Abstract
The clinical utility of serum procalcitonin (PCT) levels continues to evolve. PCT is regarded as a promising candidate marker for making a diagnosis and antibiotic stewardship in patients with systemic infections. The aim of this review is to summarize the current evidence for PCT in different infections and clinical settings, and to discuss the reliability of this marker when used with validated diagnostic algorithms.
Key words: procalcitonin, bacterial infections, antibiotic stewardship.
1. INTRODUCTION
Infectious agents are the most common causes of febrile illness (30%–40%). Therefore, in the first contact with febrile patient doctors have doubts concerning the etiology of the disease and the need for giving empirical antibiotic therapy. Only one hour of delaying adequate antimicrobial therapy increases the mortality of septic patients for 5% -10% (1, 2).
On the other hand, up to 50% of antimicrobial agents that are treated in stationary facilities are unnecessary and inappropriate (3). For example, viruses are the most common cause of bronchitis, in spite of this more than 80% of patients will get antibiotics (4). Also, the length of treatment for most infections is not clearly defined, and it is likely that antibiotic treatment often takes unusually long period (5).
The isolation of causes is considered as the golden standard of diagnosing bacterial infections and sepsis. However, due to the time required for the implementation and interpretation of the results of blood and other bacterial cultures, insufficient sensitivity (blood culture) and low specificity caused by contamination (sputum), under the modern understanding of the definition, identification of bacterial pathogen today does not present the condition that determines the diagnosis of sepsis (6). About 70% of radiologically proven pneumonia and up to 80% of clinically suspected bacteremia remain microbiologically undifferentiated (7).
In medical practice there is an emphasized tendency of doctors– clinicians to define reliable diagnostic parameters that would allow quick confirmation of bacterial infections in the early stage, when the disease manifests itself only as a vague fever, with no other general or local symptoms.
The results of researches, which in recent years have been focused on the acute phase reactants of inflammation, indicate considerable diagnostic potential of procalcitonin (PCT) in the early detection of bacterial infection and its correlation with disease severity and outcome of treatment.
What is procalcitonin?
In 1993 the French authors offered the first presentation of the usefulness of PCT in differentiating bacterial infections and non-bacterial inflammatory conditions (8). Research, intensified during the last 15 years, have confirmed its potential in the diagnosis of bacterial infection and sepsis, and have placed it among the most useful markers (9). This is the only marker that was included in the guidelines for diagnosis and therapy of sepsis by the German Sepsis Society and the German Interdisciplinary Society of Intensive Care and Emergency Medicine (10).
PCT is a peptide composed of 116 amino acid, prohormone, calcitonin precursor. In the normal state of the organism it is synthesized by C-cells of the thyroid gland and, less significantly, by neuroendocrine cell lung and small intestine (9). Serum concentrations in healthy individuals are extremely low, <0.05 ng / ml, or even immeasurable (11). In systemic inflammation, particularly in bacterial infections, under the influence of inflammatory cytokines and bacterial endotoxin, it is produced in a number of tissues (lung, liver, kidney, adipose tissue) and goes in circulation, when its level can increase up to 1000 times (9,12). The first measurable values are found even 2-4 hours after stimulation, and the maximum within 6-24 hours (9,12). In comparison, the level of CRP (C-reactive protein) starts to rise 12-24 hours after stimulation, reaching a maximum value after 48 hours (9). PCT is a stable marker, whose concentration is not affected by neutropenia, immunodeficiency conditions and the use of nonsteroid and steroid anti-inflammatory drugs, which is not the case with CRP (7). The level of PCT follows the intensity of the inflammatory response and the severity of infection, so the increase in concentration or persistence of high values is considered as prognostic indicator for severe forms of the disease with an adverse outcome (13). Depending on the method used, the level of PCT in the patient’s blood can be determined in a range from 19 minutes to 2.5 hours (10, 12). The concentration of circulating PCT is cut in halves 24 hours since the beginning of controlling the infection, either with the immune system or a prescribed antibiotic (14), which makes it an indicator of effectiveness of applied therapy. It has been shown that the inclusion of PCT in therapeutic guidelines reduce the use of antibiotics, with no negative effects on the final outcome of the disease, both in inpatient and outpatient practice (15, 16).
PCT is a biomarker that has diagnostic and therapeutic significance in various indication areas, but only if it is used in combination with other relevant laboratory parameters, as a supplement to detailed clinical observation of doctors (17).
2. DIAGNOSTIC IMPORTANCE OF PROCALCITONIN
Fever of unknown etiology
The role of serum PCT in the early triage of febrile illness is still not clearly defined.
The group of Spanish authors analyzed 52 patients hospitalized in an internal department for vague fever. They failed to prove discriminatory importance of PCT in the global differentiating bacterial from febrile conditions of other etiology. As a possible explanation they mentioned etiological heterogeneity of “febrile conditions” and clinical variations of “bacterial infections” which included severe bacteremia with high PCT, as well as localized infections with normal values of PCT. However, in subgroup analysis, significantly higher PCT values were in the group of patients with infectious diseases (PCT 1.012 ng / ml, 95% CI, 0.342 to 1.683), compared to an immune inflammatory conditions (PCT 0.187 ng / ml, 95% CI , 0-0.427), and in subjects with bacterial, compared to the viral febrile illness (double Welch t-test t = 2.76, DF = 37.423, p = 0.009) (18).
Meningitis
Several studies have examined the usability of PCT in acute meningitis. In a prospective study of Viallona and associates (19), among 254 patients with an acute form of meningitis, it is proved that the concentration of lactate in the cerebrospinal fluid and serum procalcitonin were the most reliable parameters in differentiating between viral and bacterial meningitis with negative direct-microscopic findings of cerebrospinal fluid (17). Sensitivity of 95% and specificity of 100%, NPV 100%, PPV 97% (“area under the receiver operating characteristic curve”– AUC, 0.99, 95% CI, 0.99 to 1) have been demonstrated for the PCT cut off of 0.28 ng / ml. Jimenez et al (20) emphasized the importance of the PCT (> 2ng/ml) and CRP (> 90mg / L) in the etiological differentiation of acute meningitis with atypical CSF findings and negative results of the Gram stain, especially with patients who were partially antibiotic treated and who had no symptoms of systemic infection).
Respiratory infections
Diagnostic, prognostic and therapeutic options of PCT are often analyzed in respiratory tract infections.
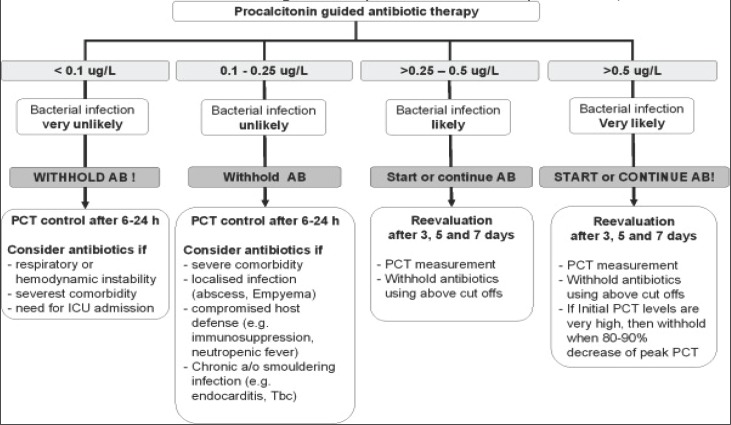
Berg and Lindhart, in a meta-analysis from 2012 (21), dealt with the results of 31 studies that examined the importance of PCT in community acquired pneumonia (CAP). Based on the collected data, they confirmed that the PCT level ≥ 0.25 ng / ml is a diagnostic threshold that suggests probable bacterial pneumonia, and requires additional microbiological analysis, in order to have exact detection of pathogens. PCT > 0.5 ng / ml is an indicator of more serious, complicated forms of the disease, with an increased risk of fatal outcome. PCT values <0.01 ml / mg definitely exclude bacterial pneumonia, even in cases with positive radiological findings (Fig 1). The level of PCT in bacterial pneumonia show significantly higher values compared to the atypical and viral pneumonia, which do not differ by the concentration of PCT, and follows the severity of CAP in accordance with the relevant scales such as CPSI (Clinical Pulmonary Infection Score) and Curb-65 (Confusion, Urea> 7 mmol / L, Respiratory rate> 30, SBP <90mmHg, DBP <60mmHg, Age> 65).
Figure 1.
Antibiotic stewardship based on procalcitonin (PCT) cut-off ranges, adopted from (35).
Due to the low percentage of positive results (from 3% to 10%), the usefulness and cost-effectiveness of routine blood culture testing in hospitalized patients with CAP is questionable. In order to rationalize the blood culture, Muller et al (22) examined in the prospective study the importance of PCT as a predictor of CAP bacteremia. Out of 925 patients with CAP, 73 (7.9%) of them had positive blood cultures. In the group of positive blood cultures the values of PCT were almost 15 times higher (5.8 ng / ml vs 0.4 ng / ml). PCT showed the highest diagnostic accuracy in predicting bacteremia (AUC 0.82, 95% CI, 0.78 to 0.87) in relation to other compared laboratory and clinical markers (L, urea, CRP, temperature, systolic blood pressure, pulse, renal dysfunction). PCT <0.25 ng / ml identified the patients with a low risk of bacteremia and excluded the need for taking blood culture which, among other things, also had financial significance. PCT level> 0.5 ng / ml, and in particular the PCT> 1ng/ml, suggested bacteremia with a high degree of probability, resulting in a useful aggressive diagnostic approach and antibiotic treatment.
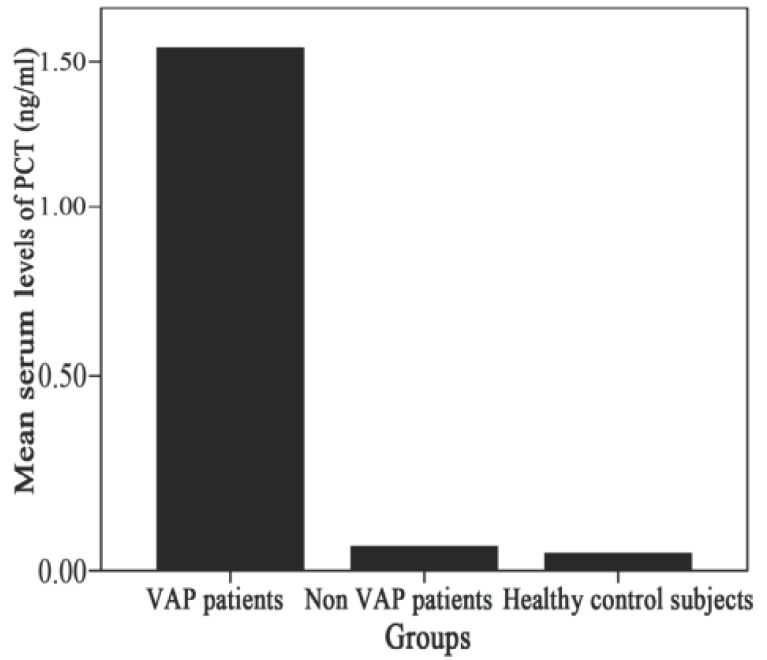
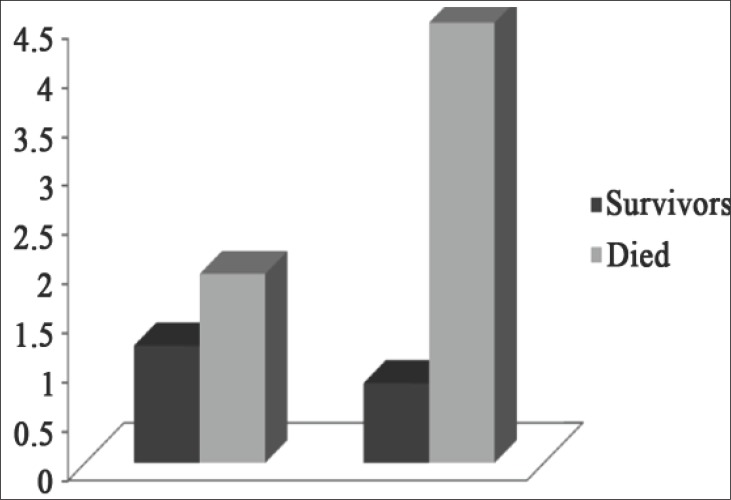
Ventilator associated pneumonia (VAP) occurs in 28% of patients, and has a high mortality rate (27% -76%). Clinical symptoms and chest radiogram in the first phase of VAP development are not typical, and etiological significance of microbial isolates from tracheobronchial secretion is questionable, with regard to the expected colonization of respiratory pathogens. El Halim et al (23) compared the mean values of PCT in the group of VAP-patients (n = 42), with values in patients on mechanical ventilation without VAP (n = 20) and control group of healthy patients (n = 20). They demonstrated a significantly higher PCT values (p <0.001) in the VAP group (1.54 ± 0.50 ng / ml), compared to patients without VAP (0.06 ± 0.03 ng / ml) and patients of control group (0.04 ± 0.02 ng / ml) (Fig. 2). Mean values of PCT measured first (1.92 ± 0.41 ng / ml vs. 1.19 ± 0.29 ng / ml) and five days after the appearance of VAP (4.49 ± 0.59 ng / ml vs 0.81 ± 0.21 ng / ml) were significantly higher (p <0.001) in the group of VAP patients who died (n = 20), compared to the VAP patients who recovered (n = 22) (Fig. 3). The authors concluded that the increase of serum procalcitonin is an important diagnostic marker in identifying and predicting the outcome of VAP, and its usefulness is confirmed by the result of many other studies (23, 24, 25).
Figure 2.
Comparison between the different subject groups re-garding initial PCT levels (ng/ml), adapted from (23). PCT 1st day PCT 5th day.
Figure 3.
Comparison between the survivor and died groups among VAP patients (first and 5th days respectively), adapted from (23).
Urinary tract infections
Manifest urinary tract infections of adults in 20% of cases are complicated bacteremia, which prolongs hospital treatment and increases the risk of fatal outcome from 0.3% to 7.5-30%. This explains the interest of clinicians to diagnose and treat complicated urinary tract infections as soon as possible. In a prospective observational multicenter study, which involved 581 adult participants, Nieuwkoop et al (26) used three different models for diagnosing urine bacteremia. They found that a model, in which the only criterion of bacteremia was PCT value > 0.25 ng / ml, had the best diagnostic performance (sensitivity 0.95, 95% CI: 0.89 to 0.98, specificity 0.50, 95% CI: 0.46 to 0.55). At the same time, this diagnostic approach, as well as in research that is related to the infection of lower respiratory tract (22) resulted in a lower number of taken blood cultures for 40%, by which each examinee could save $ 36, while maintaining the percentage of detection bacteremia for 94-99%. They proved that a shorter time required for the isolation of bacteria from blood cultures followed higher PCT values, thus suggesting the importance of PCT as an indicator of high bacterial load (viral-load).
Burns
Infections are major problem for therapies of extensive burns. It is believed that burns themselves lead to an increase in the value of the PCT, which is why it is not used as a marker of infection in these conditions. The research of Russian authors announced new diagnostic capabilities of PCT. Budkevich et al (27) showed that there was no increase of PCT> 0.5 ng / ml in the first 24 hours of the trauma, in a group of 50 children (6 months to 14 years old), with burns without affection of the respiratory tract. They also found that the growth of PCT in cases of burns without inhalation lesions is a reliable sign of bacterial complications. By the pathohistological analysis of the burn samples, they found that the depth of the propagation of infection in traumatized tissue fairly accurately correlates with the increase of serum procalcitonin, where they additionally emphasized its diagnostic value in identifying the infectious process.
Blood stream infections
Kim et al (28) have analyzed the level of PCT in group of 300 febrile patients, where the largest number had localized infection (137), 58 patients had proven bacteremia, 90 patients had a temperature of non-infectious etiology, while in 15 cases cause of fever could not be determined. The authors have shown that the values of PCT were significantly higher in patients with bacteremia, compared to non-bacteremia patients (11.9 ± 25.1 vs 2.5 ± 14.7 ng / ml, p <0.001) with AUC 0.753 in relation to 0,696. At the same time, the value of CRP showed no significant difference in these groups (p = 0.298). The sensitivity, specificity, PPV and NPV for the nine cut-off values of PCT showed the highest NPV (95.4%) for the cut-off level of PCT <0.4 ng / ml, which reliably excluded the diagnosis of bacteremia. The sensitivity and specificity for the PCT cut-off of 0.5 ng / ml were 74.2% and 70.1%.
In a prospective study of authors from Albania (29), the importance of the PCT, CRP and total leukocyte count (L) are compared among 99 patients who on admission in emergency department (ED) and intensive care unit (ICU) had at least two of the five symptoms of SIRS (Systemic inflammatory response syndrome). According to the clinical findings and the available diagnostic procedures, without the results of the markers of inflammation, the examinees have been selected in a group with a high risk for sepsis (60 patients) and in a group with no suspicion of sepsis (39 patients). The mean PCT value for the first group was 11.128 ng / ml, compared to 0.272 ng / ml in the second group of patients. The sensitivity and specificity of the cut-off value of PCT of 0.5 ng / ml for differentiating SIRS and sepsis were 97.4% and 96.6%, while the value of PCT of 2 and > 2 showed 100% specificity in the confirmation of sepsis diagnosis. The authors assume that, because of the high sensitivity and specificity, PCT is a useful marker in their management of septic patients. They also proved significant correlation (p 0,001) of high values of PCT with the severity of the disease, which was determined by APACH II or SOFA score.
Prognostic value of procalcitonin
The PCT values have prognostic implications, considering the fact that they correlate with bacterial load and the severity of infection (30). Dynamics of the PCT value movement may be a predictor of disease outcome. Ruiz-Rodriguez et al (31) applied a new concept of monitoring the dynamics by determining the PCT clearance (PCT-c). They measured PCT in severe septic patients 12 hours after the appearance of septic shock symptoms (SS) and MODS-a (multi-organ dysfunction), and followed its movements in survivors, after 24, 48 and 72 hours, at the same time determining PCT-c. They concluded that the persistence of PCT high values is a predictor of unfavorable outcome of the disease, and that the movement dynamics is more credible indicator than individual initial values of PCT.
Therapeutic significance of procalcitonin
Previous studies have confirmed that the inclusion of PCT in therapeutic guidelines of bacterial infections effectively and safely reduces unnecessary administration of antibiotics and duration of antibiotic treatment, with no adverse effects on the ultimate outcome of the disease (6).
Christ-Crain et al (32) published the results of a study conducted among 302 patients with CAP, in which the control group subjects (151) had antibiotic treatment according to the usual clinical practice, while patients in PCT group (151) had treatment according to the value of the PCT (PCT <0.1 ng / ml and < 0.25 ng / ml–“no antibiotic”; PCT > 0.5 ng / ml– “mandatory antibiotic”). In the PCT group there was significantly reduced antibiotic exposure, primarily at the expense of individual shortening the duration of antibiotic therapy, on average, from 12 to 5 days. According to laboratory and clinical parameters the outcome of treatment in both groups was similar.
The significance of the PCT algorithm for the treatment of lower respiratory tract infections was confirmed by the results of a large multicenter randomized controlled studies of ProHOSP (33) with 1359 respondents, according to which the average antibiotic exposure in the PCT group was lower by 35%, with an approximate rate of mortality, admission on intensive care unit and the percentage of infection recurrence.
Studies that are focused on the intensive care unit, evaluate the role and importance of PCT as an indicator for the ending of antibiotic treatment of patients with clinical signs of recovery. In a large multicenter French study (PRORATA) with over 600 patients in the ICU with symptoms of sepsis or septic shock, the examinees of PCT group are significantly shorter treated with antibiotics (11.6 ± 8.2 vs 14.3 ± 9.1), without being inferior to the control group in comparison to mortality and relapse percentage (15).
The influence of PCT to rationalize antibiotic therapy was examined by Maravic-Stojkovic et al. (34) in a prospective study which included 205 cardiac patients in the early stages of recovery after open heart surgery. Antibiotics were given to 47% patient of the standard and 19% of PCT group. There was no significant difference in the length of treatment in the intensive care unit and the mortality rate in PCT and standard patients. The cost mean value of antibiotics per subject in the PCT group was 193.3 ± 636.6 euros (€) and 372.1 ± 841.1 € in the standard group, while the mean cost value per hospital day was 8.0 ± 18.4 € in PCT and 17.8 ± 36.3 € in the standard group. The authors believe that the antibiotic treatment, based on measuring the concentration of PCT, is reliable and as such provides a significant reduction in the cost of treatment after cardiac surgeries.
3. CONCLUSION
The PCT is a reliable marker that contributes to the early diagnosis of invasive bacterial infections and evaluation of their severity and prognosis. Sequenced way of measuring PCT provides objective data which, along with clinical evaluation, form the basis on which the PCT guidelines of antibiotic therapy is based. Respectable number of randomized controlled trials confirmed that treating lower respiratory tract infections and sepsis, according to the PCT antibiotic stewardship, reduces antibiotic exposure, without compromising safety of patients and the treatment outcome. Inclusion of PCT in diagnostic and therapeutic protocols of bacterial infections reduces the cost of treatment and the emergence of multi-resistant strains of bacteria. The impression is that methodologically valid studies on the possible use of PCT in surgical and gynecological infections are missing, as well as reports on the results of the application of PCT algorithm in “real life”, with daily medical practice, outside clinical studies.
Conflict of interest
None declared.
REFERENCES
- 1.Reinhart K, Meisner M. Biomarkers in the Critically Ill Patient: Procalcitonin. Crit Care Clin. 2011;27(2):253–263. doi: 10.1016/j.ccc.2011.01.002. doi:10.1016/j.ccc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Gille-Johnson P, Hansson E.K, Gardlund B. Clinical and laboratory variables identifying bacterial infection and bacteraemia in the emergency department. Scand J Infect Dis. 2012;44(10):745–752. doi: 10.3109/00365548.2012.689846. doi:10.3109/00365548.2012.689846. [DOI] [PubMed] [Google Scholar]
- 3.John JF, Fishman NO. Programmatic role of the infectious diseases physician in controlling antimicrobial costs in the hospital. Clin Infect Dis. 1997;24:471–85. doi: 10.1093/clinids/24.3.471. [DOI] [PubMed] [Google Scholar]
- 4.Wenzel RP, Fowler AA. Acute bronchitis. N Engl J Med. 2006;355:2125–2130. doi: 10.1056/NEJMcp061493. [DOI] [PubMed] [Google Scholar]
- 5.Hayashi Y, Paterson DL. Strategies for reduction in duration of antibiotic use in hospitalized patients. Clin Infect Dis. 2011;52:1232–1240. doi: 10.1093/cid/cir063. [DOI] [PubMed] [Google Scholar]
- 6.Schuez P, Albrich W, Mueller B. Procalcitonin for diagnosis of infection guide to antibiotic decisinon: past, present and future. BMC Medicine. 2011;9:107. doi: 10.1186/1741-7015-9-107. http://www.biomedcentral.com/1741-7015/9/107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz P, Christ-Crain M, Muller B. Procalcitonin and other biomarkers to improve assessments and antibiotic stewardship in infections – hope for hype? Swiss Med WKLY. 2009;139(23-24):318–326. doi: 10.4414/smw.2009.12584. www.smw.ch . [DOI] [PubMed] [Google Scholar]
- 8.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341(8844):515–518. doi: 10.1016/0140-6736(93)90277-N. PMID: 8094770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agency for Healthcare Research and Quality. Effective Health Care Program. EPC Project. Project Title: Procalcitonin for diagnosis and Management of Sepsis. Research protocol. 2011. Apr, Available at:http://www.effectivehealthcare.ahrq.gov .
- 10.Kosanke R, Beier W, Lipecky R, Meisner M. Clinical Benefit of Procalcitonin. Tanaffos. 2008;7(1):14–18. [Google Scholar]
- 11.Suberviola B, Castellanos-Ortega A, Gonzalez-Castro A, Garcia-Astudillo LA, Fernandez-Miret B. Prognostic value of procalcitonin, C-reactive protein and leukocytes in septic shock. Med Intensiva. 2012;36(3):177–184. doi: 10.1016/j.medin.2011.09.008. doi: 10.1016/j.medin.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Chan T, Gu F. Early Diagnosis of Sepsis Using Serum Biomarkers. Expert Rev Mol Diagn. 2011;11(5):487–496. doi: 10.1586/erm.11.26. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson S, Heikkinen M, Pettila V, Alila S, Vaisanen S, Pulkki K, Kolho E, Ruokonen E. Predictive value of procalcitonin decrease in patients with sever sepsis: a prospective observational study. Critical Care. 2010;14(6):205. doi: 10.1186/cc9327. doi:10.1186/cc9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schuettz P, Chiappa V, Briel M, Greenwald JL. Procalcitonin Algorithms for Antibiotic Therapy Decisions. Arch Intern Med. 2011;171(15):1322–1331. doi: 10.1001/archinternmed.2011.318. [DOI] [PubMed] [Google Scholar]
- 15.Bouadma L, Luyt C, Tubach F, Cracco C, Alvarez A, Schwebel C, et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. doi:10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 16.Albrich CW, Dusemund F, Bucher B, Meyer S, Thomann R, Kuhn F, Bassetti Sprenger M, et al. Effectiveness and Safety of Procalcitonin-Guided Antibiotic Therapy in Lower Respiratory Tract Infections in “Real Life”. Arch Intern Med. 2012;172(9):715–722. doi: 10.1001/archinternmed.2012.770. [DOI] [PubMed] [Google Scholar]
- 17.Jongwutiwes U. Procalcitonin; A New Marker for Bacterial Infection. Siriraj Med J. 2007;59(7):389–394. [Google Scholar]
- 18.Ruiz-Esteban R, Sarabia PR, Delgado EG, Aguado CB, Cuervo-Arango JA, Varela M. Procalcitonin and C-reactive protein levels as diagnostic tools in febrile patients admitted to a General Internal Medicine ward. Clinical Biochemistry. 2012;45:22–25. doi: 10.1016/j.clinbiochem.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Viallon A, Desseigne N, Marjollet O, Birynczyk A, Belin M, Guyomarch S, Borg J, Pozetto B, Bertrand JC, Zeni F. Meningitis in adult patients with a negative direct cerebrospinal fluid examination: value of cytochemical markers for differential diagnosis. Critical Care. 2011;15(3):136. doi: 10.1186/cc10254. doi: 10.1186/cc10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Julián-Jiménez A, Chacartegui MF, Palomo de los Reyes MJ, Brea-Zubigaray S. Usefulness of procalcitonin and C-reactive protein in acute meningitis in the emergency department. Neurolgia. 2013;28:189–190. doi: 10.1016/j.nrl.2011.09.009. doi: 10.1016/j.nrleng.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Berg P, Lindhardt ØB. The role of procalcitonin in adult patients with community-acquired pneumonia – a systematic review. Dan Med J. 2012;59(3):A4357. [PubMed] [Google Scholar]
- 22.Müller F, Christ-Crain M, Bregenzer T, Krause M, Zimmerli W, Mueller B, Schuetz P. Procalcitonin Levels Predict Bacteremia in Patients With Community-Acquired Pneumonia. Chest. 2010;138(1):121–129. doi: 10.1378/chest.09-2920. [DOI] [PubMed] [Google Scholar]
- 23.El Halim AA, Attia A, Zytoun T, Salah HE. The Diagnostic and Prognostic Value of Serum Procalcitonin among Ventilator Associated Pneumonia Patients. OJRD. 2013;3:73–78. doi:10.4236/ojrd.2013.32012. [Google Scholar]
- 24.Seligman R, Seligman BGS, Teixeira PJZ. Comparing the accuracy of predictors of mortality in ventilator-associated pneumonia. J Bras Pneumol. 2011;37(4):495–503. doi: 10.1590/s1806-37132011000400012. http://www.jornaldepneumologia.com.br . [DOI] [PubMed] [Google Scholar]
- 25.Charles PE, Kus E, Aho S, Prin S, Doise JM, Olsson NO, Blettery B, Quenot JP. Serum procalcitonin for the early recognition of nosocomial infection in the critically ill patients: a preliminary report. BMC Infectious Diseases. 2009;9:49. doi: 10.1186/1471-2334-9-49. doi:10.1186/1471-2334-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Nieuwkoop C, Bonten TN, van´tWout JW, Kuijper EJ, Groeneveld GH, Becker MJ, Koster T, Wattel-Louis GH, Delfos NM, Ablij HC, Leyten EMS, van Dissel JT. Procalcitonin reflects bacteremia and bacterial load in urosepsis syndrome: a prospective observational study. Critical Care. 2010;14:206. doi: 10.1186/cc9328. doi:10.1186/cc9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Budkevich LI, Lecmanov AU, Kolokolchikova EG, Soshkina VV. Comparing the Morphological Changes in Burn Wound Tissues and the Procalcitonin Concentration. International Journal of BioMedicine. 2013;3(1):23–26. [Google Scholar]
- 28.Kim MH, Lim G, Kang SY, Lee WI, Suh JT, Lee HJ. Utility of Procalcitonin as an Early Diagnostic Marker of Bacteremia in Patients with Acute Fever. Yonsei Med J. 2011;52(2):276–281. doi: 10.3349/ymj.2011.52.2.276. doi: 10.3349/ymj.2011.52.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beqja-Lika A, Bulo-Kasneci A, Refatllari E, Heta-Alliu N, Rucaj- Barbullushi A, Mone I, Mitre A. Serum Procalcitonine Levels as an Early Diagnostic Indicator of Sepsis. Mat Soc Med. 2013;25(1):23–25. doi: 10.5455/msm.2013.25.23-25. doi: 10.5455/msm.2013.25.23-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuetz P, Haubitz S, Mueller B. Do sepsis biomarkers in the emergency room allow transition from bundled sepsis care to personalized patient care? Curr Opin Crit Care. 2012;18:328–354. doi: 10.1097/MCC.0b013e328354b2c8. doi:10.1097/MCC.0b013e328354b2c8. [DOI] [PubMed] [Google Scholar]
- 31.Ruiz-Rodríguez JC, Caballero J, Ruiz-Sanmartin A, Ribas VJ, Pérez M, Bóveda JL, Rello J. Usefulness of procalcitonin clearance as a prognostic biomarker in septic shock. A prospective pilot study. Med Intensiva. 2012;36(7):475–480. doi: 10.1016/j.medin.2011.11.024. doi:10.1016/j.medin.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 32.Christ-Crain M, Stolz D, Bingisser R, Muller C, Miedinger D, Huber PR, Zimmerli W, Harbarth S, Tamm M, Muller B. Procalcitonin Guidance of Antibiotic Therapy in Community-acquired Pneumonia. A Randomized Trial. Am J Respir Crit Care Med. 2006;174:84–93. doi: 10.1164/rccm.200512-1922OC. doi: 10.1164/rccm.200512-1922OC. [DOI] [PubMed] [Google Scholar]
- 33.Schuetz P, Christ-Crain M, Thomann R, et al. Effect of Procalcitonin- Based Guidelines vs Standard Guidelines on Antibiotic Use in Lower Respiratory Tract Infections: The ProHOSP Randomized Controlled Trial. JAMA. 2009;302(10):1059–1066. doi: 10.1001/jama.2009.1297. doi:10.1001/jama.2009.1297. [DOI] [PubMed] [Google Scholar]
- 34.Maravić-Stojković V, Lausević-Vuk Lj, Jović M, Ranković A, Borzanović M, Marinković J. Procalcitonin-based therapeutic strategy to reduce antibiotic use in patients after cardiac surgery: a randomized controlled trial. Srp Arh Celok Lek. 2011;139(11-12):736–742. doi: 10.2298/SARH1112736M. [PubMed] [Google Scholar]
- 35.Schuetz P, Christ-Crain M, Wolbers M, Schild U, Thomann R and the ProHOSP study group. Procalcitonin guided antibiotic therapy and hospitalization in patients with lower respiratory tract infections: a prospective, multicenter, randomized controlled trial. BMC Health Services Research. 2007;7:102. doi: 10.1186/1472-6963-7-102. doi:10.1186/1472-6963-7-102. [DOI] [PMC free article] [PubMed] [Google Scholar]