi: 10.1172/JCI108355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett W. M., Pulliam J. P. Cyclosporine nephrotoxicity. Ann Intern Med. 1983 Dec;99(6):851–854. doi: 10.7326/0003-4819-99-6-851. [DOI] [PubMed] [Google Scholar]
- Cohen D. J., Loertscher R., Rubin M. F., Tilney N. L., Carpenter C. B., Strom T. B. Cyclosporine: a new immunosuppressive agent for organ transplantation. Ann Intern Med. 1984 Nov;101(5):667–682. doi: 10.7326/0003-4819-101-5-667. [DOI] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Gerkens J. F., Bhagwandeen S. B., Dosen P. J., Smith A. J. The effect of salt intake on cyclosporine-induced impairment of renal function in rats. Transplantation. 1984 Oct;38(4):412–417. doi: 10.1097/00007890-198410000-00019. [DOI] [PubMed] [Google Scholar]
- Gerkens J. F., Branch R. A. The influence of sodium status and furosemide on canine acute amphotericin B nephrotoxicity. J Pharmacol Exp Ther. 1980 Aug;214(2):306–311. [PubMed] [Google Scholar]
- Joss D. V., Barrett A. J., Kendra J. R., Lucas C. F., Desai S. Hypertension and convulsions in children receiving cyclosporin A. Lancet. 1982 Apr 17;1(8277):906–906. doi: 10.1016/s0140-6736(82)92171-7. [DOI] [PubMed] [Google Scholar]
- Kline R. L., Mercer P. F. Functional reinnervation and development of supersensitivity to NE after renal denervation in rats. Am J Physiol. 1980 May;238(5):R353–R358. doi: 10.1152/ajpregu.1980.238.5.R353. [DOI] [PubMed] [Google Scholar]
- Loughran T. P., Jr, Deeg H. J., Dahlberg S., Kennedy M. S., Storb R., Thomas E. D. Incidence of hypertension after marrow transplantation among 112 patients randomized to either cyclosporine or methotrexate as graft-versus-host disease prophylaxis. Br J Haematol. 1985 Mar;59(3):547–553. doi: 10.1111/j.1365-2141.1985.tb07342.x. [DOI] [PubMed] [Google Scholar]
- Recordati G. M., Moss N. G., Genovesi S., Rogenes P. R. Renal receptors in the rat sensitive to chemical alterations of their environment. Circ Res. 1980 Mar;46(3):395–405. doi: 10.1161/01.res.46.3.395. [DOI] [PubMed] [Google Scholar]
- Recordati G., Genovesi S., Cerati D. Renorenal reflexes in the rat elicited upon stimulation of renal chemoreceptors. J Auton Nerv Syst. 1982 Sep;6(2):127–142. doi: 10.1016/0165-1838(82)90046-7. [DOI] [PubMed] [Google Scholar]
- Recordati G., Moss N. G., Genovesi S., Rogenes P. Renal chemoreceptors. J Auton Nerv Syst. 1981 Apr;3(2-4):237–251. doi: 10.1016/0165-1838(81)90066-7. [DOI] [PubMed] [Google Scholar]
- Rogenes P. R. Single-unit and multiunit analyses of renorenal reflexes elicited by stimulation of renal chemoreceptors in the rat. J Auton Nerv Syst. 1982 Sep;6(2):143–156. doi: 10.1016/0165-1838(82)90047-9. [DOI] [PubMed] [Google Scholar]
- Zambraski E. J., Dibona G. F., Kaloyanides G. J. Effect of sympathetic blocking agents on the antinatriuresis of reflex renal nerve stimulation. J Pharmacol Exp Ther. 1976 Aug;198(2):464–472. [PubMed] [Google Scholar]


