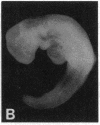
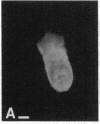
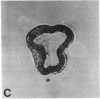
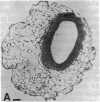
Abstract
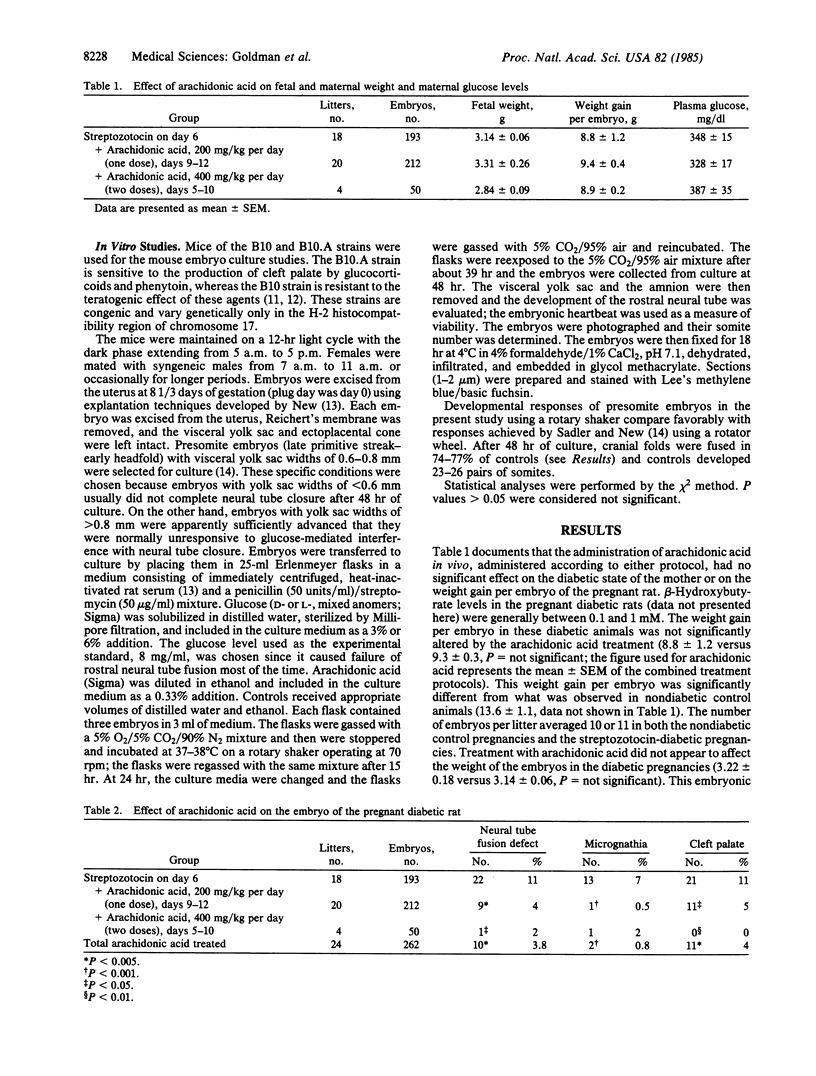
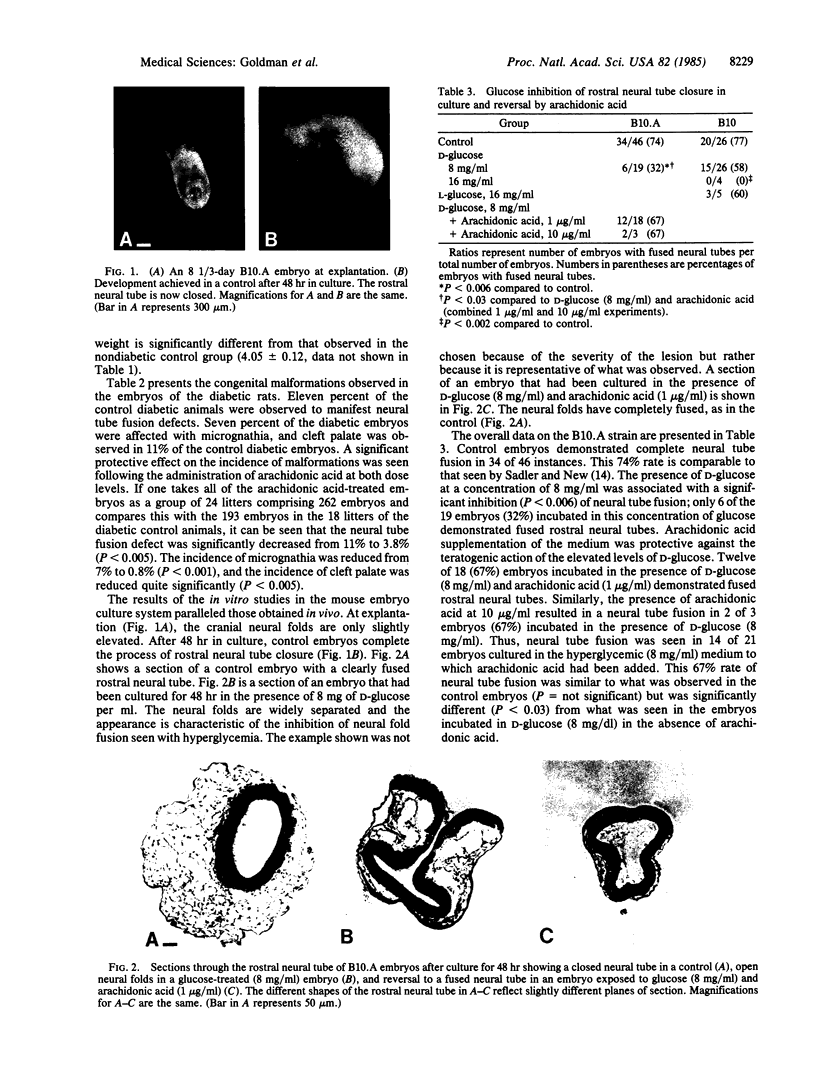
Congenital malformations now represent the largest single cause of mortality in the infant of the diabetic mother. The mechanism by which diabetes exerts its teratogenic effects is not known. This study evaluated whether arachidonic acid might be involved, a possibility raised by the role of arachidonic acid in palatal elevation and fusion, processes analogous to neural tube folding and fusion. This hypothesis was tested in two animal models of diabetic embryopathy, the in vivo pregnant diabetic rat and the in vitro hyperglycemic mouse embryo culture. The subcutaneous injection of arachidonic acid (200-400 mg/kg per day) into pregnant diabetic rats during the period of organ differentiation (days 6-12) did not alter the maternal glucose concentration, the maternal weight gain, or the weight of the embryos. However, the incidence of neural tube fusion defects was reduced from 11% to 3.8% (P less than 0.005), the frequency of cleft palate was reduced from 11% to 4% (P less than 0.005), and the incidence of micrognathia was reduced from 7% to 0.8% (P less than 0.001). The addition of arachidonic acid to B10.A mouse embryos in culture also resulted in a reversal of hyperglycemia-induced teratogenesis. The teratogenic effect of D-glucose (8 mg/ml) in the medium resulted in normal neural tube fusion in only 32% of the embryos (P less than 0.006 when compared to controls). Arachidonic acid supplementation (1 or 10 micrograms/ml) produced a rate of neural tube fusion (67%) that was not significantly different from that observed in controls. The evidence presented indicates that arachidonic acid supplementation exerts a significant protective effect against the teratogenic action of hyperglycemia in both in vivo (rat) and in vitro (mouse) animal models. These data therefore suggest that the mechanism mediating the teratogenic effect of an increased glucose concentration involves a functional deficiency of arachidonic acid at a critical stage of organogenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker L., Egler J. M., Klein S. H., Goldman A. S. Meticulous control of diabetes during organogenesis prevents congenital lumbosacral defects in rats. Diabetes. 1981 Nov;30(11):955–959. doi: 10.2337/diab.30.11.955. [DOI] [PubMed] [Google Scholar]
- Cockroft D. L., Coppola P. T. Teratogenic effects of excess glucose on head-fold rat embryos in culture. Teratology. 1977 Oct;16(2):141–146. doi: 10.1002/tera.1420160205. [DOI] [PubMed] [Google Scholar]
- Eriksson U. J., Dahlström V. E., Styrud J. Metabolically determined teratogenesis: malformations and maternal diabetes. Biochem Soc Trans. 1985 Feb;13(1):79–82. doi: 10.1042/bst0130079. [DOI] [PubMed] [Google Scholar]
- Eriksson U., Dahlström E., Larsson K. S., Hellerström C. Increased incidence of congenital malformations in the offspring of diabetic rats and their prevention by maternal insulin therapy. Diabetes. 1982 Jan;31(1):1–6. doi: 10.2337/diab.31.1.1. [DOI] [PubMed] [Google Scholar]
- Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980 Dec;29(12):1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- Fuhrmann K., Reiher H., Semmler K., Fischer F., Fischer M., Glöckner E. Prevention of congenital malformations in infants of insulin-dependent diabetic mothers. Diabetes Care. 1983 May-Jun;6(3):219–223. doi: 10.2337/diacare.6.3.219. [DOI] [PubMed] [Google Scholar]
- Gabbe S. G. Congenital malformations in infants of diabetic mothers. Obstet Gynecol Surv. 1977 Mar;32(3):125–132. doi: 10.1097/00006254-197703000-00001. [DOI] [PubMed] [Google Scholar]
- Garnham E. A., Beck F., Clarke C. A., Stanisstreet M. Effects of glucose on rat embryos in culture. Diabetologia. 1983 Sep;25(3):291–295. doi: 10.1007/BF00279946. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Baker M. K., Gasser D. L. Susceptibility to phenytoin-induced cleft palate in mice is influenced by genes linked to H-2 and H-3. Immunogenetics. 1983;18(1):17–22. doi: 10.1007/BF00401352. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Baker M. K., Piddington R., Herold R. Inhibition of programmed cell death in mouse embryonic palate in vitro by cortisol and phenytoin: receptor involvement and requirement of protein synthesis. Proc Soc Exp Biol Med. 1983 Nov;174(2):239–243. doi: 10.3181/00379727-174-41731. [DOI] [PubMed] [Google Scholar]
- Goldman A. S. Biochemical mechanism of glucocorticoid-and phenytoin-induced cleft palate. Curr Top Dev Biol. 1984;19:217–239. doi: 10.1016/s0070-2153(08)60401-9. [DOI] [PubMed] [Google Scholar]
- Goldman A. S., Herold R., Piddington R. Inhibition of programmed cell death in the fetal palate by cortisol. Proc Soc Exp Biol Med. 1981 Mar;166(3):418–424. doi: 10.3181/00379727-166-41084. [DOI] [PubMed] [Google Scholar]
- Grix A., Jr Malformations in infants of diabetic mothers. Am J Med Genet. 1982 Oct;13(2):131–137. doi: 10.1002/ajmg.1320130205. [DOI] [PubMed] [Google Scholar]
- Gupta C., Katsumata M., Goldman A. S. H-2 influences phenytoin binding and inhibition of prostaglandin synthesis. Immunogenetics. 1984;20(6):667–676. doi: 10.1007/BF00430325. [DOI] [PubMed] [Google Scholar]
- Gupta C., Katsumata M., Goldman A. S., Herold R., Piddington R. Glucocorticoid-induced phospholipase A2-inhibitory proteins mediate glucocorticoid teratogenicity in vitro. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1140–1143. doi: 10.1073/pnas.81.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii K. I., Watanabe G. I., Ingalls T. H. Experimental diabetes in pregnant mice. Prevention of congenital malformations in offspring by insulin. Diabetes. 1966 Mar;15(3):194–204. doi: 10.2337/diab.15.3.194. [DOI] [PubMed] [Google Scholar]
- Horton W. E., Jr, Sadler T. W. Effects of maternal diabetes on early embryogenesis. Alterations in morphogenesis produced by the ketone body, B-hydroxybutyrate. Diabetes. 1983 Jul;32(7):610–616. doi: 10.2337/diab.32.7.610. [DOI] [PubMed] [Google Scholar]
- Katsumata M., Baker M. K., Goldman A. S., Gasser D. L. Influence of H-2-linked genes on glucocorticoid receptors in the fetal mouse palate. Immunogenetics. 1981;13(4):319–325. doi: 10.1007/BF00364497. [DOI] [PubMed] [Google Scholar]
- Miller E., Hare J. W., Cloherty J. P., Dunn P. J., Gleason R. E., Soeldner J. S., Kitzmiller J. L. Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. N Engl J Med. 1981 May 28;304(22):1331–1334. doi: 10.1056/NEJM198105283042204. [DOI] [PubMed] [Google Scholar]
- Mills J. L., Baker L., Goldman A. S. Malformations in infants of diabetic mothers occur before the seventh gestational week. Implications for treatment. Diabetes. 1979 Apr;28(4):292–293. doi: 10.2337/diab.28.4.292. [DOI] [PubMed] [Google Scholar]
- Moran D., Rice R. W. An ultrastructural examination of the role of cell membrane surface coat material during neurulation. J Cell Biol. 1975 Jan;64(1):172–181. doi: 10.1083/jcb.64.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New D. A. Whole-embryo culture and the study of mammalian embryos during organogenesis. Biol Rev Camb Philos Soc. 1978 Feb;53(1):81–122. doi: 10.1111/j.1469-185x.1978.tb00993.x. [DOI] [PubMed] [Google Scholar]
- Piddington R., Herold R., Goldman A. S. Further evidence for a role of arachidonic acid in glucocorticoid teratogenic action in the palate. Proc Soc Exp Biol Med. 1983 Dec;174(3):336–342. doi: 10.3181/00379727-174-41745. [DOI] [PubMed] [Google Scholar]
- Sadler T. W. Effects of maternal diabetes on early embryogenesis: II. Hyperglycemia-induced exencephaly. Teratology. 1980 Jun;21(3):349–356. doi: 10.1002/tera.1420210311. [DOI] [PubMed] [Google Scholar]
- Sadler T. W., Horton W. E., Jr Effects of maternal diabetes on early embryogenesis. The role of insulin and insulin therapy. Diabetes. 1983 Nov;32(11):1070–1074. doi: 10.2337/diab.32.11.1070. [DOI] [PubMed] [Google Scholar]
- Sadler T. W., New D. A. Culture of mouse embryos during neurulation. J Embryol Exp Morphol. 1981 Dec;66:109–116. [PubMed] [Google Scholar]
- Schlüter G. Ultrastructural observations on cell necrosis during formation of the neural tube in mouse embryos. Z Anat Entwicklungsgesch. 1973;141(3):251–264. doi: 10.1007/BF00519046. [DOI] [PubMed] [Google Scholar]
- Soler N. G., Walsh C. H., Malins J. M. Congenital malformations in infants of diabetic mothers. Q J Med. 1976 Apr;45(178):303–313. [PubMed] [Google Scholar]
- Tzortzatou G. G., Goldman A. S., Boutwell W. C. Evidence for a role of arachidonic acid in glucocorticoid-induced cleft palate in rats. Proc Soc Exp Biol Med. 1981 Mar;166(3):321–324. doi: 10.3181/00379727-166-41067. [DOI] [PubMed] [Google Scholar]
- WATANABE G., INGALLS T. H. Congenital malformations in the offspring of alloxan-diabetic mice. Diabetes. 1963 Jan-Feb;12:66–72. doi: 10.2337/diab.12.1.66. [DOI] [PubMed] [Google Scholar]
- Waterman R. E., Ross L. M., Meller S. M. Alterations in the epithelial surface of A-Jax mouse palatal shelves prior to and during palatal fusion: a scanning electron microscopic study. Anat Rec. 1973 Jul;176(3):361–375. doi: 10.1002/ar.1091760311. [DOI] [PubMed] [Google Scholar]