Abstract
Context
A marked lack of empathy is a hallmark characteristic of individuals with psychopathy. However, neural response associated to empathic processing has not yet been directly examined in psychopathy especially in response to the perception of other people in pain and distress.
Objective
To identify potential differences in patterns of neural activity in incarcerated psychopaths and incarcerated controls during the perception of empathy-eliciting stimuli depicting other people in pain.
Design
In a case-control study, brain activation patterns elicited by dynamic stimuli depicting individuals being harmed and facial expression of pain were compared between incarcerated psychopaths and incarcerated controls.
Setting
Participants were scanned in on the grounds of a correctional facility using the Mind Research Network's mobile 1.5 T MRI system.
Participants
Eighty incarcerated males were classified according to scores on the Hare Psychopathy Checklist-Revised (PCL-R) as high (n = 27; PCL-R =30), intermediate (n = 28; PCL-R between 21–29), or low (n = 25; PCL-R ≤20) on psychopathy.
Main Outcome Measure
Neuro-hemodynamic response to empathy-eliciting dynamic scenarios revealed by functional magnetic resonance imaging.
Results
Psychopaths exhibited significantly less activation in the ventromedial prefrontal cortex, lateral orbitofrontal cortex, and periaqueductal gray relative to controls, but showed greater activation in the insula.
Conclusion
In response to pain cues expressed by others, psychopaths exhibit deficits in vmPFC and OFC regardless of stimulus type, but display selective impairment in processing facial cues of distress in regions associated with cognitive mentalizing.
INTRODUCTION
Psychopathy is a personality disorder characterized by affective and interpersonal deficits as well as social deviance and poor behavioral control. As measured by the Hare Psychopathy Checklist-Revised (PCL-R),1 psychopathy is comprised of interpersonal, affective (Factor 1), and lifestyle and antisocial features (Factor 2) features. The interpersonal/affective component of psychopathy is largely defined by a lack of empathy, attachment, and a callous lack of regard for others.2 Importantly, empathy, the natural capacity to share and understand the affective states of others,3 is at the heart of the first of the disorder's two core components.
The construct of empathy is complex and involves social, emotional, and motivational facets.3–5 A primary component of empathy, empathic sensitivity (or empathic arousal) refers to the automatic sharing of the affective states of others, and is a crucial prerequisite to the experience of empathic concern (i.e., another-oriented emotional response congruent with the perceived welfare of someone in need).4 Inter-connected subcortical regions including brainstem, amygdala, and hypothalamus, and cortical regions like the insula, orbitofrontal cortex (OFC) and ventromedial prefrontal cortex (vmPFC) form the essential neural circuit of empathy.3–5 Empathic sensitivity is a phylogenetically ancient and basic form of intraspecies communication, and it is the first component of empathy to develop in children.4,6,7 The vicarious sharing of another's negative state provides a strong signal which can promote empathic concern, and the lack of such signals during development can impede the process of normal socialization.7,8 To be motivated to help another, one needs to be affectively, empathically aroused, and to anticipate the cessation of mutually-experienced personal distress.9,10 Empathic sensitivity may thus serve as a catalyst in promoting empathic concern for others: the lack of this signal would make the engagement of empathic concern and prosocial behavior much less likely.4, 11
The perception of others' pain or physical distress usually acts as a prosocial signal, notifying others that their conspecific is at risk, attracting their attention and motivating helping behavior,12 and has become a fruitful avenue to investigate the neural mechanisms underpinning affective processing and empathy.13
In healthy participants, functional magnetic resonance imaging (fMRI) studies of empathy have demonstrated reliable activation of a neural network that overlaps substantially with regions engaged when one experiences pain and when one perceives, anticipates or even imagines pain happening to others. 6,8,13–19 The activated neural network includes the anterior insular cortex (AIC), dorsal anterior cingulate cortex (dACC), anterior midcingulate cortex (aMCC), supplementary motor area (SMA), amygdala, periaqueductal gray (PAG), and vmPFC.20
The neural response to the distress of others, such as pain, is thought to reflect an aversive response in the observer, which may act as a trigger to inhibit aggression or prompt motivation to help.3–8 Hence examining the neural response of psychopaths as they view individuals being hurt or expressing pain may be an effective probe into the neural processes underlying affective and empathic deficits in psychopathy.
So far no fMRI study has investigated the neural response to empathy-eliciting stimuli in incarcerated psychopaths. Previous research showed that individuals with psychopathy understand the emotional state of others without `sharing' their feelings or being aroused by their emotional states.21–23 Thus one can anticipate different hemodynamic response in the neural network involved in the perception of pain between individuals with psychopathy, especially for participants scoring high on the PCL-R. An alternative hypothesis draws on research showing that children and adolescents with callous–unemotional traits are reward-oriented, insensitive to punishment cues, lack emotional responsiveness to distress cues and may show both reactive and instrumental aggression.24 In support of this hypothesis, one study found that male adolescent offenders with high callous-unemotional traits exhibit atypical neural dynamics of pain empathy processing (measured with event-related brain potentials) in the early stages of affective arousal, coupled with relative insensitivity to actual pain.23 Another neuroimaging study also documented strong activation of the amygdala (as well as the pain network), which correlated with a measure of sadism, in youth with aggressive conduct disorder when they observed people being hurt.25
To investigate the neural mechanisms underlying empathy for pain in adults with psychopathy, 80 incarcerated male volunteers were scanned using fMRI, stratified into three groups. Participants classified as psychopaths (n=27) were those who scored 30 or above on the PCL-R (out of a possible 40), those classified as intermediate (n=28) scored between 21–29, and volunteers scoring 20 or below (n=25) were classified as lowpsychopathy controls. The well-matched groups from the prison population are used to isolate differences due to psychopathy and eliminate confounds possible in the direct comparison of incarcerated psychopaths with community controls.
Further, the inclusion of participants from across the scoring spectrum allowed us to investigate differences at a groupwise and at a continuous level, using both PCL-R total and Factor 1 and 2 scores. The neuro-hemodynamic activity was measured while participants attended to visual scenarios depicting individuals being physically hurt and dynamic facial expressions of pain, as these stimuli have been used in numerous fMRI studies investigating the neural underpinnings of empathy for pain in healthy children, adolescents and adults.6,8,13–19,23,25–30 Moreover, having two sets of stimuli, i.e., pain interactions (two person interacting without the faces of the protagonists) and facial expressions of pain may help us identify which component of empathy is dysfunctional in psychopathy. The former class of stimuli requires a cognitive understanding of a social interaction with a negative outcome, which is associated with the engagement of the network supporting mental state inference and the perception of pain in others,8 while the latter also induces activation in the OFC and vmPFC, prefrontal regions that play a pivotal role in adaptive responses to emotionally relevant situations and the production of an affective state.31,32
METHODS
Participants
Eighty adult males incarcerated in a medium-security North American correctional facility between the ages of 18 and 50 volunteered for the research study and provided informed consent to the procedures described here, which were approved by the Institutional Review Boards of the University of New Mexico and the University of Chicago. Volunteers underwent the PCL-R, including file review and interview, conducted by trained research assistants under the supervision of Dr. Kiehl. Those scoring 30 and above on the PCL-R were assigned to the high-psychopathy group (n=27). To create the low- and medium-psychopathy groups, two groups of volunteers matched to high scorers on age, race and ethnicity, IQ, comorbidity for DSM-IV Axis II disorders,1,33 and past drug abuse and dependence, from volunteers scoring at or below 20 on the PCL-R (n=28) and volunteers scoring between 21 and 29 (n=25), respectively. The sample size for each group was determined by a power analyses based on prior studies by Dr. Kiehl. Participants were paid one dollar per hour for their participation in the study, a typical rate for institutional labor compensation.
MRI Acquisition
Scanning was conducted on a 1.5 Tesla Siemens Magnetom Avanto mobile unit equipped with advanced SQ gradients and a twelve element head coil. Functional images were collected using an EPI gradient-echo pulse sequence with TR/TE = 2000/39 ms, flip angle = 90°, field of view = 240×240 mm, matrix = 64×64 cm, in-plane resolution = 3.4×3.4 mm, slice thickness = 5mm, and 30 slices, full-brain coverage. Task presentation was implemented using the commercial software package E-Prime (Psychology Software Tools, Inc., Pittsburgh PA).
High-resolution T1-weighted structural MRI scans were acquired using a multiecho MPRAGE pulse sequence (repetition time = 2530 ms, echo times = 1.64 ms, 3.50 ms, 5.36 ms, 7.22 ms, inversion time = 1100 ms, flip angle = 7°, slice thickness = 1.3 mm, matrix size = 256 × 256) yielding 128 sagittal slices with an in-plane resolution of 1.0 mm × 1.0 mm.
Task design
Participants completed two counterbalanced tasks to assess neural processes involved in empathy through the observation of individuals experiencing pain.
Pain Interactions Task
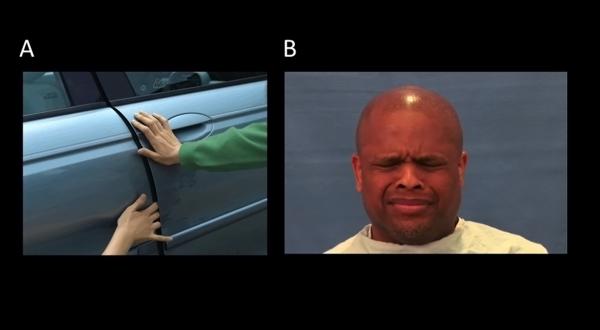
In this task, used previously in several fMRI studies,6,8,14,25 participants viewed ninety-six short dynamic visual stimuli depicting persons harming one another, presented in a pseudo-randomized rapid event-related design (Figure 1A). Timing parameters were generated using Optimize Design.34 To verify the participants' attention to the task, eight randomized trials were followed with the question, “Did a person in the previous picture feel pain?”, after which the subject had 6 seconds to answer by pressing the correct button.
Figure 1.
In the Pain Interactions task, 48 visual scenarios depicting pain and 48 control scenarios without pain were used. Each scenario consisted of a three-part frame capture taken from videos of live actors, presented at the rate of 1000ms, 200ms, and 1000ms to simulate biological motion (A for an example of the last frame). The scenarios depicted people intentionally harming another by striking, cutting, pinching, crushing, etc, the hands, feet, arms, legs, fingers, or toes. Control stimuli included sequences in which two people interacted, but no harm or pain occurred. No heads or faces were visible in the scenarios. Data were collected in two runs of seven minutes each.
In the Pain Expression Task (B for an example), video clips showed a natural pain response in which individuals displayed brow lowering, orbit tightening, and either cursing/pressing of the lips or mouth opening/stretching. These movements have consistently been attributed to the facial expression of pain. After eight of the clips, participants were asked whether the previous clip had featured a male or a female subject. Data in this task were acquired in one eight-minute run.
Pain Expressions Task
The second functional task examined neural responses during the viewing of dynamic facial expressions of pain (Figure 1B). Participants were presented with sixty-four video clips of expression stimuli 2.2-seconds in duration, interspersed with thirty-two instances of a dynamically scrambled baseline stimulus.
Image processing and analysis
The functional images were processed using SPM8 (Wellcome Department of Imaging Neuroscience, London, UK) in Matlab (Mathworks Inc., Sherborn, MA, USA). For each participant, functional data were realigned to the first image acquisition of the series and re-sampled to a voxel size of 2×2×2 mm3. Structural T1 images were co-registered to the mean functional image and segmented using the `New Segment' routine. A group-level structural template and individual flow fields were created using DARTEL, and the flow fields were in turn were used to spatially normalize functional images to standard MNI space. Data were smoothed with an 8 mm full-width at half maximum (FWHM) isotropic Gaussian kernel. Ten participants were eliminated from further analysis due to image quality issues related to movement or image quality, leaving N=70 (n=22, 24, 24 for low, intermediate, and high psychopathy, respectively).
Statistics were calculated at the first level using the general linear model. The design matrix included three regressors for each stimulus category (detailed above), representing the event onsets and their time and dispersion derivatives. Movement parameters from the realignment output were included as regressors of no interest. All participants were entered into two second-level pooled analyses (one for the Pain Interactions task and one for the Pain expressions task), and full brain results were reported at a statistical cutoff of FWE-corrected p<0.05.
Second level analyses were conducted by comparing the extremes of the sample distribution of PCL-R scores, and then as a continuous regressor using the entire sample. Participants with PCL-R total score at or above 30 were selected for the psychopathy group, while participants scoring at 20 or below comprised the incarcerated control group. For these analyses, regions of interest (ROIs) were created from the existing literature. For the pain interactions task, coordinates for ROIs were taken from previous fMRI studies that used the same task paradigm utilized here6,8,14,25 and from a meta-analysis of 32 fMRI studies of empathy for pain.20 For the pain expressions task, coordinates were taken from studies that reported functional neuroimaging results for the perception of facial expressions of pain.27–30,35,36 ROI data are reported for significant contrast image peaks within 8mm of these a priori coordinates. Beyond existing literature on the processing of empathy-inducing stimuli in healthy populations, there may be additional cortical or subcortical brain regions that contribute to abnormal processing of these regions in psychopathy. For this reason, additional regions of note that survive statistical cutoff of p<0.001 uncorrected and a spatial extent threshold of k=100 voxels are also reported in the groupwise analysis.
To explore whether results found in the groupwise analysis may be due to PCL-R factor 1, factor 2, or both, the regions reported above were tested for significant correlation with PCL-R factor scores. Corresponding t-values for subfactor covariates within 5mm of the ROIs above, if significant, were reported for each factor and task.
RESULTS
A summary table including regions of interest for all tasks is included in the manuscript as Table 1. Additional detailed results are included as supplementary Tables where noted.
Table 1.
Groupwise results and Factor subscore correlations (MNI coordinates).
| Pain Interactions Task | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Region of interest | x | y | z | peak T | Factor 1 | Peak T | Factor 2 | Peak T | |||||
| Controls > Psychopaths | |||||||||||||
|
| |||||||||||||
| R | ventromedial prefrontal cortex | 2 | 16 | −28 | 3.45 | 4 | 16 | −26 | 2.62 | 4 | 16 | −26 | 2.40 |
| R | lateral orbitofrontal cortex | 26 | 70 | −12 | 2.69 | n.s. | n.s. | ||||||
| L | periaqueductal gray | −6 | −30 | −12 | 2.56 | −6 | −30 | −14 | 2.60 | −6 | −30 | −12 | 2.90 |
|
| |||||||||||||
| Psychopaths > Controls | |||||||||||||
|
| |||||||||||||
| R | inferior frontal gyrus | 56 | 10 | 6 | 3.37 | 56 | 10 | 6 | 3.46 | 54 | 16 | 16 | 2.40 |
| L | inferior frontal gyrus | −50 | 14 | 14 | 2.46 | −54 | 18 | 14 | 2.84 | n.s. | |||
| R | dorsomedial prefrontal cortex | 6 | 58 | 16 | 2.60 | n.s. | n.s. | ||||||
| R | dorsal anterior cingulate gyrus | 4 | 50 | 16 | 2.41 | 6 | 38 | 20 | 2.84 | n.s. | |||
| L | dorsal anterior cingulate gyrus | −8 | 38 | 26 | 2.21 | −6 | 32 | 32 | 2.72 | n.s. | |||
| R | anterior midcingulate cortex | 2 | 16 | 28 | 3.13 | 6 | 8 | 26 | 2.49 | n.s. | |||
| R | supplementary motor area | 8 | 18 | 54 | 2.46 | 12 | 8 | 58 | 2.43 | n.s. | |||
| R | anterior insula | 36 | 16 | −8 | 3.65 | 34 | 16 | −6 | 3.31 | 36 | 16 | −6 | 3.36 |
| L | anterior insula | 36 | 16 | −8 | 2.52 | n.s. | n.s. | ||||||
| R | Post. superior temporal sulcus | 60 | −46 | 18 | 3.65 | 60 | −44 | 14 | 3.40 | 58 | −46 | 22 | 2.82 |
| L | supramarginal gyrus | −54 | −42 | 28 | 2.85 | −54 | −40 | 26 | 2.96 | 60 | −38 | 44 | 2.71 |
| L | dorsal striatum (globus pallidus) | −14 | 8 | 2 | 2.98 | −12 | 8 | 4 | 3.49 | n.s. | |||
| R | dorsal striatum (globus pallidus) | 14 | 4 | −2 | 2.56 | 16 | 2 | 0 | 3.67 | n.s. | |||
| Pain Expressions Task | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Region of interest | x | y | z | peak T | Factor 1 | Peak T | Factor 2 | Peak T | |||||
| Controls > Psychopaths | |||||||||||||
|
| |||||||||||||
| R | inferior frontal gyrus | 50 | 5 | 15 | 4.32 | 52 | 5 | 15 | 3.61 | 50 | 5 | 15 | 3.94 |
| L | inferior frontal gyrus | −38 | 5 | 25 | 3.69 | −38 | 5 | 25 | 3.41 | −35 | 7 | 25 | 3.40 |
| R | ventromedial prefrontal cortex | 8 | 30 | −10 | 3.25 | n.s. | n.s. | ||||||
| R | lateral orbitofrontal cortex | 30 | 55 | −10 | 3.51 | 30 | 55 | −10 | 3.05 | n.s. | |||
| R | dorsomedial prefrontal cortex | 8 | 58 | 35 | 2.39 | n.s. | n.s. | ||||||
| L | dorsomedial prefrontal cortex | −10 | 40 | 45 | 2.61 | −8 | 42 | 40 | 2.41 | −7 | 37 | 42 | 3.26 |
| L | dorsal anterior cingulate gyrus | −2 | 35 | 5 | 2.38 | n.s. | 5 | 32 | 15 | 2.59 | |||
| L | middle cingulate cortex | −18 | 8 | 42 | 4.26 | −18 | 8 | 45 | 3.96 | −15 | 7 | 42 | 4.06 |
| R | post. superior temporal sulcus | 42 | −40 | 2 | 3.05 | n.s. | 50 | −65 | 12 | 3.37 | |||
| L | post. superior temporal sulcus | −52 | −72 | 20 | 3.02 | −50 | −42 | −2 | 2.77 | n.s. | |||
| R | supramarginal gyrus | 68 | −25 | 38 | 3.05 | n.s. | 55 | −45 | 27 | 2.69 | |||
| R | inferior parietal lobule | 52 | −65 | 28 | 3.60 | 52 | −65 | 28 | 3.27 | n.s. | |||
| L | inferior parietal lobule | −50 | −77 | 32 | 2.54 | −47 | −72 | 42 | 2.33 | −47 | −75 | 35 | 2.37 |
| R | dorsal striatum (globus pallidus) | 15 | 8 | −2 | 2.43 | n.s. | 15 | 7 | 5 | 2.48 | |||
| R | dorsal striatum (putamen) | 28 | 12 | 12 | 3.24 | n.s. | 27 | 15 | 12 | 3.04 | |||
|
| |||||||||||||
| Psychopaths > Controls | |||||||||||||
|
| |||||||||||||
| R | anterior insula | 28 | 28 | 0 | 3.25 | 28 | 32 | −2 | 3.04 | n.s. | |||
| L | anterior insula | −32 | 30 | 0 | 2.83 | −32 | 28 | 0 | 3.26 | −32 | 30 | 0 | 2.59 |
Perceiving pain interactions
When participants viewed dynamic stimuli depicting individuals being physically injured, significant signal increase was detected in a number of clusters surviving a statistical cutoff of FWE-corrected p<0.05, which were located bilaterally in the AIC, dACC, aMCC, SMA, supramarginal gyrus and inferior parietal lobule, precuneus and posterior cingulate gyrus. Significant subcortical activations were also seen bilaterally in the thalamus and globus pallidus. At a slightly relaxed cutoff of p<0.0001 with a spatial extent threshold of k=100 voxels, additional activations were seen in the amygdala, orbitofrontal cortex (OFC), and vmPFC (eTable 1 for full results).
Participants from within the pooled analysis were selected from the extremes of the PCL-R total score distribution to comprise a psychopathy (PCL-R score ≥30) and a control group (PCL-R ≤ 20). At a cutoff threshold of p<0.05, corrected for family wise error (FWE) for a priori regions of interest, control participants had greater activation in PAG, vmPFC, and lateral OFC (eTable 2). High-scoring psychopaths exhibited greater activation in a priori regions including the SMA, dACC, bilateral AIC, dorsal striatum, inferior frontal gyrus (IFG), medial prefrontal cortex, pSTS, postcentral gyrus, and supramarginal gyrus (eTable 3).
Correlations with PCL-R
To examine the extent to which the results seen in the group-wise comparison were driven by scores on factor 1 (representing deficits in affective and interpersonal components) or factor 2 (measuring deficits in behavioral controls and impulsivity) of the PCL-R, each cluster was tested for significant correlations with PCL-R factor scores. For those clusters more active in the control group, three were significantly, negatively correlated with both factor scores: PAG, the vmPFC, and the superior temporal pole. Activity in the lateral OFC was not significantly correlated with either factor score (eTable 4).
For those clusters found to be significantly more active in the psychopath group, several were significantly correlated with both factor scores, including both right AIC, right IFG, right pSTS, right superior frontal gyrus, right dorsomedial prefrontal cortex (dmPFC), and left precuneus. Several clusters were correlated only with Factor 1 scores (but not significantly correlated with Factor 2 scores), including the right SMA, bilateral dorsal ACC, bilateral dorsal striatum, IFG, and somatosensory cortex (eTable 5).
Perceiving facial expressions of pain
In the pain expressions task, participants showed robust hemodynamic activation in the face network of expected cortical and subcortical brain regions during the perception of facial expression of pain.37 At the full-group level (n=70) clusters (FWE-corrected p<0.05) were detected bilaterally in the fusiform gyrus, occipital regions, pSTS, and IFG. At a slightly relaxed cutoff of p<0.0001 with a spatial extent threshold of k=100 voxels, additional activations were seen bilaterally in the AIC, aMCC, right hemisphere parietal regions, thalamus, and striatum (eTable 6 for full results).
In direct comparison between groups, control participants had greater activation bilaterally in the IFG, middle cingulate cortex, angular gyrus, putamen, pSTS, supramarginal gyrus, dmPFC, globus pallidus, and dorsal ACC. At a relaxed whole-brain cutoff of p<0.001, uncorrected, additional clusters of greater activation in the control group were observed in vmPFC and medial OFC (eTable 7). Psychopaths exhibited greater activation in a priori regions in the AIC, postcentral gyrus, inferior parietal lobule, and precentral gyrus (eTable 8).
PCL-R correlations
A number of clusters found to be significantly more active in the control group, including the middle cingulate cortex, IFG, dmPFC, and left angular gyrus, were negatively correlated with both factor 1 and 2 scores. The right angular gyrus and left pSTS were correlated with Factor 1 only, and right STS, dorsal ACC, and striatum were correlated with Factor 2 only (eTable 9). In the reverse direction, AIC activity was positively correlated with both factor 1 and 2 scores. Two clusters from left postcentral gyrus and right precentral gyrus were correlated with factor 1 scores only (eTable 10).
Between-task comparisons
Three regions mentioned previously were congruent, between tasks, in the direction of differences between psychopaths and controls, while four others were different but task-dependent. Psychopaths had significantly greater activation than controls while viewing others in pain in the AIC, whether the pain was in the form of facial expressions or people interacting (Figure 2). Controls, conversely, had greater activation than psychopaths during each task in the right vmPFC and the right lateral OFC (Figure 3). In addition, four regions were more active in psychopaths for painful interactions, but more active in controls for facial expressions of pain. This pattern was observed in the dmPFC, angular gyrus, pSTS, and IFG (Figure 4).
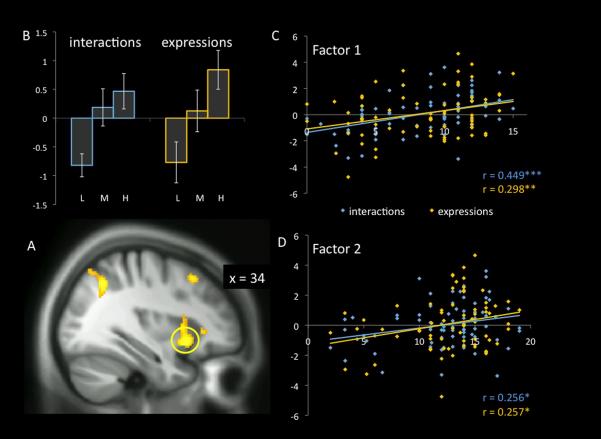
Figure 2.
Groupwise and continuous measures of hemodynamic response in the right anterior insular cortex. BOLD response increased as a function of the degree of psychopathy (as measured by the PCL-R) during the viewing of both types of empathy-eliciting stimuli, interactions in which one person caused pain to another (interactions) and facial expressions of pain (expressions). (A) Anatomical location of cluster of interest (circled), superimposed on the sample-specific DARTEL-normalized T1 template. (B) Histogram of responses of all participants stratified into three groups. L= low-psychopathy (PCL-R total score ≤20), M=intermediate (PCL-R above 20 but below 30), H=high psychopathy (PCL-R ≥30). Values used for (B-D) are the contrast estimates per subject averaged over the 3mm sphere centered on the cluster peak at MNI [26, 28, %#x2212;8], from the contrast of scenarios with pain/harm content versus scenarios with no pain versus dynamic baseline stimuli in the “expressions” task. Error bars are +/− the standard error of the mean. (C,D) The groupwise effects seen in (B) are expanded to examine the contribution of continuous Factor 1 (C) and Factor 2 (D) PCL-R sub-scores, representing the Affective/Interpersonal and Lifestyle/Behavioral features of psychopathy, respectively. *, p<0.05; **, p<0.01; ***, p<0.001.
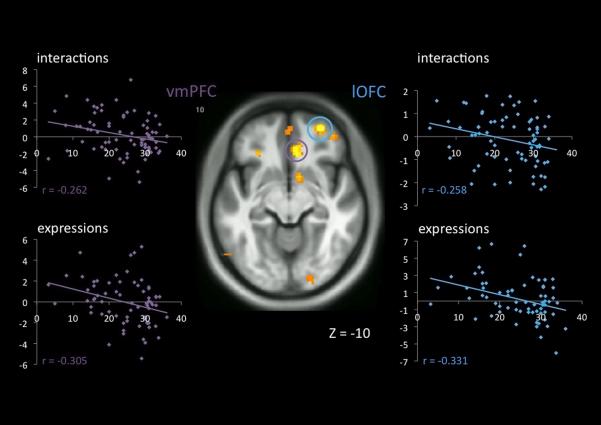
Figure 3.
Neuro-hemodynamic activity in the ventromedial prefrontal cortex (vmPFC) and lateral orbitofrontal cortex (lOFC) decreases as a function of total psychopathy score during the viewing of two types of empathy-eliciting stimuli, interactions in which one person caused pain to another (“interactions”) and facial expressions of pain (“expressions”). At the center, the clusters illustrated in the figure are indicated on the study-specific T1 template, circled in violet for the vmPFC and blue for the lOFC. At left and right, per-subject contrast estimates averaged over the 3-mm sphere surrounding the peak voxel in each cluster (MNI [8, 30, −10] for the vmPFC and [42, 48, −12] for the lOFC) are expanded for the entire (n=70) sample as a function of PCL-R total score.
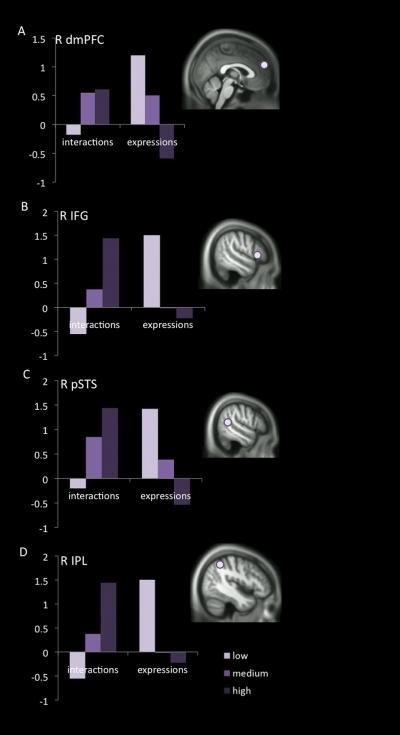
Figure 4.
The direction of differences among PCL-R stratified groups was stimulus-type dependent in four regions of interest. In (A) the dorsomedial prefrontal cortex (dmPFC), (B) the inferior frontal gyrus (IFG), (C) the posterior superior temporal sulcus (pSTS), and (D) the inferior parietal lobule (IPL), a pattern was identified in which BOLD signal during the passive viewing of empathy-eliciting stimuli increased or decreased as a function of degree of psychopathy depending on the type of stimuli being viewed. In each of these regions, activity was greater in psychopaths when viewing people interacting, resulting in pain to one person (“interactions”) but reduced in psychopaths when viewing facial expressions of people in pain (“expressions”). MNI coordinates for all clusters of interest are listed in Table 1.
DISCUSSION
In order to better understand the deficits in socioemotional information processing in individuals with psychopathy, the current study used two classes of stimuli that have been extensively employed during the past decade to chart out the neural networks underpinning empathy in healthy adults and children.6,8,13,14,27–30
In the pooled analyses of all participants, collapsed across PCL-R scores (n=70), expected patterns of activation were observed during perception of people being hurt and facial expressions of pain. The former elicited activity in the AIC, dACC, aMCC, amygdala, and SMA, and latter recruited activity in the fusiform gyrus, AIC, pSTS, and IFG.
There were significant differences, however, in a number of brain regions engaged between the two extreme groups. When viewing people being hurt, psychopaths showed greater activation in the AIC, as well as in the dorsal striatum, dmPFC and pSTS, three regions involved in the cognitive dimension of mentalizing.38 Control participants showed greater signal increase in the PAG, vmPFC and lateral OFC, a circuit with reciprocal connections with the amygdala and hypothalamus involved in the regulation and mediation of emotional and affective behavior.9,10
When viewing facial expressions of pain, fusiform gyrus activity was equivalent between groups. The high-scoring psychopath group again displayed greater activation bilaterally in the AIC. However in this case, low-scoring incarcerated control participants had greater activation than psychopaths in regions involved in both emotional and cognitive aspects of mentalizing, including vmPFC, OFC, pSTS, dmPFC, IPL, dACC, and dorsal striatum.
The amplified involvement of the AIC in participants with psychopathy is surprising due to the well documented role of this region in the experience of empathy (Figure 2). The AIC is polysensory cortex involved in mapping internal states of bodily and subjective feeling. With extensive reciprocal connections with limbic forebrain areas,39 it is the most consistently-activated region across all studies of empathy for pain,20 even when there is no explicit cognitive demand to empathize with another individual.40 Moreover, gray matter reduction has been observed in the insula in individuals scoring high on psychopathy,41 although the stereotaxic coordinates are different between their study and ours (posterior insula vs. anterior insula respectively). A previous fMRI study of empathy with children with aggressive conduct disorder (CD) and psychopathic tendencies, using similar stimuli, reported similar findings. 25 Increase activity was detected in the AIC as well as reduced response in the OFC when children with CD were presented with stimuli depicting others in pain. Importantly, a recent case study reported a patient who, despite a complete destruction of the insula, experiences all aspects of feelings and emotions including empathy.42 This indicates that the role of the insula in emotion and empathy is complex and far being understood. In addition, it has been proposed from network analysis that the insula and ACC form the core of a network that facilitates the detection of important environmental stimuli.43 The pSTS and medial prefrontal cortex are part of the cognitive mentalizing network (processing intentions and understanding social interaction), and have been reported in previous research using the similar stimuli.8,14 The augmented involvement of these regions, including the AIC, in individuals with psychopathy supports a cognitive assessment strategy of these scenarios rather than an affective processing.
Relative to participants with psychopathy, controls showed greater activation in the orbital and ventromedial prefrontal cortex when perceiving individuals being injured as well as during facial expressions of pain (Figure 3). This result is in agreement with the affective neuroscience literature on psychopathy. These regions, important for monitoring ongoing behavior, estimating consequences and incorporating emotional learning into decision-making, have consistently been featured in theories of psychopathy, and remain the most common prefrontal regions implicated in neuroimaging investigations of the condition.21,22 Structural and functional deficits in the vmPFC and OFC have been reported in individuals with high psychopathic traits and criminal convictions.21,22,43–47 The fundamental role of the OFC in empathy is supported by fMRI studies of healthy children6,8,49,50 and adults,20,51 and by brain lesions in neurological patients.31,32,52,53 Of particular interest, one recent study examined affective versus cognitive theory of mind processing in criminal offenders diagnosed with antisocial personality disorder with high psychopathy features as well as participants with localized lesions in the OFC or dorsolateral prefrontal cortex.54 The authors found that individuals with psychopathy and those with OFC lesions were impaired on the affective but not cognitive dimension of theory of mind.
Major task-dependent differences were found between groups in four brain regions (Figure 4). The dmPFC, IPL, pSTS, and IFG were significantly less active during the viewing facial expression of pain in the psychopath group, but significantly more active than controls while watching individuals hurting others. According to one network model,38 ventral regions such as the OFC and vmPFC are recruited to process affective aspects of mentalizing, while dorsal regions such as the dmPFC, ACC, and dorsal striatum are recruited for cognitive mentalizing, and pSTS and IPL are engaged in either. When dealing with either faces or social interactions, empathy-eliciting stimuli lead to a significantly dampened response in the affective mentalizing regions in the psychopath group. However, cognitive mentalizing areas were selectively impaired in the faces task only. This pattern of results suggests not an overall deficit in the theory of mind network but a stimulus class-specific failure of this network to be triggered by facial expressions of pain.
Interestingly, there were differential contributions of PCL-R factor 1 and factor 2 scores to the differences uncovered in the groupwise analysis. In previous research, PCL-R factor 1 and 2 have been demonstrated to differentially contribute to abnormalities in brain function in functional imaging assessments of criminal psychopaths.55 In the current study, clusters that were more active in the control group than the psychopath group were generally correlated either with both factor scores or with factor 2 scores only. Conversely, clusters that were more active in the psychopathy group were influenced mainly by factor 1 scores. Further, between the two tasks used in the current investigation, the direction of differences between groups was unequally distributed. When looking at facial expressions of pain, the bulk of differences seen between groups were deficits in the psychopathy group, and were driven to a greater extent by Factor 2, whereas when looking at pain interactions, the bulk of differences observed were in the direction of greater activation in the psychopath group, and these differences were driven to a greater extent by Factor 1. This is particularly interesting in light of research regarding the relationship of Factor 1 scores to instrumental aggression in psychopathy.56 Instrumental or predatory aggression is controlled, purposeful aggression used to attain a desired external goal, and in multiple studies involving adult and adolescent psychopaths instrumental aggression has been linked more strongly to Factor 1 scores on psychopathy than to Factor 2.57,58 Factor 1 items include conning and manipulation, lying, glibness, and superficial charm, skills by which psychopaths may achieve external goals through selfish interactions with others. Thus, greater Factor 1-related activity when watching social interactions resulting in harm may reflect a propensity for or interest in this type of behavior. Facial expressions of pain, devoid of any additional contextual information, may not be sufficient to engage similar patterns of processing. Hence in the current Pain Expressions task, the pattern of deficits in the psychopath group related to both factors or to Factor 2 alone may be a purer measure of deficits in empathic sensitivity.
Overall, the results indicate that the major difference in pattern of brain response between psychopaths compared to controls during the perception of others in pain is the lack of engagement of regions in the brainstem and OFC/vmPFC. Animal research has clearly shown that the ability to share and be affected by the emotional state of another is organized by basic systems subserving attachment-related processes, involving the brainstem, thalamus, and paralimbic areas.3,59 The OFC/vmPFC are essential for being able to represent a particular reward or punishment level with an object, and integrating mental representations with affective value. Such interplay between basic affective mechanisms and higher order computations in the OFC plays a crucial role in the experience of empathy and feeling concern for others. Future work is necessary to elucidate the respective contribution of the lateral and medial aspects of the OFC and connectivity with brainstem nuclei in psychopathy.
Limitations of the study
Our study has several limitations that are worth noting. First, the tasks used here explicitly focused on passive viewing of empathy-eliciting stimuli, and as such do not permit assessments of explicit cognitive and behavioral responses. These tasks were selected because they have been extensively employed in neuroimaging studies with typically developing children and adults, and reliably document a network involved in processing distress cues. No tasks, however, can capture the entire range of affective, cognitive, and behavioral components of what the concept of empathy emcompasses,3–6,60 and links between empathic sensitivity, as studied here, and downstream behavioral sequelae, remain to be investigated in this population. A second limitation may stem from the absence of sufficient amygdala activation in either task to allow assessment of deficits in this region in the psychopaths as anticipated by the extant literature.22,47 Bilateral amygdala activation was observed in the pooled results of the Pain Interactions task, but power was not sufficient to detect significant activation in the pooled Pain Expressions task, or in any groupwise analysis. Activity in the amygdala is frequently but not always detected in response to the distress or pain of others in healthy participants (see20 for a meta-analysis), so in assessing this region it may be of particular importance when working with incarcerated populations to use stimuli that are sufficiently salient, perhaps requiring the creation of materials that are more extreme in both valence and arousal than those used in typical populations.
Supplementary-Material
Acknowledgements
This study was supported by NIMH R01 grant 1R01MH087525-01A2 (J. Decety, PI) and by NIMH R01 grant MH070539-01 and NIDA 1R01DA026505-01A1 (K. Kiehl, PI).
Dr. Decety takes full responsibility for the integrity of the data and the accuracy of the data analysis. All authors had full access to all the data in the study.
Footnotes
Dr. Decety, Dr. Skelly, and Dr. Kiehl have no conflicts of interest to disclose.
References
- 1.Hare RD. The Hare Psychopathy Checklist: Revised. Multi-Health Systems; New York: 2003. [Google Scholar]
- 2.Hare RD. Without conscience: The disturbing world of the psychopaths among us. Guilford; New York: 1999. [Google Scholar]
- 3.Decety J, Norman GJ, Berntson GG, Cacioppo JT. A neurobehavioral evolutionary perspective on the mechanisms underlying empathy. Progress in Neurobiology. 2012;98:38–48. doi: 10.1016/j.pneurobio.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Decety J, Sveltova M. Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental Cognitive Neuroscience. 2012;2:1–24. doi: 10.1016/j.dcn.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Light S, Zahn-Waxler C. Nature and forms of empathy in the first years of life. In: Decety J, editor. Empathy from Bench to Bedside. MIT Press; Cambridge: 2012. pp. 109–130. [Google Scholar]
- 6.Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev Sci. 2010;13:886–899. doi: 10.1111/j.1467-7687.2009.00940.x. [DOI] [PubMed] [Google Scholar]
- 7.Blair RJR. A cognitive developmental approach to morality: Investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- 8.Decety J, Michalska KJ, Akitsuki Y. Who caused the pain? A functional MRI investigation of empathy and intentionality in children. Neuropsychologia. 2008;46:2607–2614. doi: 10.1016/j.neuropsychologia.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Decety J. The neurodevelopment of empathy in humans. Dev Neurosci. 2010;32(4):257–67. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blair RJR. Neurobiological bases of psychopathy. British Journal of Psychiatry. 2003;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- 11.Roth-Hanania R, Davidov M, Zahn-Waxler C. Empathy development from 8 to 16 months: Early signs of concern for others. Infant Behavior and Development. 2011;34:447–458. doi: 10.1016/j.infbeh.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Craig KD. The social communication model of pain. Can Psychol. 2009;50:22–32. [Google Scholar]
- 13.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others: A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Akitsuki Y, Decety J. Social context and perceived agency modulate brain activity in the neural circuits underpinning empathy for pain: an event-related fMRI study. Neuroimage. 2009;47(7):722–734. doi: 10.1016/j.neuroimage.2009.04.091. [DOI] [PubMed] [Google Scholar]
- 15.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain: An event-related fMRI study. Neuropsychologia. 2006;44:752–61. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? J Cogn Neurosci. 2010;2:362–376. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- 17.Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not the sensory components of pain. Science. 2004;303:1157–1161. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 18.Cheng Y, Chen CY, Lin CP, Chou KH, Decety J. Love hurts: an fMRI study. Neuroimage. 2010;51:923–929. doi: 10.1016/j.neuroimage.2010.02.047. 2010. [DOI] [PubMed] [Google Scholar]
- 19.Benuzzi F, Lui F, Duzzi D, Nichelli PF, Porro CA. Does it look painful or disgusting? Ask your parietal and cingulate cortex. Journal of Neuroscience. 2008;28:923–931. doi: 10.1523/JNEUROSCI.4012-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Anderson NE, Kiehl KA. The psychopath magnetized: insights from brain imaging. TICS. 2011;16:52–60. doi: 10.1016/j.tics.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair RJR. The amygdala and ventromedial prefrontal cortex in morality and psychopathy. TICS. 2007;11:387–392. doi: 10.1016/j.tics.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Cheng Y, Hung A, Decety J. Dissociation between affective sharing and emotion understanding in juvenile psychopaths. Dev Psychopathol. 2012;24:623–636. doi: 10.1017/S095457941200020X. [DOI] [PubMed] [Google Scholar]
- 24.de Wied M, Gispen-de Wied C, van Boxtel A. Empathy dysfunction in children and adolescents with disruptive behavior disorders. Eur J Pharmacol. 2010;626:97–103. doi: 10.1016/j.ejphar.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Decety J, Michalska KJ, Akitsuki Y, Lahey B. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80:203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harenski CL, Thornton DM, Harenski KA, Decety J, Kiehl KA. Increased fronto-temporal activation during pain observation in sexual sadism. Archives of General Psychiatry. 2012;69:283–292. doi: 10.1001/archgenpsychiatry.2011.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decety J, Echols SC, Correll J. The blame game: The effect of responsibility and social Stigma on empathy for pain. Journal of Cognitive Neuroscience. 2010;22:985–997. doi: 10.1162/jocn.2009.21266. [DOI] [PubMed] [Google Scholar]
- 28.Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007;19:42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Saarela MV, Hluschuk Y, Williams AC, Schurmann M, Lalso E, Hari R. The compassionate brain: humans detect intensity of pain from another's face. Cerebral Cortex. 2007;17:230–237. doi: 10.1093/cercor/bhj141. [DOI] [PubMed] [Google Scholar]
- 30.Simon D, Craig KD, Miltner WH, Rainville P. Brain responses to dynamic facial expressions of pain. Pain. 2006;126:309–318. doi: 10.1016/j.pain.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 31.Gleichgerrcht E, Torralva T, Roca M, Pose M, Manes F. The role of social cognition in moral judgment in frontotemporal dementia. Social Neuroscience. 2011;6:113–122. doi: 10.1080/17470919.2010.506751. [DOI] [PubMed] [Google Scholar]
- 32.Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–2946. doi: 10.1093/brain/awl254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. American Psychiatric Association; Washington, DC: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- 34.Wager TD, Nichols TE. Optimization of experimental design in fMRI: a general framework using a genetic algorithm. Neuroimage. 2003;18:293–309. doi: 10.1016/s1053-8119(02)00046-0. [DOI] [PubMed] [Google Scholar]
- 35.Budell L, Jackson PL, Rainville P. Brain responses to facial expression of pain: emotional or motor mirroring? Neuroimage. 2010;53:355–363. doi: 10.1016/j.neuroimage.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 36.Botvinick M, Jha AP, Bylsma LM, Fabian SA, Solomon PE, Prkachin KM. Viewing facial expressions of pain engages cortical areas involved in the direct experience of pain. Neuroimage. 2005;25:312–319. doi: 10.1016/j.neuroimage.2004.11.043. [DOI] [PubMed] [Google Scholar]
- 37.Skelly LR, Decety J. Passive and motivated perception of emotional faces: qualitative and quantitative changes in the face processing network. PLoS One. 2012;7(6):e40371. doi: 10.1371/journal.pone.0040371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. 2011;49(11):2971–2984. doi: 10.1016/j.neuropsychologia.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 39.Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 40.Gu X, Liu X, Guise KG, Naidich TP, Hof PR, Fan J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J Neurosci. 2010;30:3739–3744. doi: 10.1523/JNEUROSCI.4844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Oliveira-Souza R, Hare RD, Bramati IE, Garrido GJ, Ignacio FA, Tovar-Moll F, Moll J. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213. doi: 10.1016/j.neuroimage.2007.12.054. [DOI] [PubMed] [Google Scholar]
- 42.Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs077. doi:10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muller JL, Sommer M, Dohnel K, Weber T, Schmidt-Wilcke T, Hajak G. Disturbed prefrontal and temporal brain function during emotion and cognition interaction in criminal psychopathy. Behavioral Sciences and the Law. 2008;26:131–150. doi: 10.1002/bsl.796. [DOI] [PubMed] [Google Scholar]
- 45.Rilling JK, Glenn AL, Jairam MR, Pagnoni G, Goldsmith DR, Elfenbein HA, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007;61:1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 46.Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Morphological alterations in the prefrontal cortex and the amygdala in unsuccessful psychopaths. J. Abnorm. Psychology. 2010;119:546–554. doi: 10.1037/a0019611. [DOI] [PubMed] [Google Scholar]
- 47.Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Res. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Müller JL, Sommer M, Wagner V, Lange K, Taschler H, Röder CH. Abnormalities in emotion processing within cortical and subcortical regions in criminal psychopaths: evidence from a functional magnetic resonance imaging study using pictures with emotional content. Biological Psychiatry. 2003;54:152–162. doi: 10.1016/s0006-3223(02)01749-3. [DOI] [PubMed] [Google Scholar]
- 49.Brink TT, Urton K, Held D, Kirilina E, Hofmann MJ, Klann-Delius G, Jacobs AM, Kuchinkle L. The role of orbitofrontal cortex in processing empathy stories in 4-8year-old children. Frontiers in Psychology. 2011;2:80. doi: 10.3389/fpsyg.2011.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decety J, Michalska KJ, Kinzler KD. The contribution of emotion and cognition to moral sensitivity: A neurodevelopmental study. Cerebral Cortex. 2012;22:209–220. doi: 10.1093/cercor/bhr111. [DOI] [PubMed] [Google Scholar]
- 51.Masten CL, Morelli SA, Eisenberger NI. An fMRI investigation of empathy for social pain and subsequent prosocial behavior. Neuroimage. 2011;55:381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 52.Blair RJR, Cipolotti L. Impaired social response reversal: a case of acquired sociopathy. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- 53.Shamay-Tsoory S. Empathic processing: Its cognitive and affective dimensions and neuroanatomical basis. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. 2009. pp. 215–232. [Google Scholar]
- 54.Shamay-Tsoory SG, Harari H, Aharon-Peretz J, Levkovitz Y. The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex. 2010;46:668–677. doi: 10.1016/j.cortex.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Juárez M, Kiehl KA, Calhoun VD. Intrinsic limbic and paralimbic networks are associated with criminal psychopathy. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22037. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glenn AL, Raine A. Psychopathy and instrumental aggression: evolutionary, neurobiological and legal perspectives. International Journal of Law and Psychiatry. 2009;32:253–258. doi: 10.1016/j.ijlp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Flight JI, Forth AE. Instrumentally violent youths: The roles of psychopathic traits, empathy, and attachment. Criminal Justice and Behavior. 2007;34:739–51. [Google Scholar]
- 58.Porter S, Birt AR, Boer DP. Investigation of the criminal and conditional release histories of Canadian federal offenders as a function of psychopathy and age. Law and Human Behavior. 2001;25:647–61. doi: 10.1023/a:1012710424821. [DOI] [PubMed] [Google Scholar]
- 59.Panksepp J. Affective neuroscience: the foundations of human and animal emotions. Oxford University Press; New York: 1998. [Google Scholar]
- 60.Batson CD. These things called empathy: eight related but distinct phenomena. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. MIT Press; Cambridge: 2009. pp. 3–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.