Abstract
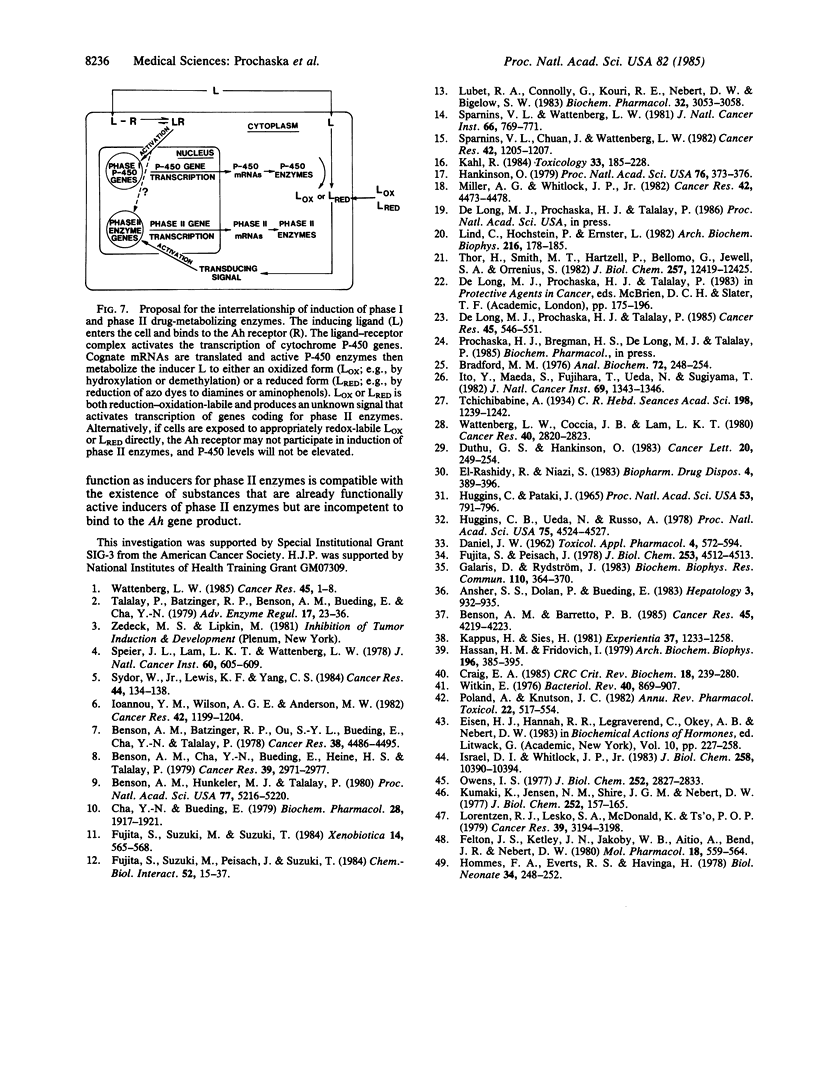
Induction of detoxification enzymes is a major mechanism whereby a wide variety of chemical agents protect rodents against neoplastic, mutagenic, and other toxicities of carcinogens. The enzyme NAD(P)H:(quinone acceptor) oxidoreductase (EC 1.6.99.2) can protect against the toxicities of quinones and is a useful marker for protective enzyme induction. Quinone reductase can be induced in murine Hepa 1c1c7 hepatoma cells and 3T3 embryo fibroblasts by compounds that are chemoprotectors in vivo, including some phenolic antioxidants, azo dyes, aromatic diamines, and aminophenols. Structurally dissimilar catechols (1,2-diphenols) and hydroquinones (1,4-diphenols) induce quinone reductase in these systems, but resorcinol (1,3-diphenol) and its substituted analogues are inactive. Furthermore, only aromatic 1,2- and 1,4-diamines and aminophenols are inducers, whereas the 1,3-diamines are completely inactive. These findings suggest that the functional capacity to form quinones or quinone-diimines, rather than the precise structure, is essential for inductive activity and that the generation of the signal for enzyme induction depends upon oxidation-reduction lability. The observations that some chemoprotective compounds (e.g., azo dyes, beta-naphthoflavone) induce both cytochromes P-450 and quinone reductase, whereas others (e.g., tert-butylhydroquinone) induce only quinone reductase, can be reconciled by the fact that inducers of the first type are metabolized by P-450 enzymes to form products that are functionally similar to compounds of the second type.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansher S. S., Dolan P., Bueding E. Chemoprotective effects of two dithiolthiones and of butylhydroxyanisole against carbon tetrachloride and acetaminophen toxicity. Hepatology. 1983 Nov-Dec;3(6):932–935. doi: 10.1002/hep.1840030608. [DOI] [PubMed] [Google Scholar]
- Benson A. M., Barretto P. B. Effects of disulfiram, diethyldithiocarbamate, bisethylxanthogen, and benzyl isothiocyanate on glutathione transferase activities in mouse organs. Cancer Res. 1985 Sep;45(9):4219–4223. [PubMed] [Google Scholar]
- Benson A. M., Batzinger R. P., Ou S. Y., Bueding E., Cha Y. N., Talalay P. Elevation of hepatic glutathione S-transferase activities and protection against mutagenic metabolites of benzo(a)pyrene by dietary antioxidants. Cancer Res. 1978 Dec;38(12):4486–4495. [PubMed] [Google Scholar]
- Benson A. M., Cha Y. N., Bueding E., Heine H. S., Talalay P. Elevation of extrahepatic glutathione S-transferase and epoxide hydratase activities by 2(3)-tert-butyl-4-hydroxyanisole. Cancer Res. 1979 Aug;39(8):2971–2977. [PubMed] [Google Scholar]
- Benson A. M., Hunkeler M. J., Talalay P. Increase of NAD(P)H:quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cha Y. N., Bueding E. Effects of 2(3)-tert-butyl-4-hydroxyanisole administration on the activities of several hepatic microsomal and cytoplasmic enzymes in mice. Biochem Pharmacol. 1979 Jun 15;28(12):1917–1921. doi: 10.1016/0006-2952(79)90645-2. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- DANIEL J. W. The excretion and metabolism of edible food colors. Toxicol Appl Pharmacol. 1962 Sep;4:572–594. doi: 10.1016/0041-008x(62)90085-6. [DOI] [PubMed] [Google Scholar]
- De Long M. J., Prochaska H. J., Talalay P. Tissue-specific induction patterns of cancer-protective enzymes in mice by tert-butyl-4-hydroxyanisole and related substituted phenols. Cancer Res. 1985 Feb;45(2):546–551. [PubMed] [Google Scholar]
- Duthu G. S., Hankinson O. The defects in all classes of aryl hydrocarbon hydroxylase-deficient mutant of mouse hepatoma line, Hepa-1, are restricted to activities catalyzed by cytochrome P-450. Cancer Lett. 1983 Oct;20(3):249–254. doi: 10.1016/0304-3835(83)90021-6. [DOI] [PubMed] [Google Scholar]
- Felton J. S., Ketley J. N., Jakoby W. B., Aitio A., Bend J. R., Nebert D. W. Hepatic glutathione transferase activity induced by polycyclic aromatic compounds. Lack of correlation with the murine Ah locus. Mol Pharmacol. 1980 Nov;18(3):559–564. [PubMed] [Google Scholar]
- Fujita S., Peisach J. Liver microsomal cytochromes P-450 and azoreductase activity. J Biol Chem. 1978 Jul 10;253(13):4512–4513. [PubMed] [Google Scholar]
- Fujita S., Suzuki M., Peisach J., Suzuki T. Induction of hepatic microsomal drug metabolism by azo compounds: a structure-activity relationship. Chem Biol Interact. 1984 Nov;52(1):15–37. doi: 10.1016/0009-2797(84)90080-2. [DOI] [PubMed] [Google Scholar]
- Fujita S., Suzuki M., Suzuki T. Structure-activity relationships in the induction of hepatic drug metabolism by azo compounds. Xenobiotica. 1984 Jul;14(7):565–568. doi: 10.3109/00498258409151449. [DOI] [PubMed] [Google Scholar]
- Galaris D., Rydström J. Enzyme induction by daunorubicin in neonatal heart cells in culture. Biochem Biophys Res Commun. 1983 Jan 27;110(2):364–370. doi: 10.1016/0006-291x(83)91157-9. [DOI] [PubMed] [Google Scholar]
- HUGGINS C., PATAKI J. AROMATIC AZO DERIVATIVES PREVENTING MAMMARY CANCER AND ADRENAL INJURY FROM 7,12-DIMETHYLBENZ(A)ANTHRACENE. Proc Natl Acad Sci U S A. 1965 Apr;53:791–796. doi: 10.1073/pnas.53.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankinson O. Single-step selection of clones of a mouse hepatoma line deficient in aryl hydrocarbon hydroxylase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):373–376. doi: 10.1073/pnas.76.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Intracellular production of superoxide radical and of hydrogen peroxide by redox active compounds. Arch Biochem Biophys. 1979 Sep;196(2):385–395. doi: 10.1016/0003-9861(79)90289-3. [DOI] [PubMed] [Google Scholar]
- Hommes F. A., Everts R. S., Havinga H. The development of DT-diaphorase in rat liver and its induction by benzo(a)pyrene. Biol Neonate. 1978;34(5-6):248–252. doi: 10.1159/000241136. [DOI] [PubMed] [Google Scholar]
- Huggins C. B., Ueda N., Russo A. Azo dyes prevent hydrocarbon-induced leukemia in the rat. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4524–4527. doi: 10.1073/pnas.75.9.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannou Y. M., Wilson A. G., Anderson M. W. Effect of butylated hydroxyanisole, alpha-angelica lactone, and beta-naphthoflavone on benzo(alpha)pyrene:DNA adduct formation in vivo in the forestomach, lung, and liver of mice. Cancer Res. 1982 Apr;42(4):1199–1204. [PubMed] [Google Scholar]
- Israel D. I., Whitlock J. P., Jr Induction of mRNA specific for cytochrome P1-450 in wild type and variant mouse hepatoma cells. J Biol Chem. 1983 Sep 10;258(17):10390–10394. [PubMed] [Google Scholar]
- Ito Y., Maeda S., Fujihara T., Ueda N., Sugiyama T. Suppression of 7,12-dimethylbenz[a]anthracene-induced chromosome aberrations in rat bone marrow cells after treatment with Sudan III and related azo dyes. J Natl Cancer Inst. 1982 Dec;69(6):1343–1346. [PubMed] [Google Scholar]
- Kahl R. Synthetic antioxidants: biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicology. 1984 Dec;33(3-4):185–228. doi: 10.1016/0300-483x(84)90038-6. [DOI] [PubMed] [Google Scholar]
- Kappus H., Sies H. Toxic drug effects associated with oxygen metabolism: redox cycling and lipid peroxidation. Experientia. 1981 Dec 15;37(12):1233–1241. doi: 10.1007/BF01948335. [DOI] [PubMed] [Google Scholar]
- Kumaki K., Jensen N. M., Shire J. G., Nebert D. W. Genetic differences in induction of cytosol reduced-NAD(P):menadione oxidoreductase and microsomal aryl hydrocarbon hydroxylase in the mouse. J Biol Chem. 1977 Jan 10;252(1):157–165. [PubMed] [Google Scholar]
- Lind C., Hochstein P., Ernster L. DT-diaphorase as a quinone reductase: a cellular control device against semiquinone and superoxide radical formation. Arch Biochem Biophys. 1982 Jun;216(1):178–185. doi: 10.1016/0003-9861(82)90202-8. [DOI] [PubMed] [Google Scholar]
- Lorentzen R. J., Lesko S. A., McDonald K., Ts'o P. O. Toxicity of metabolic benzo(a)pyrenediones to cultured cells and the dependence upon molecular oxygen. Cancer Res. 1979 Aug;39(8):3194–3198. [PubMed] [Google Scholar]
- Lubet R. A., Connolly G., Kouri R. E., Nebert D. W., Bigelow S. W. Biological effects of the Sudan dyes. Role of the Ah cytosolic receptor. Biochem Pharmacol. 1983 Oct 15;32(20):3053–3058. doi: 10.1016/0006-2952(83)90248-4. [DOI] [PubMed] [Google Scholar]
- Miller A. G., Whitlock J. P., Jr Efficient metabolism of benzo(a)pyrene at nanomolar concentrations by intact murine hepatoma cells. Cancer Res. 1982 Nov;42(11):4473–4478. [PubMed] [Google Scholar]
- Owens I. S. Genetic regulation of UDP-glucuronosyltransferase induction by polycyclic aromatic compounds in mice. Co-segregation with aryl hydrocarbon (benzo(alpha)pyrene) hydroxylase induction. J Biol Chem. 1977 May 10;252(9):2827–2833. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Sparnins V. L., Chuan J., Wattenberg L. W. Enhancement of glutathione S-transferase activity of the esophagus by phenols, lactones, and benzyl isothiocyanate. Cancer Res. 1982 Apr;42(4):1205–1207. [PubMed] [Google Scholar]
- Sparnins V. L., Wattenberg L. W. Enhancement of glutathione S-transferase activity of the mouse forestomach by inhibitors of Benzo[a]pyrene-induced neoplasia of the forestomach. J Natl Cancer Inst. 1981 Apr;66(4):769–771. [PubMed] [Google Scholar]
- Speier J. L., Lam L. K., Wattenberg L. W. Effects of administration to mice of butylated hydroxyanisole by oral intubation on benzo[a]pyrene-induced pulmonary adenoma formation and metabolism of benzo[a]pyrene. J Natl Cancer Inst. 1978 Mar;60(3):605–609. doi: 10.1093/jnci/60.3.605. [DOI] [PubMed] [Google Scholar]
- Sydor W., Jr, Lewis K. F., Yang C. S. Effects of butylated hydroxyanisole on the metabolism of benzo(a)pyrene by mouse lung microsomes. Cancer Res. 1984 Jan;44(1):134–138. [PubMed] [Google Scholar]
- Talalay P., Batzinger R. P., Benson A. M., Bueding E., Cha Y. N. Biochemical studies on the mechanisms by which dietary antioxidants suppress mutagenic activity. Adv Enzyme Regul. 1978;17:23–36. doi: 10.1016/0065-2571(79)90006-2. [DOI] [PubMed] [Google Scholar]
- Thor H., Smith M. T., Hartzell P., Bellomo G., Jewell S. A., Orrenius S. The metabolism of menadione (2-methyl-1,4-naphthoquinone) by isolated hepatocytes. A study of the implications of oxidative stress in intact cells. J Biol Chem. 1982 Oct 25;257(20):12419–12425. [PubMed] [Google Scholar]
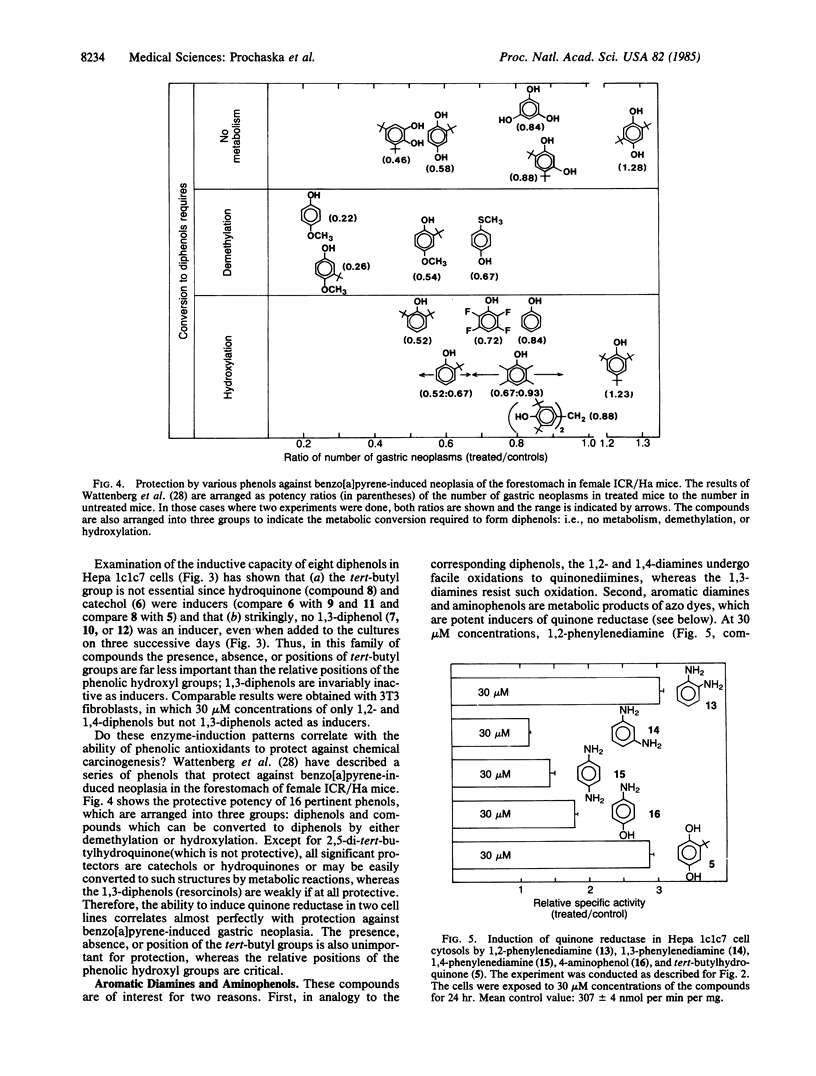
- Wattenberg L. W. Chemoprevention of cancer. Cancer Res. 1985 Jan;45(1):1–8. [PubMed] [Google Scholar]
- Wattenberg L. W., Coccia J. B., Lam L. K. Inhibitory effects of phenolic compounds on benzo(a)pyrene-induced neoplasia. Cancer Res. 1980 Aug;40(8 Pt 1):2820–2823. [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


